Published online Feb 14, 2017. doi: 10.3748/wjg.v23.i6.935
Peer-review started: August 10, 2016
First decision: September 5, 2016
Revised: September 7, 2016
Accepted: January 18, 2017
Article in press: January 18, 2017
Published online: February 14, 2017
Processing time: 188 Days and 23.8 Hours
Familial pancreatic cancer (FPC) is broadly defined as two first-degree-relatives with pancreatic cancer (PC) and accounts for 4%-10% of PC. Several genetic syndromes, including Peutz-Jeghers syndrome, hereditary pancreatitis, hereditary breast-ovarian cancer syndrome (HBOC), Lynch syndrome, and familial adenomatous polyposis (FAP), also have increased risks of PC, but the narrowest definition of FPC excludes these known syndromes. When compared with other familial tumors, proven genetic alterations are limited to a small proportion (< 20%) and the familial aggregation is usually modest. However, an ethnic deviation (Ashkenazi Jewish > Caucasian) and a younger onset are common also in FPC. In European countries, “anticipation” is reported in FPC families, as with other hereditary syndromes; a trend toward younger age and worse prognosis is recognized in the late years. The resected pancreases of FPC kindred often show multiple pancreatic intraepithelial neoplasia (PanIN) foci, with various K-ras mutations, similar to colorectal polyposis seen in the FAP patients. As with HBOC patients, a patient who is a BRCA mutation carrier with unresectable pancreatic cancer (accounting for 0%-19% of FPC patients) demonstrated better outcome following platinum and Poly (ADP-ribose) polymerase inhibitor treatment. Western countries have established FPC registries since the 1990s and several surveillance projects for high-risk individuals are now ongoing to detect early PCs. Improvement in lifestyle habits, including non-smoking, is recommended for individuals at risk. In Japan, the FPC study group was initiated in 2013 and the Japanese FPC registry was established in 2014 by the Japan Pancreas Society.
Core tip: The incidence of pancreatic cancer increases with the number of family members with pancreatic cancer (PC). Familial pancreatic cancer (FPC) is defined as at least two first-degree relatives with PC that does not meet the criteria of other hereditary cancer syndromes. FPC has some epidemiological, pathological, and therapeutic characteristics. Since the 1990s, FPC registries have been established for use in studies to follow up high-risk individuals with family history of PC and hereditary cancer syndromes. Japan initiated a nationwide FPC registry in 2014, and several projects are expected at both the clinical and basic levels.
- Citation: Matsubayashi H, Takaori K, Morizane C, Maguchi H, Mizuma M, Takahashi H, Wada K, Hosoi H, Yachida S, Suzuki M, Usui R, Furukawa T, Furuse J, Sato T, Ueno M, Kiyozumi Y, Hijioka S, Mizuno N, Terashima T, Mizumoto M, Kodama Y, Torishima M, Kawaguchi T, Ashida R, Kitano M, Hanada K, Furukawa M, Kawabe K, Majima Y, Shimosegawa T. Familial pancreatic cancer: Concept, management and issues. World J Gastroenterol 2017; 23(6): 935-948
- URL: https://www.wjgnet.com/1007-9327/full/v23/i6/935.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i6.935
Today, in both Japan and Unites States, the number of patients with pancreatic cancer (PC) is gradually increasing[1,2]. The nationwide cancer deaths due to PC is now total over 30000, so that PC ranks fourth among all human cancers (http://ganjoho.jp/reg_stat/statistics/dl/index.html#mortality)[1]. A survey by the Japanese Pancreas Society (2012) indicated an overall 5 year survival for PC patients of only 13.0%. However, when treated when the tumor size is ≤ 10 mm or within UICC-Stage 0, the 5 year survival increases to 80.4% and 85.8%, respectively[2]. The best strategy for curing this deadly cancer is currently thought to be early detection by following high-risk individuals and resection at a suitable time.
The risk factors for PC include image-detectable pancreatic diseases and lifestyle factors. The former includes pancreatic cysts[3,4], pancreatic duct dilation[3], intraductal papillary mucinous neoplasm (IPMN)[5], and chronic pancreatitis[6,7], while the latter includes smoking[8-10], diabetes mellitus[10-12], obesity[13,14], and low vitamin intake[15], among others. A family history of PC is another known risk, and one that cannot be modified by individual effort or by medicine.
Various human cancers show family history as a risk of the same cancer developing in related family members[16-18]. Several case-control studies and cohort studies have demonstrated an increased risk of PC in those who have a first degree relative (FDR) who is a PC patient [2.1[19]-5.3[20] of odds ratio (OR) and 1.5[21]-1.7[22] of relative risk (RR)[23]]. The incidence of PC increases with the number of family members with PC, so that persons with one FDR with PC have a 4.5 fold increased risk of PC, those with two FDRs have a 6.4 fold increased risk, and those with three or more FDRs have up to a 32 fold risk[24]. The presence of two or more pancreatic cancer patients within FDRs, and without association with known hereditary genetic syndromes, is defined as familial pancreatic cancer (FPC).
The incidence of FPC among total cases of PC is 4%-10%. However, highly affected families are rare (i.e., families with three or more PC cases within FDRs account for only 0.5% of all PC cases in Japan)[10], and their inherited risk is not as high as that of other human malignancies (e.g., melanoma, prostate cancer, ovarian cancer, and breast cancer) as confirmed by a study of a large number of twins in Nordic countries[25]. Several environmental factors (tobacco smoke, asbestos, radon)[10,26] have been reported in cases of FPC, and we must bear in mind that "familial PC" is not a synonym for "inherited PC". With the mentioned criteria, pathogenic germline mutation has been proven in less than 20% of FPC cases, and this is far lower than is observed in other familial cancers associated with the pancreatic neoplasms, such as multiple endocrine neoplasia type 1 (MEN1) and von Hippel-Lindau disease.
Higher risks of PC are also associated with some inherited syndromes, such as Peutz-Jeghers syndrome (PJS)[27], hereditary pancreatitis (HP)[28-31], familial atypical multiple mole melanoma (FAMMM)[32,33], hereditary breast-ovarian cancer (HBOC)[34-37], hereditary nonpolyposis colorectal cancer [HNPCC, Lynch syndrome (LS)][38,39], familial adenomatous polyposis (FAP)[40], and Werner syndrome[41] (Table 1). However, these syndromes are excluded from the definition of FPC in its narrowest meaning. In western countries, high risk individuals (HRI) with a family history of PC and hereditary cancer syndromes have been participating in nationwide or institutional FPC registries[42], and clinical surveillance and basic research have been performed to detect PC in its early stage. This review has focused on the concept and the current outcomes of surveillance of HRI.
| Inherited syndrome | Relative risk | Cumulative risk of PC | Responsible gene |
| Peutz-Jeghers syndrome[27] | 132 | 11%-36% | STK11 |
| Hereditary pancreatitis[28-31] | 53-87 | 40%-55% | PRSS1 |
| Familial atypical multiple mole melanoma[32,33] | 13-22 | 17% | CDKN2A |
| Hereditary breast-ovarian cancer syndrome[34-37,73] | 4-13 | 2%-7% | BRCA1, BRCA2 |
| Lynch syndrome[38,39] | 5-9 | 4% | MLH1, MSH2, MSH6, PMS2 |
| Familial adenomatous polyposis[40] | 5 | - | APC, MUTYH |
FPC has several epidemiological features that distinguish it from ordinary PC. Similar to other familial cancers, FPC shows a trend toward a younger onset [FPC: age 58[43]-68[44], compared to sporadic PC (SPC): age 61[43]-74[44]] and an ethnic deviation (Ashkenazi Jewish > Caucasian)[34]. The lifetime risk of PC also increases with decreasing age of onset of PC in family members[44,45]. Meanwhile, similar to the sporadic cases, smoking (especially current smoking)[10,26] and diabetes (recent onset of diabetes)[10] are also risks for FPC.
A pedigree of FPC also incurs an increased risk of developing cancer or cancer death from diseases other than PC, such as in melanoma (OR = 16.8, P < 0.0001), endometrial cancer (OR = 5.26, P = 0.034), breast cancer [weighted standardized mortality ratio (wSMR): 1.7], ovarian cancer (wSMR: 2.1), and bile duct cancer (wSMR: 3.0)[46]. Several studies have also demonstrated an unexplained worse prognosis in familial cases than in sporadic cases[26,47], albeit some showed no difference[48]. Surprisingly, two European registries (EUROPAC[30] and FaPaCa[49,50]) that analyzed 106 FPC families (264 affected individuals) through three generations [dates of birth: 1900-1919, 1920-1939, 1940-1969] observed "anticipation" in the affected kindred of FPC patients[51]; that is, a trend existed toward younger age and worse prognosis in the latest generation.
As is found with colorectal polyposis in numerous FAP patients, the pancreatic histology of FPC kindred often demonstrates multiple precancerous lesions[48] or pancreatic intraepithelial neoplasias (PanINs)[52,53]. PanINs with various mutations of KRAS codon 12 are frequently recognized in the vicinity of ordinary PC[54]; however, they are 2.75-fold more frequent in the FPC than in the SPC pancreas[55]. These precursor lesions sometimes appear in the clinical image as small cystic lesions[52,56] and are more often recognized in the pancreases of FPC families than in those of CDKN2A/p16 mutation carriers (By contrast, PC is 10 times more frequent in the latter group)[57]. These lesions in FPC kindred are associated with lobular parenchymal atrophy and chronic pancreatitis-like changes observable by endoscopic ultrasonography (EUS)[53].
Despite the difference in the numbers of precursor lesions[48,53], a blind review of histological observation of 519 FPCs and 561 SPCs by expert pathologists did not show any significant difference in terms of tumor size, location, neural invasion, angiolymphatic invasion, lymph nodal metastasis, and pathological stage[58]. The genome-wide allelic status[59,60], and genetic and epigenetic alterations[61] are also similar between SPC and FPC.
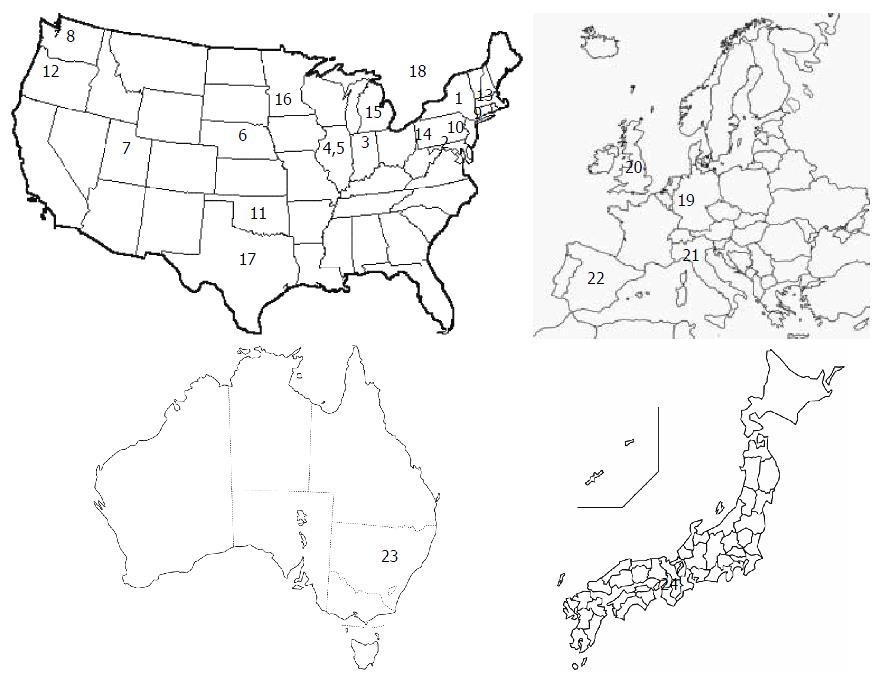
Figure 1 shows a global map of the institutional and nationwide pancreatic cancer registries, including FPC registries. The National Familial Pancreas Tumor Registry (NFPTR) (http://pathology.jhu.edu/pancreas/nfptr/history.php) was founded in 1994 at Johns Hopkins University (Baltimore, United States)[62]. This was followed by the European Registry of Hereditary Pancreatitis and Familial Pancreas Cancer (EUROPAC: http://www.europac-org.eu/)[30] (1997) at Liverpool University (Liverpool, United Kingdom) and the German National Case Collection for Familial Pancreatic Carcinoma (FaPaCa: http://www.fapaca.de/)[49,50] (1999) at Phillips University (Marburg, Germany). The NFPTR had enrolled 4322 families as of 2012; of these, 1376 families had one or more cases of PC in their FDRs. The FaPaCa had 452 registered FPC families as of 2009[49]. National FPC registries have also been established in Italy (2007)[63] and in Spain (2009)[64]. In Japan, a kickoff meeting was held at Kyoto among international experts in October 2012[65]. A committee was assembled in 2013 and the nationwide registry of FPC (Japanese Familial Pancreatic Cancer Registry: JFPCR: http://jfpcr.com) was officially established in 2014 by the Japan Pancreas Society.
Consortiums and symposiums have also been organized among several high volume centers and/or FPC registries in North America [Pancreatic Cancer Genetic Epidemiology Consortium (PACGENE) in 2002, funded from the National Cancer Institute][62] and across the globe [International Symposium on Inherited Diseases of the Pancreas[66] initiated in 1997, Pancreatic Cancer Cohort Consortium (PanScan) in 2006[8,67], and International Cancer of the Pancreas Screening Consortium (CAPS) in 2011[68]]. The aim has been to gather information on patients and families of PC and to study the cause of PC, with the ultimate goal of improving the clinical practice of counseling and screening of the HRIs, and to devise new early detection methods for PC and better treatments. To date, a large number of clinical studies have been conducted under the FPC registries, mostly concerning risk assessment and screening of family members of FPC patients, in parallel with basic research on pancreatic carcinogenesis[69].
The establishment of FPC registries was followed by a long period of basic research on FPC, as well as pursuit of its causative genes[62]. As already mentioned, several hereditary cancer syndromes have increased risks for the development of PC (Table 1)[23,70]. Genes responsible for FPC have included ATM (mutation rate: 2.4%)[71], BRCA1 (0-1%)[72,73], BRCA2 (8%-19%)[35,74,75], CHEK2 (2.9%)[76], and PALB2 (3.1%-3.7%)[77,78]. However, the known germline mutations account for less than 20% of FPC cases. These genes all function in the homologous recombination of the double strand DNA repair system, or the so-called Fanconi anemia (FA) pathway[79,80], and their germline mutations have also been reported in familial breast cancers[81].
BRCA1/2 mutation carriers have a mild to moderate level of risk for PC (relative risks: 2-8, lifetime risks: 2%-17%), but some specific mutation types may have further increased risks. For instance, BRCA2 6174delT, which is a Jewish founder mutation, was detected in 13% (3/23) of Jewish PC cases and the odds for having PC was 12.8[82]. Similarly, the BRCA2 K3326X mutation was detected in 5.6% (5/144) of American FPC cases[83]. A murine model confirmed that a germline BRCA2 mutation suffices to promote carcinogenesis by the KRAS mutation[84], which is recognized in nearly 90% of PC cases[54]. This may also explain the function of BRCA2 mutation in FPC. Other genes working in conjunction with FA complementation groups, such as FANCA[85], FANCC[86], and FANCG[86], have been reported to show very low incidences of mutation in FPC (0%-0.5%).
Most recently, the PACGENE study group, which included six American and Canadian institutions, used custom genotyping arrays (iSelect Collaborative Oncological Gene-Environment Study array: iCOGS array) to analyze a single nucleotide polymorphism of 985 PC cases [906 cases with a family history of PC and 79 cases with early-onset (≤ 50 years old)]. This group discovered evidence supporting an association of two genetic loci with PC: 7p21.1 (HDAC9) and 21q22.3 (COL6A2)[87].
Screening the high-risk population is thought to be an effective strategy for early diagnosis of PC; however, several issues concerning screening have been raised[68]. These include the nature of the pathological lesion that represents the best target for surgical resection, the degree of risk expected for the screening, the best modality or combination of multiple modalities, the best age for initiating screening, the optimal screening interval, and the cost benefit and mental burden for the subjects.
Targeted pathological lesions: The CAPS consortium summit held in Baltimore (2011) concluded that the success of a screening program for HRIs is defined as the detection and treatment of high-grade precursors (PanIN[52] and IPMN[88]) - UICC-stage IA PC (T1N0M0; limited to the pancreas and no more than 2 cm in size)[68]. Today, the overall survival of UICC-stage IA cancer is unsatisfactory (5-year survival: 68.7%). The ideal for a targeted lesion is thought as high-grade precursors - UICC-stage 0 PC (5-year survival: 85.8%)[2].
Screening candidates and lifestyle guidance at surveillance: A high predictive value can be obtained by surveillance if the conditions of the high-risk group enrolled in a screening protocol are well examined. This is important from the viewpoint of the advantage-disadvantage balance, especially concerning the economic and mental burden placed on the individuals who undergo this surveillance.
The risk level of the candidate individual is assessed based on the numbers of affected family members[24] and hereditary syndromes (Table 1). "PancPro"[89,90] is free software for estimating PC risk (based mainly on hereditary risk) that uses prospective data obtained from 961 families enrolled in the NFPTR; this software is actually applied to the screening programs in Italy[90]. The international consortiums recommended that an individual who had a 5[68,91] to 10[66,92-95] fold risk undergo PC screening. However, we must bear in mind that a complete view of the genetic susceptibility of PC is still unavailable and huge amounts of data from whole genome sequencing are needed for accurate assessment. At present, the CAPS consortium has proposed nine conditions for candidate HRIs (Table 2), within a setting of greater than a 5-fold risk or a 5% of lifetime risk of PC[68].
| Individuals with ≥ 3 affected relatives, with ≥ 1 affected FDR |
| Individuals with ≥ 2 affected FDRs with PC, with ≥ 1 affected FDR, reaching a certain age |
| Individuals with ≥ 2 affected relatives with PC, with ≥ 1 affected FDR |
| Peutz-Jeghers syndrome patients, regardless of family history of PC |
| CDKN2A mutation carriers with one affected FDR |
| BRCA2 mutation carriers with one affected FDR |
| BRCA2 mutation carriers with two affected family member pf PC |
| PALB2 mutation carriers with one affected FDR |
| Mismatch repair gene mutation carrier (lynch syndrome) with one affected FDR |
A screening strategy should also evaluate the risk factors of lifestyle and pancreatic diseases, such as smoking[8,10,26,66,96], obesity[13,14,66], physical inactivity[14], diabetes[10-12,66], chronic pancreatitis[6,7,66], IPMN[88], pancreatic cyst[3,4], pancreatic duct ectasia[3], etc. (Table 3). For instance, a patient with diabetes mellitus and a smoking history and a patient with one FDR with PC each showed a 10-fold risk when compared with negative controls[10]. The initial counseling should be used to present modifiable risks related to the lifestyle to HRIs and their improvement should be recommended; i.e., smoking cessation, a healthy diet high in fruits and vegetables, higher intakes of vitamin D (> 600 IU)[15], and regular exercise to control weight (body mass index: < 25 kg/m2)[66].
Modalities of screening: Consensus could not be reached at the international consortium regarding the modality that is the most suitable for screening[66]. Many institutions currently use EUS as their standard modality[70], based on its ability to detect small pancreatic lesions (< 1 cm)[97-100]. Kamata et al[100] prospectively compared the sensitivity of detecting a PC using EUS, enhanced computed tomography (CT), or magnetic resonance imaging (MRI) during the screening of 167 consecutive cases of IPMN; these authors concluded that EUS had the best sensitivity. EUS is also superior at detecting risk findings frequently seen in HRIs, such as duct ectasia, cysts[3], and subtle parenchymal findings of the pancreas[53,97,100-102]. However, agreement is poor in terms of these characteristic findings, even among expert endosonographers[103]. First, visualization by EUS largely depends on the operator's skill[104]. The choice of EUS scopes is also contentious[105], as convex and radial types each have their own different peculiarities[106]. Other drawbacks of EUS include the necessity for a relatively long-time fasting period and conscious sedation, with a limited observation area in cases with a reconstructed upper gastrointestinal tract. In this sense, abdominal ultrasonography is a handy tool that may substitute for EUS if visualization of the pancreas is good without any blind spots[3], for the subjects with slim abdominal trunk.
MRI or magnetic resonance cholangiopancreatography (MRCP) is good at visualization of the pancreatic ductal systems. Dilation of the pancreatic duct and cyst formation are risk factors for PC[3,4] and are actually frequently recognized in HRIs (cyst in 38.9% and duct ectasia in 2.3%)[102], making MRCP a promising tool for assessing the risk level of HRIs. CT scans have a high spatial resolution; however, the healthy examiners are exposed to radiation. Long-term screening for breast cancer with low-dose radiation may possibly increase the incidence of cancer in BRCA mutation carriers (BRCA1: < 2%, BRCA2: < 4%)[107]. This risk is especially high when radiation exposure occurs at age 20 or younger (OR = 2.0, 95%CI: 1.3-3.1) or is repeated five or more times (OR = 1.8, 95%CI: 1.1-3.0)[108]. Excessive use of CT should be avoided in a BRCA mutant cohort. Endoscopic retrograde cholangiopancreatography (ERCP) is too invasive for routine screening and carries its own risk of procedure-associated pancreatitis; nevertheless, it is used for further investigation as it has the advantage of obtaining pathological samples[42,109-111]. Repeated pancreatic juice cytology with placement of endoscopic naso-pancreatic duct drainage is effective for detecting early pancreatic carcinoma or carcinoma in situ spreading within the pancreatic duct[109]. EUS-guided fine needle aspiration (EUS-FNA) can target small invasive carcinoma, although only limited tissues can be obtained from carcinoma in situ, and dissemination is a risk[112].
In summary, EUS and MRI are considered the most accurate image tools[100-102,113] with high agreement among the consortium experts (agreement, EUS: 83.7% and MRI/MRCP: 73.5%)[68]. EUS-FNA and ERCP are applicable when abnormal findings or their changes are observed in other images[42,97]. In addition to image analysis, serum tumor markers, including elevated carcinoembryonic antigen and CA19-9, should be checked each time[42,49,50,68].
When to start screening: Screening in many institutions is started at 40 years of age[64,97] or 10 years younger than the age of the youngest relative with PC[42,49]. As PC develops in cases of PJS at a young age (40.8 years)[27], screening is started at 30 years old[97]. However, detection of pancreatic lesions increases after age 50-60[102,114]. No consensus has been reached regarding the age to initiate screening and more than half (51%) of the experts in CAPS consortium voted the initial screening at age 50[68].
Screening interval: Many institutions opt for yearly screening[42,50,95,97,114] if the latest EUS and/or CT is normal (73.5% of agreement by CAPS consortium)[68]. Once an abnormal finding is observed, subsequent screening is done every 3-6 mo[50,97] or 3-12 mo[42,68]. The endorsed screening interval for a non-suspicious cyst is 6-12 mo (agree: 83.7%), 3 mo for a newly detected solid lesion if surgery is not imminent (agree: 85.7%), and 3 mo for an indeterminate main pancreatic duct stricture (agree: 95.9%)[68]. The natural history and progression of FPC still require study to determine the appropriate duration for screening intervals in relation to the risk level.
Surgical indications and procedures: As already mentioned, the characteristics of pancreatic histology in FPC kindred are multifocal PanINs or IPMNs[55] associated with duct ectasia and parenchymal atrophy[53]. The surgical indication for IPMN lesions can be determined according to established Fukuoka guidelines[88]. However, detection of PanIN3 (carcinoma in situ) or minimally invasive cancer is difficult, as these cancers are tiny and do not form a solid mass or a nodule.
The extent of resection is controversial, depending on the therapeutic concept. The choices are to remove all precancerous lesions[42] or to resect only a targeted area that includes nodular or cystic lesions[97,115]. In cases of HBOC with the BRCA mutation, risk-reducing salpingo-oophorectomy is affordable and has an acceptable level of complications[116]. However, for the pancreas, total pancreatectomy (TP) has severe complications, including a considerable level of postsurgical in-hospital mortality (cf. nationwide: 23%, high-volume hospital: 5%, in Germany)[117,118] and subsequent serious glycemic control failure (mortality: 4%-8% per year)[119]. A secondary pancreatectomy for the remnant pancreas can be conducted without increasing morbidity and mortality[120], so resection of the target area, rather than TP, has been preferable thus far.
For many years, TP with pancreatic transplantation has been conducted in patients with type 1 diabetes[119] and TP combined with islet autotransplantation has been performed on chronic pancreatitis patients with intractable pain[121]. However, most recently, due to the improvements in post-surgical quality of life, these treatment procedures have been considered and actually indicated for FPC kindred with premalignant lesions[119,122,123]. Further improvements are expected in the future.
Several surveillance results have been reported from single or collaborated FPC registries in western countries; their protocol conditions and outcomes are summarized in Table 4[42,50,91,92,94,95,97,101,114,124-127]. Some of the cases from the same registry may appear in more than one report; therefore, interpretation of cumulative data needs caution. About 5%-20% of the screened HRIs underwent surgery for suspected lesions. Roughly one third of the resected cases were benign lesions that underwent unnecessary treatment, and only less than one fifth were borderline precursors and carcinoma in situ, or definitive targets of the surveillance (Table 4). A small proportion of PC was resected at an early phase (T1N0M0)[94], but some PC cases were detected at the advanced unresectable stage. These outcomes testified to the difficulty of providing an accurate diagnosis of PCs at the curative stage.
| Ref. | Year | Country/registry | Entry period | Subjects conditions | Age (range), yr | n | Duration (mo) | Modality | Ratio of surgical cases (n) | Pathology of the pancreatic lesion: n | Ratio of unresectable advanced PC (n) | ||
| (surveillance→examination) | Benign1 | Border/CIS2 | PC | ||||||||||
| Brentnall et al[42] | 1999 | United States | NA | FPC kindred | 41 (28-65) | 14 | 15 | EUS, CT → ERCP | 50.0% (7) | 0 | 75 | 0 | 0% (0) |
| Canto et al[101] | 2004 | United States | 1998-2001 | FPC kindred, PJS | 58 (NA) | 38 | 22 | EUS → CT, EUS-FNA, ERCP | 18.4% (7) | 4 | 2 | 1 | 0% (0) |
| Canto et al[97] | 2006 | United States | 2001-2004 | FPC kindred, PJS | 52 (32-77) | 78 | 12 | EUS, CT → EUS-FNA, ERCP | 9.0% (7) | 4 | 3 | 0 | 1.3% (1) |
| Langer et al[50] | 2009 | FaPaCa | 1999-2007 | FPC kindred, BRCA2 (+)4, CDKN2A (+) FAMMM family | 60 (35-85) | 76 | NA | EUS, MRI → EUS-FNA | 9.2% (7)3 | 6 | 0 | 0 | 0% (0) |
| Poley et al[95] | 2009 | Netherlands | 2005-2007 | FPC kindred, HP, PJS, FAMMM, BRCA1/2 (+), TP53 (+) | 50 (32-75) | 44 | Initial6 | EUS → CT, MRI | 6.8% (3) | 0 | 0 | 3 | 0% (0) |
| Verna et al[124] | 2010 | United States | 2005-2008 | FPC kindred, BRCA1/2 (+), LS, FAMMM | 52 (29-77) | 51 | Initial | EUS, MRI → EUS-FNA, ERCP | 9.8% (5) | 4 | 0 | 1 | 2.0% (1) |
| Ludwig et al[114] | 2011 | United States | 2002-2009 | FPC kindred, BRCA1/2(+) | 54 (33-86) | 109 | Initial | MRI → EUS, EUS-FNA | 5.5% (6) | 3 | 2 | 1 | 0% (0) |
| Vasen et al[125] | 2011 | Netherlands | 2000-2010 | CDKN2A-Leiden (+) | 56 (39-72) | 79 | 48 | MRI | 6.3% (5) | 0 | 0 | 5 | 2.5% (2) |
| Zubarik et al[126] | 2011 | United States | 2006-2009 | FDR of PC with sCA19-9↑ | 59 (NA) | 26 | NA | EUS → EUS-FNA | 11.5% (3) | 2 | 0 | 1 | 0% (0) |
| Al-Sukhni et al[127] | 2012 | Canada | 2003-2011 | FPC kindred, PJS, HP, CDKN2A (+), BRCA1/2 (+), STK11 (+) | 54 (22-89) | 262 | 50 | MRI → MRI, EUS, EUS-FNA, ERCP | 1.5% (4) | 3 | 0 | 1 | 0.8% (2) |
| Sud et al[91] | 2014 | United States | 2008-2011 | FPC kindred, HP, CDKN2A (+), BRCA1/2 (+), PJS, LS | 51 (20-75) | 16 | NA | EUS → EUS-FNA | 18.8% (3) | 1 | 0 | 2 | 0% (0) |
| Del Chiaro et al[92] | 2015 | Sweden | 2010-2013 | FPC kindred, individuals with increased genetic risk | 50 (23-76) | 40 | 13 | MRI → EUS, EUS-FNA | 12.5% (5) | 2 | 0 | 3 | 0% (0) |
| Vasen et al[94] | 2016 | FaPaCa | 2000-2015 | FPC kindred, CDKN2A (+), BRCA1/2 (+), PALB2 (+) | 46-56 (25-81) | 411 | 16-53 | MRI ± EUS → EUS, CT → EUS-FNA | 7.3% (30) | 15 | 4 | 11 | 1.0% (4) |
Screening participants who are FPC kindred commonly express grief from the experience of family death due to PC[128-130], and are distressed by the high mortality and uncertainty related to prevention and early detection[128]. Their motivation for participating in surveillance is "possible early detection of (a precursor stage of) PC" (95%-100%)[131], and they want to control their cancer risk by seeking information and resources to prevent PC[128]. Research conducted by the Mayo Clinic indicated that 67% (238/361) of FPC kindred felt they had a higher lifetime risk of PC when compared to people of the same age, race, and gender, and 95% were likely to undergo blood test surveillance and 75% were likely to undergo EUS surveillance[130]. A study at the University of Toronto revealed that the perception of PC risk was higher in FPC kindred than in BRCA2 mutation carriers (42% vs 15%)[129]. Most participants had anxiety and worry at the beginning, although only occasionally or sometimes[128,130]; however, this gradually decreased as surveillance progressed (over a 3-year period of follow-up)[129,131]. This trend was significant in younger participants[132]. The German FaPaCa registry showed that only 39% (80/205) of HRIs participated in the recommended surveillance. The psychological status of these non-participants is still unknown.
Several studies have analyzed the cost-effectiveness of the PC surveillance of HRIs; however, they are not consistent in terms of the applied modality and the target group. For example, Rulyak et al[133] evaluated a one-time screening by EUS and ERCP and reported an incremental cost-effectiveness ratio of $16885/life-year saved. They concluded that surveillance remained cost-effective if the prevalence of dysplasia was at least 16% or if the sensitivity of EUS was at least 84%. Bruenderman et al[134] estimated costs per year of life of MRI/MRCP surveillance for CDKN2A (p16)-Leiden mutation carriers at $4545, and concluded it to be affordable. By contrast, Latchford et al[135] estimated a life-saved cost of over $350000 for total surveillance of PJS patients that followed the American Gastroenterology Association guidelines and recommended its performance only on a research basis. Rubenstein et al[136] applied a Markov model to FPC kindred in a setting of 45-year-old-males with positive EUS findings of chronic pancreatitis and compared four different strategies: doing nothing, prophylactic TP, annual EUS surveillance, and annual EUS-FNA surveillance. The "doing nothing" strategy provided the lowest cost, the greatest remaining years of life, and the best quality-adjusted life years, when compared to the smallest benefit in these aspects obtained with prophylactic TP.
For unresectable PC, on the basis of current evidence, FOLFIRINOX (fluorouracil, folic acid, irinotecan, and oxaliplatin) and gemcitabine-based regimens are standard choices of chemotherapy (median survival: 11 mo and 6-9 mo, respectively)[70]. However, in agreement with the response observed in HBOC patients[137-139], PC patients with BRCA1/2 mutation carriers respond well to platinum-based chemotherapy[140] and poly (ADP-ribose) polymerase (PARP) inhibitors[138,141], as determined in several studies. For example, Golan et al[140] compared overall survival (OS) of 43 patients with stage III-IV PC with BRCA mutation carriers in terms of their chemotherapy regimen-either platinum or non-platinum. Superior OS was observed for patients treated with platinum chemotherapy (n = 22) than with non-platinum (n = 21) (22 mo vs 9 mo, P = 0.039). A similar effect was confirmed in an experiment using xenografts by Lohse et al[142], who reported that PC xenografts harvested from BRCA mutation carriers and implanted into nude mice showed sensitivity to both gemcitabine and cisplatin. By contrast, xenografts from BRCA wild cases showed sensitivity only to gemcitabine. A joint study by Johns Hopkins University and the MD Anderson Cancer Center[143] analyzed effectiveness of platinum-based chemotherapy in metastatic PC patients (n = 549) by familial cancer history, although germline BRCA status was not described, and demonstrated a superior OS in patients with family history of either breast, ovarian, or pancreatic cancer (HR = 0.49, P = 0.003). Survival was strongly associated with the number of relatives with BRCA-related malignancy (P = 0.009).
Kaufman et al[138] reported that a PARP inhibitor (PARPi) treatment induced a 22% response ratio with 4.6 mo of progression-free survival in BRCA-mutant PC patients who had already showed progression resistant to the gemcitabine treatment. PARPi is effective for PC cases with deficiency in the homologous recombination pathway; i.e., in cases with either mutation of ATM, BRCA1, BRCA2, or CHEK2. This outcome is explained by a synthetic lethal theory, where apoptosis is induced by blocking both the single- and double-strand DNA break repair system[139]. Currently, data are lacking with respect to PARPi use against FPC in causative mutation carriers. Future outcomes are expected.
In addition to classical risk factors, hereditary factors including family history of pancreatic cancer and some genetic syndromes must be taken into account when screening to detect early pancreatic cancer. Since the 1990s, basic and clinical research has accumulated much scientific data on FPC. However, to date, screening of HRIs has had unsatisfactory outcomes. In 2014, the JFPCR was established in Japan, and projects have just begun for early detection and better outcomes of PC. Success in this venture will depend on improvement of all aspects, including genetic medicine, screening and treatment methods, and better understanding of what determines a HRI.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kamisawa T, Zhang ZM S- Editor: Gong ZM L- Editor: A E- Editor: Liu WX
| 1. | American Cancer Society. Cancer Facts and Figures 2016. Available from: http://www.cancerorg/research/cancerfactsstatistics/2016. |
| 2. | Egawa S, Toma H, Ohigashi H, Okusaka T, Nakao A, Hatori T, Maguchi H, Yanagisawa A, Tanaka M. Japan Pancreatic Cancer Registry; 30th year anniversary: Japan Pancreas Society. Pancreas. 2012;41:985-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Tanaka S, Nakao M, Ioka T, Takakura R, Takano Y, Tsukuma H, Uehara H, Suzuki R, Fukuda J. Slight dilatation of the main pancreatic duct and presence of pancreatic cysts as predictive signs of pancreatic cancer: a prospective study. Radiology. 2010;254:965-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | Matsubara S, Tada M, Akahane M, Yagioka H, Kogure H, Sasaki T, Arizumi T, Togawa O, Nakai Y, Sasahira N. Incidental pancreatic cysts found by magnetic resonance imaging and their relationship with pancreatic cancer. Pancreas. 2012;41:1241-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Tanno S, Nakano Y, Koizumi K, Sugiyama Y, Nakamura K, Sasajima J, Nishikawa T, Mizukami Y, Yanagawa N, Fujii T. Pancreatic ductal adenocarcinomas in long-term follow-up patients with branch duct intraductal papillary mucinous neoplasms. Pancreas. 2010;39:36-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 6. | Lowenfels AB, Maisonneuve P, Cavallini G, Ammann RW, Lankisch PG, Andersen JR, Dimagno EP, Andrén-Sandberg A, Domellöf L. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433-1437. [PubMed] |
| 7. | Talamini G, Falconi M, Bassi C, Sartori N, Salvia R, Caldiron E, Frulloni L, Di Francesco V, Vaona B, Bovo P. Incidence of cancer in the course of chronic pancreatitis. Am J Gastroenterol. 1999;94:1253-1260. [PubMed] |
| 8. | Lynch SM, Vrieling A, Lubin JH, Kraft P, Mendelsohn JB, Hartge P, Canzian F, Steplowski E, Arslan AA, Gross M. Cigarette smoking and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium. Am J Epidemiol. 2009;170:403-413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 244] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 9. | Bosetti C, Lucenteforte E, Silverman DT, Petersen G, Bracci PM, Ji BT, Negri E, Li D, Risch HA, Olson SH. Cigarette smoking and pancreatic cancer: an analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4). Ann Oncol. 2012;23:1880-1888. [PubMed] [DOI] [Full Text] |
| 10. | Matsubayashi H, Maeda A, Kanemoto H, Uesaka K, Yamazaki K, Hironaka S, Miyagi Y, Ikehara H, Ono H, Klein A. Risk factors of familial pancreatic cancer in Japan: current smoking and recent onset of diabetes. Pancreas. 2011;40:974-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Huxley R, Ansary-Moghaddam A, Berrington de González A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076-2083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 758] [Cited by in RCA: 780] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 12. | Ben Q, Xu M, Ning X, Liu J, Hong S, Huang W, Zhang H, Li Z. Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. Eur J Cancer. 2011;47:1928-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 473] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 13. | Larsson SC, Orsini N, Wolk A. Body mass index and pancreatic cancer risk: A meta-analysis of prospective studies. Int J Cancer. 2007;120:1993-1998. [PubMed] |
| 14. | Stolzenberg-Solomon RZ, Adams K, Leitzmann M, Schairer C, Michaud DS, Hollenbeck A, Schatzkin A, Silverman DT. Adiposity, physical activity, and pancreatic cancer in the National Institutes of Health-AARP Diet and Health Cohort. Am J Epidemiol. 2008;167:586-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Skinner HG. Vitamin D for the treatment and prevention of pancreatic cancer. Cancer Biol Ther. 2008;7:437-439. [PubMed] |
| 16. | Ait Ouakrim D, Lockett T, Boussioutas A, Hopper JL, Jenkins MA. Screening participation for people at increased risk of colorectal cancer due to family history: a systematic review and meta-analysis. Fam Cancer. 2013;12:459-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Turati F, Edefonti V, Talamini R, Ferraroni M, Malvezzi M, Bravi F, Franceschi S, Montella M, Polesel J, Zucchetto A. Family history of liver cancer and hepatocellular carcinoma. Hepatology. 2012;55:1416-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | Win AK, Reece JC, Ryan S. Family history and risk of endometrial cancer: a systematic review and meta-analysis. Obstet Gynecol. 2015;125:89-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Inoue M, Tajima K, Takezaki T, Hamajima N, Hirose K, Ito H, Tominaga S. Epidemiology of pancreatic cancer in Japan: a nested case-control study from the Hospital-based Epidemiologic Research Program at Aichi Cancer Center (HERPACC). Int J Epidemiol. 2003;32:257-262. [PubMed] |
| 20. | Falk RT, Pickle LW, Fontham ET, Correa P, Fraumeni JF. Life-style risk factors for pancreatic cancer in Louisiana: a case-control study. Am J Epidemiol. 1988;128:324-336. [PubMed] |
| 21. | Coughlin SS, Calle EE, Patel AV, Thun MJ. Predictors of pancreatic cancer mortality among a large cohort of United States adults. Cancer Causes Control. 2000;11:915-923. [PubMed] |
| 22. | Hemminki K, Li X. Familial and second primary pancreatic cancers: a nationwide epidemiologic study from Sweden. Int J Cancer. 2003;103:525-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Matsubayashi H. Familial pancreatic cancer and hereditary syndromes: screening strategy for high-risk individuals. J Gastroenterol. 2011;46:1249-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Klein AP, Brune KA, Petersen GM, Goggins M, Tersmette AC, Offerhaus GJ, Griffin C, Cameron JL, Yeo CJ, Kern S. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64:2634-2638. [PubMed] |
| 25. | Mucci LA, Hjelmborg JB, Harris JR, Czene K, Havelick DJ, Scheike T, Graff RE, Holst K, Möller S, Unger RH. Familial Risk and Heritability of Cancer Among Twins in Nordic Countries. JAMA. 2016;315:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 611] [Cited by in RCA: 620] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 26. | Yeo TP, Hruban RH, Brody J, Brune K, Fitzgerald S, Yeo CJ. Assessment of “gene-environment” interaction in cases of familial and sporadic pancreatic cancer. J Gastrointest Surg. 2009;13:1487-1494. [PubMed] |
| 27. | Giardiello FM, Brensinger JD, Tersmette AC, Goodman SN, Petersen GM, Booker SV, Cruz-Correa M, Offerhaus JA. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119:1447-1453. [PubMed] |
| 28. | Whitcomb DC, Applebaum S, Martin SP. Hereditary pancreatitis and pancreatic carcinoma. Ann N Y Acad Sci. 1999;880:201-209. [PubMed] |
| 29. | Rebours V, Boutron-Ruault MC, Schnee M, Férec C, Maire F, Hammel P, Ruszniewski P, Lévy P. Risk of pancreatic adenocarcinoma in patients with hereditary pancreatitis: a national exhaustive series. Am J Gastroenterol. 2008;103:111-119. [PubMed] |
| 30. | Howes N, Lerch MM, Greenhalf W, Stocken DD, Ellis I, Simon P, Truninger K, Ammann R, Cavallini G, Charnley RM. Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin Gastroenterol Hepatol. 2004;2:252-261. [PubMed] |
| 31. | Lowenfels AB, Maisonneuve P, DiMagno EP, Elitsur Y, Gates LK, Perrault J, Whitcomb DC. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst. 1997;89:442-446. [PubMed] |
| 32. | Lynch HT, Fusaro RM, Lynch JF, Brand R. Pancreatic cancer and the FAMMM syndrome. Fam Cancer. 2008;7:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Vasen HF, Gruis NA, Frants RR, van Der Velden PA, Hille ET, Bergman W. Risk of developing pancreatic cancer in families with familial atypical multiple mole melanoma associated with a specific 19 deletion of p16 (p16-Leiden). Int J Cancer. 2000;87:809-811. [PubMed] |
| 34. | Lynch HT, Deters CA, Lynch JF, Brand RE. Familial pancreatic carcinoma in Jews. Fam Cancer. 2004;3:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Murphy KM, Brune KA, Griffin C, Sollenberger JE, Petersen GM, Bansal R, Hruban RH, Kern SE. Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: deleterious BRCA2 mutations in 17%. Cancer Res. 2002;62:3789-3793. [PubMed] |
| 36. | Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999;91:1310-1316. [PubMed] |
| 37. | Brose MS, Rebbeck TR, Calzone KA, Stopfer JE, Nathanson KL, Weber BL. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst. 2002;94:1365-1372. [PubMed] |
| 38. | Kastrinos F, Mukherjee B, Tayob N, Wang F, Sparr J, Raymond VM, Bandipalliam P, Stoffel EM, Gruber SB, Syngal S. Risk of pancreatic cancer in families with Lynch syndrome. JAMA. 2009;302:1790-1795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 377] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 39. | Aarnio M, Sankila R, Pukkala E, Salovaara R, Aaltonen LA, de la Chapelle A, Peltomäki P, Mecklin JP, Järvinen HJ. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999;81:214-218. [PubMed] |
| 40. | Giardiello FM, Offerhaus GJ, Lee DH, Krush AJ, Tersmette AC, Booker SV, Kelley NC, Hamilton SR. Increased risk of thyroid and pancreatic carcinoma in familial adenomatous polyposis. Gut. 1993;34:1394-1396. [PubMed] |
| 41. | Chun SG, Yee NS. Werner syndrome as a hereditary risk factor for exocrine pancreatic cancer: potential role of WRN in pancreatic tumorigenesis and patient-tailored therapy. Cancer Biol Ther. 2010;10:430-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Brentnall TA, Bronner MP, Byrd DR, Haggitt RC, Kimmey MB. Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Ann Intern Med. 1999;131:247-255. [PubMed] |
| 43. | James TA, Sheldon DG, Rajput A, Kuvshinoff BW, Javle MM, Nava HR, Smith JL, Gibbs JF. Risk factors associated with earlier age of onset in familial pancreatic carcinoma. Cancer. 2004;101:2722-2726. [PubMed] |
| 44. | Brune KA, Lau B, Palmisano E, Canto M, Goggins MG, Hruban RH, Klein AP. Importance of age of onset in pancreatic cancer kindreds. J Natl Cancer Inst. 2010;102:119-126. [PubMed] |
| 45. | Del Chiaro M, Zerbi A, Falconi M, Bertacca L, Polese M, Sartori N, Boggi U, Casari G, Longoni BM, Salvia R. Cancer risk among the relatives of patients with pancreatic ductal adenocarcinoma. Pancreatology. 2007;7:459-469. [PubMed] |
| 46. | Wang L, Brune KA, Visvanathan K, Laheru D, Herman J, Wolfgang C, Schulick R, Cameron JL, Goggins M, Hruban RH. Elevated cancer mortality in the relatives of patients with pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:2829-2834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 47. | Ji J, Forsti A, Sundquist J, Lenner P, Hemminki K. Survival in familial pancreatic cancer. Pancreatology. 2008;8:252-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 48. | Humphris JL, Johns AL, Simpson SH, Cowley MJ, Pajic M, Chang DK, Nagrial AM, Chin VT, Chantrill LA, Pinese M. Clinical and pathologic features of familial pancreatic cancer. Cancer. 2014;120:3669-3675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Schneider R, Slater EP, Sina M, Habbe N, Fendrich V, Matthäi E, Langer P, Bartsch DK. German national case collection for familial pancreatic cancer (FaPaCa): ten years experience. Fam Cancer. 2011;10:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 50. | Langer P, Kann PH, Fendrich V, Habbe N, Schneider M, Sina M, Slater EP, Heverhagen JT, Gress TM, Rothmund M. Five years of prospective screening of high-risk individuals from families with familial pancreatic cancer. Gut. 2009;58:1410-1418. [PubMed] |
| 51. | McFaul CD, Greenhalf W, Earl J, Howes N, Neoptolemos JP, Kress R, Sina-Frey M, Rieder H, Hahn S, Bartsch DK. Anticipation in familial pancreatic cancer. Gut. 2006;55:252-258. [PubMed] |
| 52. | Takaori K, Hruban RH, Maitra A, Tanigawa N. Pancreatic intraepithelial neoplasia. Pancreas. 2004;28:257-262. [PubMed] |
| 53. | Brune K, Abe T, Canto M, O’Malley L, Klein AP, Maitra A, Volkan Adsay N, Fishman EK, Cameron JL, Yeo CJ. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am J Surg Pathol. 2006;30:1067-1076. [PubMed] |
| 54. | Matsubayashi H, Watanabe H, Yamaguchi T, Ajioka Y, Nishikura K, Iwafuchi M, Yamano M, Kijima H, Saito T. Multiple K-ras mutations in hyperplasia and carcinoma in cases of human pancreatic carcinoma. Jpn J Cancer Res. 1999;90:841-848. [PubMed] |
| 55. | Shi C, Klein AP, Goggins M, Maitra A, Canto M, Ali S, Schulick R, Palmisano E, Hruban RH. Increased Prevalence of Precursor Lesions in Familial Pancreatic Cancer Patients. Clin Cancer Res. 2009;15:7737-7743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 56. | Kimura W, Nagai H, Kuroda A, Muto T, Esaki Y. Analysis of small cystic lesions of the pancreas. Int J Pancreatol. 1995;18:197-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 269] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 57. | Potjer TP, Schot I, Langer P, Heverhagen JT, Wasser MN, Slater EP, Klöppel G, Morreau HM, Bonsing BA, de Vos Tot Nederveen Cappel WH. Variation in precursor lesions of pancreatic cancer among high-risk groups. Clin Cancer Res. 2013;19:442-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 58. | Singhi AD, Ishida H, Ali SZ, Goggins M, Canto M, Wolfgang CL, Meriden Z, Roberts N, Klein AP, Hruban RH. A histomorphologic comparison of familial and sporadic pancreatic cancers. Pancreatology. 2015;15:387-391. [PubMed] [DOI] [Full Text] |
| 59. | Abe T, Fukushima N, Brune K, Boehm C, Sato N, Matsubayashi H, Canto M, Petersen GM, Hruban RH, Goggins M. Genome-wide allelotypes of familial pancreatic adenocarcinomas and familial and sporadic intraductal papillary mucinous neoplasms. Clin Cancer Res. 2007;13:6019-6025. [PubMed] |
| 60. | Norris AL, Roberts NJ, Jones S, Wheelan SJ, Papadopoulos N, Vogelstein B, Kinzler KW, Hruban RH, Klein AP, Eshleman JR. Familial and sporadic pancreatic cancer share the same molecular pathogenesis. Fam Cancer. 2015;14:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 61. | Brune K, Hong SM, Li A, Yachida S, Abe T, Griffith M, Yang D, Omura N, Eshleman J, Canto M. Genetic and epigenetic alterations of familial pancreatic cancers. Cancer Epidemiol Biomarkers Prev. 2008;17:3536-3542. [PubMed] |
| 62. | Petersen GM, de Andrade M, Goggins M, Hruban RH, Bondy M, Korczak JF, Gallinger S, Lynch HT, Syngal S, Rabe KG. Pancreatic cancer genetic epidemiology consortium. Cancer Epidemiol Biomarkers Prev. 2006;15:704-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 63. | Del Chiaro M, Zerbi A, Capurso G, Zamboni G, Maisonneuve P, Presciuttini S, Arcidiacono PG, Calculli L, Falconi M; Italian Registry for Familial Pancreatic Cancer. Familial pancreatic cancer in Italy. Risk assessment, screening programs and clinical approach: a position paper from the Italian Registry. Dig Liver Dis. 2010;42:597-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 64. | Mocci E, Guillen-Ponce C, Earl J, Marquez M, Solera J, Salazar-López MT, Calcedo-Arnáiz C, Vázquez-Sequeiros E, Montans J, Muñoz-Beltrán M. PanGen-Fam: Spanish registry of hereditary pancreatic cancer. Eur J Cancer. 2015;51:1911-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 65. | Wada K, Takaori K, Traverso LW, Hruban RH, Furukawa T, Brentnall TA, Hatori T, Sano K, Takada T, Majima Y. Clinical importance of Familial Pancreatic Cancer Registry in Japan: a report from kick-off meeting at International Symposium on Pancreas Cancer 2012. J Hepatobiliary Pancreat Sci. 2013;20:557-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 66. | Brand RE, Lerch MM, Rubinstein WS, Neoptolemos JP, Whitcomb DC, Hruban RH, Brentnall TA, Lynch HT, Canto MI. Advances in counselling and surveillance of patients at risk for pancreatic cancer. Gut. 2007;56:1460-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 217] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 67. | Jacobs EJ, Chanock SJ, Fuchs CS, Lacroix A, McWilliams RR, Steplowski E, Stolzenberg-Solomon RZ, Arslan AA, Bueno-de-Mesquita HB, Gross M. Family history of cancer and risk of pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Int J Cancer. 2010;127:1421-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 68. | Canto MI, Harinck F, Hruban RH, Offerhaus GJ, Poley JW, Kamel I, Nio Y, Schulick RS, Bassi C, Kluijt I, Levy MJ, Chak A, Fockens P, Goggins M, Bruno M; International Cancer of Pancreas Screening (CAPS) Consortium. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut. 2013;62:339-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 546] [Cited by in RCA: 564] [Article Influence: 47.0] [Reference Citation Analysis (1)] |
| 69. | Hruban RH, Canto MI, Goggins M, Schulick R, Klein AP. Update on familial pancreatic cancer. Adv Surg. 2010;44:293-311. [PubMed] |
| 70. | Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 71. | Roberts NJ, Jiao Y, Yu J, Kopelovich L, Petersen GM, Bondy ML, Gallinger S, Schwartz AG, Syngal S, Cote ML. ATM mutations in patients with hereditary pancreatic cancer. Cancer Discov. 2012;2:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 378] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 72. | Axilbund JE, Argani P, Kamiyama M, Palmisano E, Raben M, Borges M, Brune KA, Goggins M, Hruban RH, Klein AP. Absence of germline BRCA1 mutations in familial pancreatic cancer patients. Cancer Biol Ther. 2009;8:131-135. [PubMed] |
| 73. | Lynch HT, Deters CA, Snyder CL, Lynch JF, Villeneuve P, Silberstein J, Martin H, Narod SA, Brand RE. BRCA1 and pancreatic cancer: pedigree findings and their causal relationships. Cancer Genet Cytogenet. 2005;158:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 74. | Goggins M, Schutte M, Lu J, Moskaluk CA, Weinstein CL, Petersen GM, Yeo CJ, Jackson CE, Lynch HT, Hruban RH. Germline BRCA2 gene mutations in patients with apparently sporadic pancreatic carcinomas. Cancer Res. 1996;56:5360-5364. [PubMed] |
| 75. | Hahn SA, Greenhalf B, Ellis I, Sina-Frey M, Rieder H, Korte B, Gerdes B, Kress R, Ziegler A, Raeburn JA. BRCA2 germline mutations in familial pancreatic carcinoma. J Natl Cancer Inst. 2003;95:214-221. [PubMed] |
| 76. | Bartsch DK, Krysewski K, Sina-Frey M, Fendrich V, Rieder H, Langer P, Kress R, Schneider M, Hahn SA, Slater EP. Low frequency of CHEK2 mutations in familial pancreatic cancer. Fam Cancer. 2006;5:305-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 77. | Jones S, Hruban RH, Kamiyama M, Borges M, Zhang X, Parsons DW, Lin JC, Palmisano E, Brune K, Jaffee EM. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science. 2009;324:217. [PubMed] |
| 78. | Slater EP, Langer P, Niemczyk E, Strauch K, Butler J, Habbe N, Neoptolemos JP, Greenhalf W, Bartsch DK. PALB2 mutations in European familial pancreatic cancer families. Clin Genet. 2010;78:490-494. [PubMed] |
| 79. | Michl J, Zimmer J, Tarsounas M. Interplay between Fanconi anemia and homologous recombination pathways in genome integrity. EMBO J. 2016;35:909-923. [PubMed] [DOI] [Full Text] |
| 80. | Renwick A, Thompson D, Seal S, Kelly P, Chagtai T, Ahmed M, North B, Jayatilake H, Barfoot R, Spanova K. ATM mutations that cause ataxia-telangiectasia are breast cancer susceptibility alleles. Nat Genet. 2006;38:873-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 549] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 81. | Mathew CG. Fanconi anaemia genes and susceptibility to cancer. Oncogene. 2006;25:5875-5884. [PubMed] [DOI] [Full Text] |
| 82. | Figer A, Irmin L, Geva R, Flex D, Sulkes J, Sulkes A, Friedman E. The rate of the 6174delT founder Jewish mutation in BRCA2 in patients with non-colonic gastrointestinal tract tumours in Israel. Br J Cancer. 2001;84:478-481. [PubMed] |
| 83. | Martin ST, Matsubayashi H, Rogers CD, Philips J, Couch FJ, Brune K, Yeo CJ, Kern SE, Hruban RH, Goggins M. Increased prevalence of the BRCA2 polymorphic stop codon K3326X among individuals with familial pancreatic cancer. Oncogene. 2005;24:3652-3656. [PubMed] |
| 84. | Skoulidis F, Cassidy LD, Pisupati V, Jonasson JG, Bjarnason H, Eyfjord JE, Karreth FA, Lim M, Barber LM, Clatworthy SA. Germline Brca2 heterozygosity promotes Kras(G12D) -driven carcinogenesis in a murine model of familial pancreatic cancer. Cancer Cell. 2010;18:499-509. [PubMed] |
| 85. | Rogers CD, Couch FJ, Brune K, Martin ST, Philips J, Murphy KM, Petersen G, Yeo CJ, Hruban RH, Goggins M. Genetics of the FANCA gene in familial pancreatic cancer. J Med Genet. 2004;41:e126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 86. | Rogers CD, van der Heijden MS, Brune K, Yeo CJ, Hruban RH, Kern SE, Goggins M. The genetics of FANCC and FANCG in familial pancreatic cancer. Cancer Biol Ther. 2004;3:167-169. [PubMed] |
| 87. | Childs EJ, Chaffee KG, Gallinger S, Syngal S, Schwartz AG, Cote ML, Bondy ML, Hruban RH, Chanock SJ, Hoover RN. Association of Common Susceptibility Variants of Pancreatic Cancer in Higher-Risk Patients: A PACGENE Study. Cancer Epidemiol Biomarkers Prev. 2016;25:1185-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 88. | Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1614] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 89. | Wang W, Chen S, Brune KA, Hruban RH, Parmigiani G, Klein AP. PancPRO: risk assessment for individuals with a family history of pancreatic cancer. J Clin Oncol. 2007;25:1417-1422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 153] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 90. | Leonardi G, Marchi S, Falconi M, Zerbi A, Ussia V, de Bortoli N, Mosca F, Presciuttini S, Del Chiaro M. “PancPro” as a tool for selecting families eligible for pancreatic cancer screening: an Italian study of incident cases. Dig Liver Dis. 2012;44:585-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 91. | Sud A, Wham D, Catalano M, Guda NM. Promising outcomes of screening for pancreatic cancer by genetic testing and endoscopic ultrasound. Pancreas. 2014;43:458-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 92. | Del Chiaro M, Verbeke CS, Kartalis N, Pozzi Mucelli R, Gustafsson P, Hansson J, Haas SL, Segersvärd R, Andren-Sandberg Å, Löhr JM. Short-term Results of a Magnetic Resonance Imaging-Based Swedish Screening Program for Individuals at Risk for Pancreatic Cancer. JAMA Surg. 2015;150:512-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 93. | Harinck F, Konings IC, Kluijt I, Poley JW, van Hooft JE, van Dullemen HM, Nio CY, Krak NC, Hermans JJ, Aalfs CM. A multicentre comparative prospective blinded analysis of EUS and MRI for screening of pancreatic cancer in high-risk individuals. Gut. 2016;65:1505-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 94. | Vasen H, Ibrahim I, Ponce CG, Slater EP, Matthäi E, Carrato A, Earl J, Robbers K, van Mil AM, Potjer T. Benefit of Surveillance for Pancreatic Cancer in High-Risk Individuals: Outcome of Long-Term Prospective Follow-Up Studies From Three European Expert Centers. J Clin Oncol. 2016;34:2010-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 269] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 95. | Poley JW, Kluijt I, Gouma DJ, Harinck F, Wagner A, Aalfs C, van Eijck CH, Cats A, Kuipers EJ, Nio Y. The yield of first-time endoscopic ultrasonography in screening individuals at a high risk of developing pancreatic cancer. Am J Gastroenterol. 2009;104:2175-2181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 96. | Lowenfels AB, Maisonneuve P, Whitcomb DC, Lerch MM, DiMagno EP. Cigarette smoking as a risk factor for pancreatic cancer in patients with hereditary pancreatitis. JAMA. 2001;286:169-170. [PubMed] |
| 97. | Canto MI, Goggins M, Hruban RH, Petersen GM, Giardiello FM, Yeo C, Fishman EK, Brune K, Axilbund J, Griffin C. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol. 2006;4:766-781; quiz 665. [PubMed] |
| 98. | Greenhalf W, Neoptolemos JP. Increasing survival rates of patients with pancreatic cancer by earlier identification. Nat Clin Pract Oncol. 2006;3:346-347. [PubMed] [DOI] [Full Text] |
| 99. | Yasuda I, Iwashita T, Doi S, Nakashima M, Moriwaki H. Role of EUS in the early detection of small pancreatic cancer. Dig Endosc. 2011;23 Suppl 1:22-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 100. | Kamata K, Kitano M, Kudo M, Sakamoto H, Kadosaka K, Miyata T, Imai H, Maekawa K, Chikugo T, Kumano M. Value of EUS in early detection of pancreatic ductal adenocarcinomas in patients with intraductal papillary mucinous neoplasms. Endoscopy. 2014;46:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 101. | Canto MI, Goggins M, Yeo CJ, Griffin C, Axilbund JE, Brune K, Ali SZ, Jagannath S, Petersen GM, Fishman EK. Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clin Gastroenterol Hepatol. 2004;2:606-621. [PubMed] |
| 102. | Canto MI, Hruban RH, Fishman EK, Kamel IR, Schulick R, Zhang Z, Topazian M, Takahashi N, Fletcher J, Petersen G. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142:796-804; quiz e14-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 487] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 103. | Topazian M, Enders F, Kimmey M, Brand R, Chak A, Clain J, Cunningham J, Eloubeidi M, Gerdes H, Gress F. Interobserver agreement for EUS findings in familial pancreatic-cancer kindreds. Gastrointest Endosc. 2007;66:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 104. | Eisen GM, Dominitz JA, Faigel DO, Goldstein JA, Petersen BT, Raddawi HM, Ryan ME, Vargo JJ, Young HS, Wheeler-Harbaugh J. Guidelines for credentialing and granting privileges for endoscopic ultrasound. Gastrointest Endosc. 2001;54:811-814. [PubMed] |
| 105. | Kanazawa K, Imazu H, Mori N, Ikeda K, Kakutani H, Sumiyama K, Hino S, Ang TL, Omar S, Tajiri H. A comparison of electronic radial and curvilinear endoscopic ultrasonography in the detection of pancreatic malignant tumor. Scand J Gastroenterol. 2012;47:1313-1320. [PubMed] [DOI] [Full Text] |
| 106. | Katanuma A, Maguchi H, Osanai M, Takahashi K. The difference in the capability of delineation between convex and radial arrayed echoendoscope for pancreas and biliary tract; case reports from the standpoint of both convex and radial arrayed echoendoscope. Dig Endosc. 2011;23 Suppl 1:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 107. | Lowry KP, Lee JM, Kong CY, McMahon PM, Gilmore ME, Cott Chubiz JE, Pisano ED, Gatsonis C, Ryan PD, Ozanne EM. Annual screening strategies in BRCA1 and BRCA2 gene mutation carriers: a comparative effectiveness analysis. Cancer. 2012;118:2021-2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 108. | Jansen-van der Weide MC, Greuter MJ, Jansen L, Oosterwijk JC, Pijnappel RM, de Bock GH. Exposure to low-dose radiation and the risk of breast cancer among women with a familial or genetic predisposition: a meta-analysis. Eur Radiol. 2010;20:2547-2556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 109. | Hanada K, Okazaki A, Hirano N, Izumi Y, Teraoka Y, Ikemoto J, Kanemitsu K, Hino F, Fukuda T, Yonehara S. Diagnostic strategies for early pancreatic cancer. J Gastroenterol. 2015;50:147-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 110. | Matsubayashi H, Sasaki K, Nagata K, Kanemoto H, Kiuchi R, Ono H. Pancreatic carcinoma mimicking diffuse-type autoimmune pancreatitis: important diagnostic role of pancreatic juice cytology using endoscopic naso-pancreatic drainage. J Dig Dis. 2012;13:287-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 111. | Ohtsuka T, Ideno N, Aso T, Nagayoshi Y, Kono H, Mori Y, Takahata S, Oda Y, Aishima S, Igarashi H. Role of endoscopic retrograde pancreatography for early detection of pancreatic ductal adenocarcinoma concomitant with intraductal papillary mucinous neoplasm of the pancreas. J Hepatobiliary Pancreat Sci. 2013;20:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 112. | Maguchi H, Takahashi K, Osanai M, Katanuma A. Small pancreatic lesions: is there need for EUS-FNA preoperatively? What to do with the incidental lesions? Endoscopy. 2006;38 Suppl 1:S53-S56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 113. | Kitano M, Kudo M, Yamao K, Takagi T, Sakamoto H, Komaki T, Kamata K, Imai H, Chiba Y, Okada M. Characterization of small solid tumors in the pancreas: the value of contrast-enhanced harmonic endoscopic ultrasonography. Am J Gastroenterol. 2012;107:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 242] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 114. | Ludwig E, Olson SH, Bayuga S, Simon J, Schattner MA, Gerdes H, Allen PJ, Jarnagin WR, Kurtz RC. Feasibility and yield of screening in relatives from familial pancreatic cancer families. Am J Gastroenterol. 2011;106:946-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 115. | Davis B, Lowy AM. Surgical management of hereditary pancreatic cancer. Med Clin North Am. 2000;84:749-759. [PubMed] |
| 116. | Kauff ND, Barakat RR. Risk-reducing salpingo-oophorectomy in patients with germline mutations in BRCA1 or BRCA2. J Clin Oncol. 2007;25:2921-2927. [PubMed] [DOI] [Full Text] |
| 117. | Nimptsch U, Krautz C, Weber GF, Mansky T, Grützmann R. Nationwide In-hospital Mortality Following Pancreatic Surgery in Germany is Higher than Anticipated. Ann Surg. 2016;264:1082-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 118. | Müller MW, Friess H, Kleeff J, Dahmen R, Wagner M, Hinz U, Breisch-Girbig D, Ceyhan GO, Büchler MW. Is there still a role for total pancreatectomy? Ann Surg. 2007;246:966-974; discussion 974-975. [PubMed] |
| 119. | Mehrabi A, Golriz M, Adili-Aghdam F, Hafezi M, Ashrafi M, Morath C, Zeier M, Hackert T, Schemmer P. Expanding the indications of pancreas transplantation alone. Pancreas. 2014;43:1190-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 120. | Miyazaki M, Yoshitomi H, Shimizu H, Ohtsuka M, Yoshidome H, Furukawa K, Takayasiki T, Kuboki S, Okamura D, Suzuki D. Repeat pancreatectomy for pancreatic ductal cancer recurrence in the remnant pancreas after initial pancreatectomy: is it worthwhile? Surgery. 2014;155:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 121. | Bellin MD, Gelrud A, Arreaza-Rubin G, Dunn TB, Humar A, Morgan KA, Naziruddin B, Rastellini C, Rickels MR, Schwarzenberg SJ. Total pancreatectomy with islet autotransplantation: summary of a National Institute of Diabetes and Digestive and Kidney diseases workshop. Pancreas. 2014;43:1163-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 122. | Heidt DG, Burant C, Simeone DM. Total pancreatectomy: indications, operative technique, and postoperative sequelae. J Gastrointest Surg. 2007;11:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 123. | Wu W, Dodson R, Makary MA, Weiss MJ, Hirose K, Cameron JL, Ahuja N, Pawlik TM, Wolfgang CL, He J. A Contemporary Evaluation of the Cause of Death and Long-Term Quality of Life After Total Pancreatectomy. World J Surg. 2016;40:2513-2518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 124. | Verna EC, Hwang C, Stevens PD, Rotterdam H, Stavropoulos SN, Sy CD, Prince MA, Chung WK, Fine RL, Chabot JA. Pancreatic cancer screening in a prospective cohort of high-risk patients: a comprehensive strategy of imaging and genetics. Clin Cancer Res. 2010;16:5028-5037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 125. | Vasen HF, Wasser M, van Mil A, Tollenaar RA, Konstantinovski M, Gruis NA, Bergman W, Hes FJ, Hommes DW, Offerhaus GJ. Magnetic resonance imaging surveillance detects early-stage pancreatic cancer in carriers of a p16-Leiden mutation. Gastroenterology. 2011;140:850-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 126. | Zubarik R, Gordon SR, Lidofsky SD, Anderson SR, Pipas JM, Badger G, Ganguly E, Vecchio J. Screening for pancreatic cancer in a high-risk population with serum CA 19-9 and targeted EUS: a feasibility study. Gastrointest Endosc. 2011;74:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 127. | Al-Sukhni W, Borgida A, Rothenmund H, Holter S, Semotiuk K, Grant R, Wilson S, Moore M, Narod S, Jhaveri K. Screening for pancreatic cancer in a high-risk cohort: an eight-year experience. J Gastrointest Surg. 2012;16:771-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 128. | Underhill M, Berry D, Dalton E, Schienda J, Syngal S. Patient experiences living with pancreatic cancer risk. Hered Cancer Clin Pract. 2015;13:13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 129. | Maheu C, Vodermaier A, Rothenmund H, Gallinger S, Ardiles P, Semotiuk K, Holter S, Thayalan S, Esplen MJ. Pancreatic cancer risk counselling and screening: impact on perceived risk and psychological functioning. Fam Cancer. 2010;9:617-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 130. | Breitkopf CR, Sinicrope PS, Rabe KG, Brockman TA, Patten CA, McWilliams RR, Ehlers S, Petersen GM. Factors influencing receptivity to future screening options for pancreatic cancer in those with and without pancreatic cancer family history. Hered Cancer Clin Pract. 2012;10:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 131. | Konings IC, Sidharta GN, Harinck F, Aalfs CM, Poley JW, Kieffer JM, Kuenen MA, Smets EM, Wagner A, van Hooft JE. Repeated participation in pancreatic cancer surveillance by high-risk individuals imposes low psychological burden. Psychooncology. 2016;25:971-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 132. | Hart SL, Torbit LA, Crangle CJ, Esplen MJ, Holter S, Semotiuk K, Borgida A, Ardiles P, Rothenmund H, Gallinger S. Moderators of cancer-related distress and worry after a pancreatic cancer genetic counseling and screening intervention. Psychooncology. 2012;21:1324-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 133. | Rulyak SJ, Kimmey MB, Veenstra DL, Brentnall TA. Cost-effectiveness of pancreatic cancer screening in familial pancreatic cancer kindreds. Gastrointest Endosc. 2003;57:23-29. [PubMed] |
| 134. | Bruenderman E, Martin RC. A cost analysis of a pancreatic cancer screening protocol in high-risk populations. Am J Surg. 2015;210:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 135. | Latchford A, Greenhalf W, Vitone LJ, Neoptolemos JP, Lancaster GA, Phillips RK. Peutz-Jeghers syndrome and screening for pancreatic cancer. Br J Surg. 2006;93:1446-1455. [PubMed] |
| 136. | Rubenstein JH, Scheiman JM, Anderson MA. A clinical and economic evaluation of endoscopic ultrasound for patients at risk for familial pancreatic adenocarcinoma. Pancreatology. 2007;7:514-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 137. | Alsop K, Fereday S, Meldrum C, deFazio A, Emmanuel C, George J, Dobrovic A, Birrer MJ, Webb PM, Stewart C. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30:2654-2663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 952] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 138. | Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmaña J, Mitchell G, Fried G, Stemmer SM, Hubert A. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1328] [Article Influence: 120.7] [Reference Citation Analysis (0)] |
| 139. | Ashworth A. A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol. 2008;26:3785-3790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 634] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 140. | Golan T, Kanji ZS, Epelbaum R, Devaud N, Dagan E, Holter S, Aderka D, Paluch-Shimon S, Kaufman B, Gershoni-Baruch R. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer. 2014;111:1132-1138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 335] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 141. | Oza AM, Cibula D, Benzaquen AO, Poole C, Mathijssen RH, Sonke GS, Colombo N, Špaček J, Vuylsteke P, Hirte H. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol. 2015;16:87-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 444] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 142. | Lohse I, Borgida A, Cao P, Cheung M, Pintilie M, Bianco T, Holter S, Ibrahimov E, Kumareswaran R, Bristow RG. BRCA1 and BRCA2 mutations sensitize to chemotherapy in patient-derived pancreatic cancer xenografts. Br J Cancer. 2015;113:425-432. [PubMed] [DOI] [Full Text] |
| 143. | Fogelman D, Sugar EA, Oliver G, Shah N, Klein A, Alewine C, Wang H, Javle M, Shroff R, Wolff RA. Family history as a marker of platinum sensitivity in pancreatic adenocarcinoma. Cancer Chemother Pharmacol. 2015;76:489-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |