Published online Feb 7, 2017. doi: 10.3748/wjg.v23.i5.906
Peer-review started: October 10, 2016
First decision: October 20, 2016
Revised: November 14, 2016
Accepted: December 8, 2016
Article in press: December 8, 2016
Published online: February 7, 2017
Processing time: 106 Days and 8.3 Hours
To treated with electrochemotherapy (ECT) a prospective case series of patients with liver cirrhosis and Vp3-Vp4- portal vein tumor thrombus (PVTT) from hepatocellular carcinoma (HCC), in order to evaluate the feasibility, safety and efficacy of this new non thermal ablative technique in those patients.
Six patients (5 males and 1 female), aged 61-85 years (mean age, 70 years), four in Child-Pugh A and two in Child-Pugh B class, entered our study series. All patients were studied with three-phase computed tomography (CT), contrast enhanced ultrasound (CEUS) and ultrasound-guided percutaneous biopsy of the thrombus before ECT. All patients underwent ECT treatment (Cliniporator Vitae®, IGEA SpA, Carpi, Modena, Italy) of Vp3-Vp4 PVTT in a single session. At the end of the procedure a post-treatment biopsy of the thrombus was performed. Scheduled follow-up in all patients entailed: CEUS within 24 h after treatment; triphasic contrast-enhanced CT and CEUS at 3 mo after treatment and every six months thereafter.
Post-treatment CEUS showed complete absence of enhancement of the treated thrombus in all cases. Post-treatment biopsy showed apoptosis and necrosis of tumor cells in all cases. The follow-up ranged from 9 to 20 mo (median, 14 mo). In 2 patients, the follow-up CT and CEUS demonstrated complete patency of the treated portal vein. Other 3 patients showed a persistent avascular non-tumoral shrinked thrombus at CEUS and CT during follow-up. No local recurrence was observed at follow-up CT and CEUS in 5/6 patients. One patient was lost to follow-up because of death from gastrointestinal hemorrage 5 wk after ECT.
In patients with cirrhosis, ECT seems effective and safe for curative treatment of Vp3-Vp4 PVTT from HCC.
Core tip: Six patients with portal vein tumor thrombus (PVTT) at hepatic hilum underwent electrochemotherapy (ECT), a new non thermal ablative technique. The follow-up ranged from 9 to 20 mo (median, 14 mo). In 2 patient, the follow-up computed tomography (CT) demonstrated complete patency of the treated portal vein. Three patients showed a persistent avascular non-tumoral shrinked thrombus at CT during follow-up. No local recurrence was observed in 5/6 patients. One patient, was lost to follow-up because of death from gastrointestinal hemorrage 5 wk after treatment. ECT seems effective and safe for curative treatment of PVTT at hepatic hilum.
- Citation: Tarantino L, Busto G, Nasto A, Fristachi R, Cacace L, Talamo M, Accardo C, Bortone S, Gallo P, Tarantino P, Nasto RA, Di Minno MND, Ambrosino P. Percutaneous electrochemotherapy in the treatment of portal vein tumor thrombosis at hepatic hilum in patients with hepatocellular carcinoma in cirrhosis: A feasibility study. World J Gastroenterol 2017; 23(5): 906-918
- URL: https://www.wjgnet.com/1007-9327/full/v23/i5/906.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i5.906
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third most frequent oncologic cause of death worldwide[1]. HCC is often diagnosed at intermediate or advanced stages, when portal vein (PV) and its branches have already been involved by the tumoral process[2,3]. It has been reported that approximately 10%-40% of HCC patients are diagnosed with portal vein tumor thrombus (PVTT)[4,5]. According to the Barcelona Clinic Liver Cancer classification system[6], HCC patients with PVTT are classified as advanced stage (or Stage C), with a rather poor prognosis and an expected median survival span of about 2.7-3.0 mo[3,6]. Current guidelines only recommend systemic chemotherapy with Sorafenib in such patients[6].
The Liver Cancer Study Group of Japan has recently proposed a macroscopic classification for PVTT categorized into five grades, defined as follows: Vp0, no PVTT; Vp1, PVTT distal to the 2nd order branches of the PV; Vp2, PVTT in the 2nd order branches of the PV; Vp3, PVTT in the 1st order branches of the PV; Vp4, PVTT in the main trunk of the PV or a PV branch contralateral to the mainly involved lobe (or both)[7].
In recently published papers[8-14], surgical hepatic resection (HR) and loco-regional therapies, such as trans-arterial chemoembolization (TACE), radiofrequency ablation (RFA), microwawe (MW) ablation, gamma-knife radiosurgery, 90Y-based transarterial radioembolization (TARE), proved to be feasible and moderately effective treatments to improve survival of HCC patients with peripheral PVTT (Vp1 and Vp2). However, all these therapies did not substantially modify prognosis in patients with PVTT in the main PV and/or its 1st order branches (Vp3 and Vp4). Some previous isolated reports advocated a possible role of percutaneous ethanol injection (PEI)[15], RFA[16,17] or interstitial laser therapy (ILT)[18] in the treatment of Vp3-Vp4 PVTT. However, the use of PEI remains unreliable in such clinical settings because of the easy escaping of the ethanol in the blood stream and the high probability of producing neoplastic thrombi. On the other hand, thermal ablation with RF and ILT are harmful techniques when applied near the hepatic hilum because of the high risk of irreversible damage to main bile ducts, main hepatic artery, gallbladder, duodenum or gastric wall[19-23]. As a matter of fact, with the only exception of the above cited studies, no other group recommended the use of these techniques in the treatment of Vp3-Vp4 PVTT.
A potential ideal ablation technique for Vp3-Vp4 PVTT should be able to kill tumor cells in the portal vessels without heat generation and without affecting patency of main bile ducts, arterial vessels and even without any damage to PV walls. Electrochemotherapy (ECT) is a non-thermal local tumor ablation modality using electroporation[24]. This is a physical method that enhances cell membrane permeability, and enables non-permeant or poorly permeant chemotherapeutic agents to enter cells, greatly enhancing their efficacy[25-28]. Safety and efficacy of the use of ECT in proximity of vascular and ductile structures of liver and pancreas have been already demonstrated in several studies[29-33].
In order to evaluate the feasibility, efficacy and safety of ECT in the treatment of Vp3-Vp4 PVTT, we present a prospective case series of patients with liver cirrhosis and extensive Vp3-Vp4 PVTT from HCC treated with ECT.
From December 2014 to December 2015, 42 patients (29 male, 13 female, age range 48-91 year) with Vp3-Vp4 PVTT were observed at our Unit of Hepatology and Interventional Ultrasound in a tertiary care Institution - A. Tortora Cancer Hospital. To be included in the present study, patients had to fulfil the following basal inclusion criteria: (1) Malignant PV thrombosis of the right and/or left portal branch and/or of the distal portion (last 2 cm) of the main PV; (2) synchronous naive HCC nodule or recurrence after previous treatments for HCC not exceeding 5 cm in size and not more than 3 nodules in number. The presence of nodules abutting the gastrointestinal tract or hepatic hilum, the diaphragm, gall-bladder, kidney and right or left branch of the PV were not considered exclusion criteria; (3) Karnofky performance status > 70; (4) severity of liver function impairment not exceeding Child-Pugh class B-8, platelet count > 50.000/mm3, INR < 1.5, total bilirubin < 1.5 mg/dL; (5) absence of ascites at the time of treatment; (6) absence of gastric varices and/or esophageal varices not exceeding grade F2 at endoscopy; and (7) absence of indications to Sorafenib therapy (Child B/C class) or intolerance to previous Sorafenib therapy.
Causes for exclusion from the treatment were: (1) extrahepatic HCC metastases; (2) heart impairment, arrythmias or presence of a cardiac pace-maker; (3) severe lung or renal or hepatic insufficiency; (4) epilepsy; (5) allergy to bleomycin; and (6) presence of prosthesis.
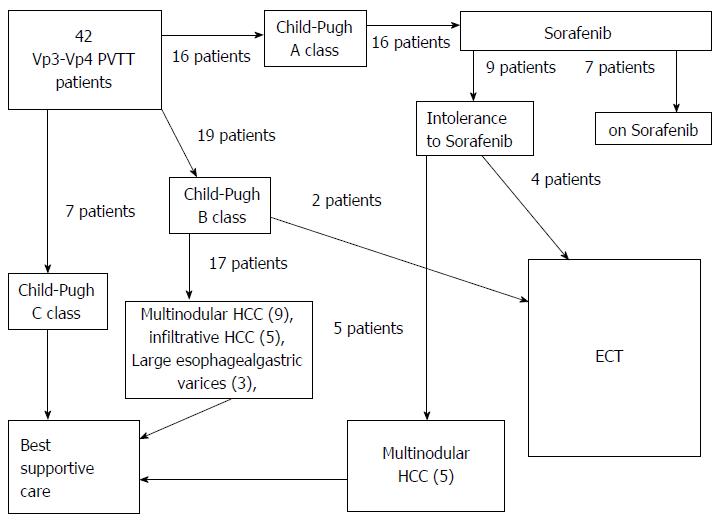
Seven patients in Child-Pugh A class had already been treated with Sorafenib at other Institutions and had stopped the treatment because of severe side effects. These patients had been addressed to our Institution for evatuation of eligibility to ECT. Other 9 Child-A-class patients were advised for systemic therapy with Sorafenib at our Institution. Two out of them stopped Sorafenib because of side effects. Seven patients remained on Sorafenib therapy. Seven Child-Pugh-C-class patients were excluded from ECT treatment because of severe impairment of hepatic function. Twenty-two patients were excluded from ECT treatment because of the presence of one or more of the following conditions (Figure 1): multinodular HCC (14 patients), extensive hepatic infiltration from HCC (5 patients), large esophageal (F3) or gastric varices (3 patients). Therefore, 36 out of 42 patients (85.7%) were excluded from ECT, and six patients with liver cirrhosis and Vp3-Vp4 PVTT from HCC fulfilled the selection criteria and were included in our series. Baseline characteristics of the six patients are reported in Table 1.
| Patient | Age/sex | PS:E/K | Cirrhosis etiology | Child-Pugh class | AFP (ng/mL) | Albumin (g/100 mL) | INR | Bilirubin (mg) | PLT (n/μL) | EGDS | Comorbidities |
| EV, GV, RS | |||||||||||
| 1 | 64 yr/M | 2/70 | HCV | A6 | 14 | 3.7 | 1.10 | 1.20% | 75000 | F1-EV, GV-, RS- | Diabetes |
| 2 | 54 yr/M | 1/80 | HCV | B7 | 84 | 3.2 | 1.40 | 1.40% | 62000 | F2-EV, GV+, RS- | Diabetes, MI, COPD |
| 3 | 85 yr/M | 1/80 | HCV | A5 | 32 | 4.2 | 1.14 | 1.20% | 125000 | F!-EV, GV-, RS- | None |
| 4 | 75 yr/M | 2/70 | HCV | A6 | 65 | 3.6 | 1.32 | 1.00% | 88000 | F2-EV, GV-, RS- | Thyroid carcinoma, COPD |
| 5 | 61 yr/F | 0/100 | HCV | A5 | 47 | 4.0 | 1.25 | 0.90% | 74000 | EV-, GV-, RS- | Diabetes, hypertension |
| 6 | 77 yr/M | 2/70 | HCV | B7 | 16 | 3.5 | 1.55 | 1.30% | 56000 | F1-EV, GV-, RS- | Diabetes |
This study was approved by the Institutional Board of our Institute and was conducted according to the declaration of Helsinki. All patients provided written, informed consent.
According to the EFSUMB (European Federation of Societies for Ultrasound in Medicine and Biology) guidelines[34,35], HCC was diagnosed on the basis of characteristic enhancement patterns (arterial enhancement and late wash-out) at three-phase contrast-enhanced computed tomography (CT) and contrast enhanced ultrasound (CEUS). In order to assess the nature of the thrombosis, pre-treatment CEUS and ultrasound (US) guided percutaneous biopsy of the thrombus were performed in all patients[36-38]. Intraoperative biopsy of the treated PVTT was also scheduled in all patients at the end of the treatment.
To be included in the study, patients had to fulfil the following inclusion criteria: (1) absence of indications to Sorafenib therapy (Child B/C class) or intolerance to previous Sorafenib therapy; (2) severity of liver function impairment not exceeding Child-Pugh class B-8; (3) platelet count > 50.000/mm3, INR < 1.5, total bilirubin < 1.5 mg/dL; (4) absence of ascites at the time of treatment; (5) absence of gastric varices and/or esophageal varices grade > F2 at endoscopy; (6) absence of extrahepatic HCC metastases; and (7) synchronous first HCC nodule or recurrence after previous treatments for HCC not exceeding 5 cm in size and not more than 3 nodules in number. The presence of nodules abutting the gastrointestinal tract or hepatic hilum, the diaphragm, gall-bladder, kidney and right or left branch of the PV were not considered exclusion criteria.
Before ECT procedure, all patients underwent: (1) serum blood tests for liver function and haemocoagulation and serum alpha-fetoprotein dosage; (2) upper gastrointestinal tract endoscopy (esophagogastroduodenoscopy) for evaluation of esophageal varices; (3) electrocardiogram; (4) chest X-ray; (5) abdominal US with a commercially available US equipment (EPIQ 7, Philips SpA, Amsterdam, The Netherlands) and a 3.5 and 5.0 MHz convex electronic probe; and (6) triphasic contrast-enhanced CT [Iomeron® 400 (iomeprol), Bracco SpA, Milan, Italy].
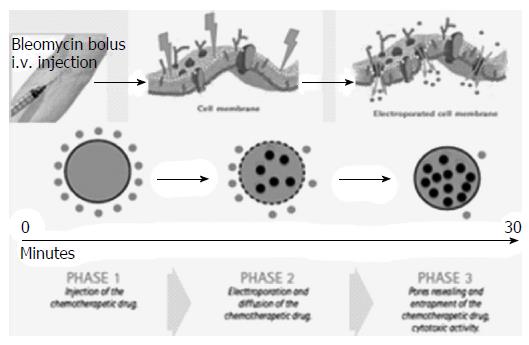
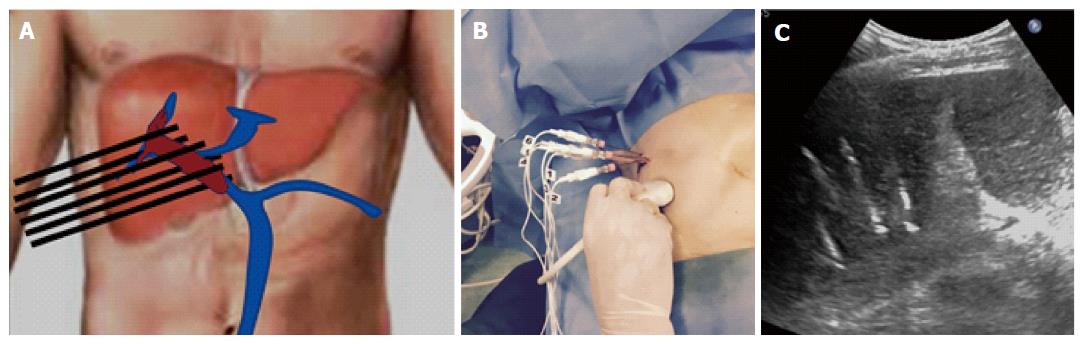
ECT is a combined use of chemotherapeutic drugs and electric pulses applied to the treated tumour nodule. Local application of electric pulses to the tumour increases drug delivery into cells, specifically at the site of electric pulse application (Figure 2). Drug uptake by delivery of electric pulses is increased for only those chemotherapeutic drugs whose transport through the plasma membrane is impeded. Among many drugs that have been tested so far, bleomycin and cisplatin found their way from preclinical testing to clinical use. We performed ECT under general anesthesia, with intubation. In order to avoid strong muscle contractions induced by electric pulses, the myorelaxant cisatracurium besylate (Nimbex®, GlaxoSmithKline, Brentford, United Kingdoms) was used. All patients underwent US guided percutaneous biopsy of the PVTT with a 21 gauge Chiba needle (ecojekt, HS Hospital service, Rome, Italy) before the start of ECT procedure. Than, under US guidance, four to six electrode-needles were inserted percutaneously along the external margin of the thrombosed portal vessel (Figure 3). The electrodes were connected to independently controlled generator outputs of the Cliniporator Vitae® (IGEA SpA, Carpi, Modena, Italy). The Cliniporator Vitae® device is a pulse generator with 6 independently controlled and electrically isolated outputs, each providing up to 3000 Volts (maximum current: 50 Amperes) and delivering 8 rectangular electrical pulses (rise time: 1 μs) of 100 μs duration at a pulse repetition frequency of 4 Hz. Eight minutes after intravenous bolus injection (15000 IU/m2) of Bleomycine sulfate (Bleoprim®, Sanofi Aventis, Paris, France), electric pulses were delivered. After electric pulses delivery, the electrodes were partially or completely withdrawn and repositioned around the HCC nodule associated with the PVTT in order to treat the tumor with ECT. Hemostasis of liver capsule, peritoneum, abdominal wall and skin was performed with a thermal track ablation by connecting every single electrode to an activated electric scalpel during the complete withdrawal of the electrodes.
US guided percutaneous cutting needle biopsy of the treated PVTT with an 18 gauge modified Menghini’s needle (Biomol, HS Hospital service, Rome, Italy) was performed in all patients after ECT. CEUS control of PVTT was performed within 24 h after the end of ECT procedure.
After procedure, all patients started Enoxaparine (4000 IU, daily). The scheduled follow-up in all patients entailed: monthly color-Doppler US (CDUS) in the first 3 mo after treatment; triphasic contrast-enhanced CT and CEUS 3 mo after treatment; afterwards, CT and CEUS controls every six months.
Six patients (5 males and 1 female), aged 54-85 years (mean age, 69 years) entered our study series. Baseline characteristics of the patients are reported in Table 1.
Four patients were in Child-Pugh A class and two in Child-Pugh B class. Four patients showed 1 (3 cases) or 2 (1 case) HCC nodules synchronous with Vp3-PVTT (all patients) and Vp4-PVTT (one case). The size of HCC nodules ranged from 2.5 to 4.5 cm (mean = 3.4 cm). Two patients, previously treated with surgery and thermal ablation, showed an isolated right branch Vp3-PVTT, without any residual or recurrent HCC nodule in the liver. Treatments’ results in the single patient are reported in Table 2.
| Patient | Previous treatments | Site of PVTT | HCC nodules/size | Results of ECT | Follow-up |
| 1 | Surgery, RFA | Right PV, distal main PV | - | Complete necrosis of Vp3-Vp4 PVTT | 20 mo |
| Complete recanalization | |||||
| 2 | None | Right PV, distal main PV | 1 nodule/3.5 cm | Complete necrosis of Vp3-Vp4 PVTT | Death for rupture of esophageal varices 14 mo after ECT |
| Complete recanalization | |||||
| 3 | None | Right PV | 1 nodule/3 cm | Complete necrosis of Vp3-Vp4 PVTT | Death for Liver failure |
| Persistent shrinked bland thrombosis | 14 mo after ECT | ||||
| 4 | RFA | RightPV, left PV, distal main PV | 2 nodules/1.5 and 2.5 cm | Complete necrosis of Vp3-Vp4 PVTT | Death 5 wk after ECT for rupture of esophageal varices |
| Persistent shrinked bland thrombosis | |||||
| 5 | None | Left PV | 1 nodule/3.5 cm | Complete necrosis of Vp3-Vp4 PVTT | 12 mo |
| Persistent shrinked bland thrombosis | |||||
| 6 | Surgery, RFA, TACE | Right PV | - | Complete necrosis of Vp3-Vp4 PVTT | 9 mo |
| Persistent shrinked bland thrombosis |
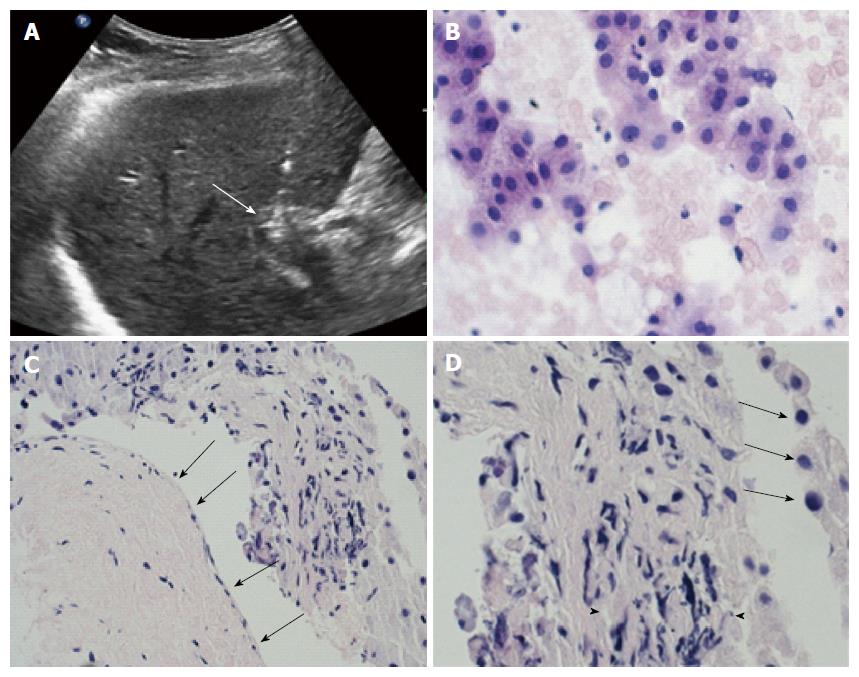
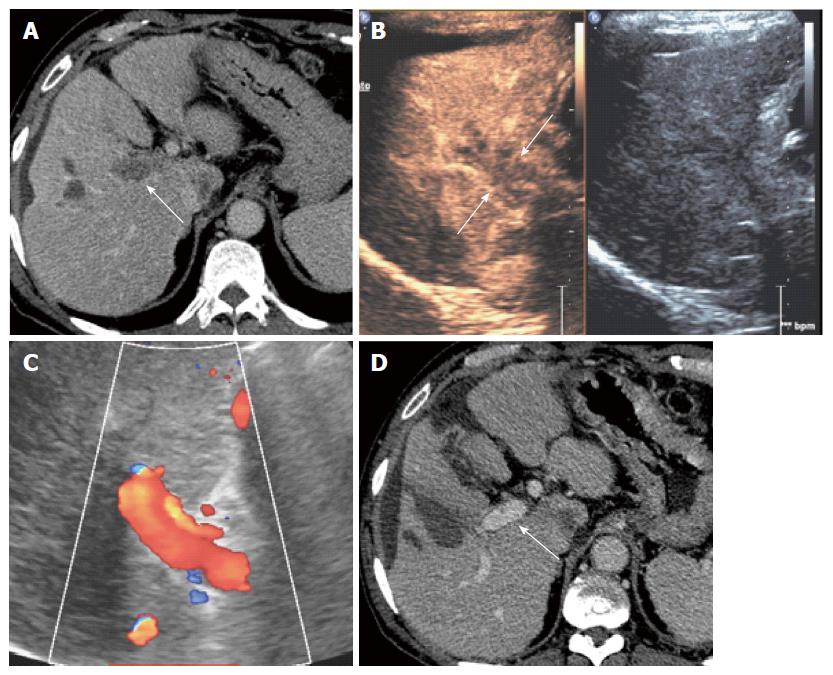
Intraoperative pre-treatment biopsy of PVTT was adequate and showed viable HCC in 5/6 cases (Figure 4A). In one patient the fine-needle biopsy only showed blood material. However, pre-treatment CEUS demonstrated hypervascular PVTT with characteristic enhancement patterns (arterial enhancement and late wash-out) (Figure 5B) in all cases (6/6), thus confirming the malignant nature of thrombosis in all patients[36,39].
Two out of six patients underwent ECT of the isolated Vp3-PVTT. Other three patients underwent ECT of PVTT and of the associated HCC nodule in the same session. In these patients, after ECT of PVTT, the electrodes were partially or completely withdrawn and were reinserted around the tumor. In one patient with 2 HCC nodules, we only treated the PVTT and planned a subsequent session of thermal ablation to treat the 2 HCC nodules (Figure 1).
Post-treatment biopsy of PVTT showed severe involutive changes of tumor cells with cellular apoptosis and areas of necrosis in all cases. In 3 cases, PV wall and periportal tissues were present in the specimen; in these portions of the specimens, PV wall showed normal findings with normal endothelium; no distortion or necrosis was detectable in the periportal structures (Figure 4B and C).
Post treatment intraoperative CEUS demonstrated complete absence of enhancement of the thrombosis (Figuer 5C) and of the treated HCC nodule in all cases.
The procedure was well tolerated in all cases. No intraoperative or post-operative major complication was reported. All patients were discharged from the hospital the day after the treatment.
The follow-up ranged from 9 to 20 mo (median, 14 mo). In two patients, CDUS showed complete recanalization of the treated PVTT at 2 and 3-mo CDUS follow-up, respectively. In these two patients, CEUS and CT confirmed complete patency of the vessel without any intravascular or perivascular recurrence during follow-up. In three patients (14, 12, and 9 mo follow-up, respectively), CT and CEUS showed permanent complete thrombosis with a persistent, shrinked, avascular thrombus into the treated vessels. In all three cases, no intravascular or perivascular enhancement consistent with residual tumor or local recurrence was detected at CT and CEUS during follow-up. In the remaining patient, 24 h post-treatment CEUS showed absence of enhancement of the treated thrombus. However, the patient was lost to follow-up because of death from gastrointestinal hemorrage five weeks after ECT treatment.
During follow-up, no local recurrences, at the site of the treated PVTT, occurred. However, 3 distant recurrences in other segments were detected in 2 patients. All 3 new lesions were treated by RFA in 2 cases and ECT in 1 case.
Patient 1: Male, 64-year-old with HCV-related Child-Pugh A6 cirrhosis. Previous RFA of a HCC nodule (diameter = 3 cm) in the IV segment in december 2012. In November 2013, laparotomy and intraoperative MW ablation for 2 HCC recurrences in I and V-VIII segments and PVTT of the segmental portal branch (Vp2) for segment V. Post treatment CT, on January 2014, demonstrated complete necrosis of the HCC nodules and of the Vp2-PVTT. In september 2014, CT showed and extensive and complete right Vp3-PVTT without any intraparenchymal recurrence of HCC. The patient started Sorafenib (Nexavar®, Bayer, Leverkusen, Germany) 400 mg daily but was forced to stop therapy because of severe side effects. In December 2014, the patient accepted ECT, as alternative therapy. Pretreatment biopsy proved PV infiltration from “moderately differentiated HCC” (Grade 2 Edmondson). The patient underwent ECT with insertion of 6 electrode-needles. The 2-mo CDUS showed complete patency of the right PV (Figure 4C). The result was confirmed at 3-mo CEUS and CT (Figure 4D). CT control at 9, 15 and 18 mo still showed a completely patent PV without any intravascular recurrence. During follow-up, US and CT controls showed recurrence of HCC in the VIII segment and in the VI segment at 6 and 12 mo respectively. Recurrences were treated by ECT and RFA respectively.
Patient 2: Male 54-year-old with HCV-related Child-Pugh B7 cirrhosis, obesity, chronic obstructive pulmonary disease (COPD), and previous myocardial infarction. The patient had been treated ten years before with coronary stents, and was on secondary ticlopidine prophylaxis. In February 2015, abdominal US, CEUS and CT showed HCC in the V segment and extensive malignant portal thrombosis of the right PV. The patient underwent ECT by percutaneous insertion of 4 electrodes. The intraoperative post treatment CEUS showed a completely avascular PV thrombus. The 3-mo CEUS and CT showed a subtotal recanalization. The 6-mo and 12-mo CT confirmed absence of local recurrence and complete recanalization of the PV. Unfortunately, the patients were lost to follow-up at 14 mo because of death after severe hemorrhage from gastroesophageal varices.
Patient 3: Male, 85-year-old with HCV-related Child-Pugh A5 cirrhosis. In december 2014, diagnosis of large infiltrating HCC (size: 4, 5 cm) in the VII segment. The patient started systemic therapy with Sorafenib (400 mg/daily) at another Institution. In April 2015, he underwent CT and MRI at our institution that detected a large infiltrating HCC in the VII segment and extensive and complete thrombosis of the right PV from its origin on the main trunk up to its bifurcation. CEUS and MRI were consistent with malignant thrombosis. The evident progression of the disease forced to stop Sorafenib therapy and the patients was advised for ECT treatment as alternative. Pretreatment biopsy proved PV infiltration from “moderately differentiated HCC” (Grade 2 Edmondson). The patient underwent ECT of both HCC nodule and Vp3-PVTT by insertion of six electrodes. Six months and 12-mo CT showed persistence of avascular, shrinked right PV thrombosis. During follow-up, a new HCC nodule was detected in the IV segment at 6 mo follow-up. The new lesion was treated by RFA. Post treatment CT demonstrated complete necrosis of the recurrence.
Patient 4: Male, 75-year-old, with HCV-related Child-Pugh A6 cirrhosis and COPD. The patient had been previously treated with radioiodine for thyroid papillary carcinoma that was in complete remission at the time of admission. Pre-treatment EGDS showed grade F2 esophageal varices and absence of gastric varices. CT in May 2015 showed 2 HCC nodules in the VIII and VII segment and Vp3-Vp4 PVTT, involving both right and left PV and the distal portion of main PV. The patient started Sorafenib (Nexavar®, Bayer, Leverkusen, Germany) 400 mg daily. Three weeks later the patient was forced to stop therapy because of severe side effects. The patient was offered a two-step treatment schedule, entailing ECT of Vp3-Vp4 PVTT as first step and RFA of HCC nodules as second step. In this patient we inserted simultaneously six electrodes in two sets of three electrodes each, in order to cover the right and main PV with three electrodes and the left PV with the other three. Post treatment CEUS showed complete absence of enhancement of thrombosis in the right, left and main PV. One month CEUS confirmed absence of enhancement in the treated Vp3-Vp4 PVTT, and also showed bland complete thrombosis of main portal trunk and upper mesenteric vein. Then, Enoxaparine dosage was increased to 8000 UI daily. The patient underwent weekly controls with CDUS that showed unchanged findings in portal vessels overtime. Unfortunately, five weeks after treatment the patient died because of a severe hemorrage from rupture of esophageal varices.
Patient 5: Female, 61-year-old, with HCV-related Child-Pugh A5 cirrhosis. In September 2011 the patient underwent liver resection for a single HCC nodule in the III segment. In may 2015, CT and CEUS showed HCC nodule in the IV segment and complete left Vp3-PVTT. The patient started systemic therapy with Sorafenib (400 mg/daily); the treatment was stopped after 3 wk because of severe side effects (weakness, diarrhea). Therefore he accepted ECT treatment. Six electrodes were inserted along the wall of the PVTT. Post-treatment intraoperative CEUS showed a large avascular area (8 cm × 7 cm) including the treated vessel and the perivascular liver parenchyma. Three months CEUS and CT follow-up showed avascular, shrinked thrombosis of the left PV. Six-months CT and nine-months CEUS confirmed the persistence of avascular thrombosis and absence of any recurrence.
Patient 6: Male, 77-years-old, with HCV-related Child-Pugh B7 cirrhosis and recurrent HCC. The patient had undergone multiple locoregional procedures (liver resection, TACE, RFA) in September 2005, June 2006, June 2008, January 2009, and July 2013 to treat multiple HCC recurrences. In August 2015, CT showed complete malignant thrombosis of the right PV. Six electrodes were inserted along the wall of the Vp3-PVTT. Post treatment CEUS, 3-mo and 9-mo CT and CEUS showed persistent, complete, shrinked avascular thrombus.
The present case series is the first, to our best knowledge, evaluating safety and efficacy of ECT for the treatment of extensive PVTT at hepatic hilum (Vp3-Vp4) in patients with HCC. We found that this mini-invasive interventional technique is feasible, safe and effective and it could represent a suitable option for the management of PVTT in this clinical setting.
With an annual incidence of more than 620000 new cases, HCC is the third most frequent cause of cancer death worldwide[1]. Its incidence is progresively increasing[2] and, despite surveillance programs, HCC is often diagnosed at an intermediate or advanced stage[3]. It has been reported that up to 44% of HCC patients are complicated with PVTT at the time of death[40]. PVTT severely affects prognosis of cirrhotic patients with HCC. The median survival time of patients with unresectable HCC without PVTT ranges from 10 to 24 mo while it is significantly reduced in cases with associated PVTT (2-4 mo). PVTT is related with poor prognosis probably because of the intensified risk of tumor spread, increased portal pressure inducing variceal bleeding and reduced portal flow and subsequent jaundice, ascites, hepatic encephalopathy and hepatic failure[4].
For HCC patients with PVTT, current guidelines recommend systemic therapy with Sorafenib, an oral multiple tyrosine-kinases inhibitor that suppresses angiogenesis and tumor cell proliferation[6]. In clinical studies[41,42]. Sorafenib proved to improve of several months survival of patients with advanced HCC. Nevertheless, subgroup analyses only showed a marginal survival benefit for Sorafenib as compared with placebo in patients with PVTT[42,43]. All currently accepted HCC treatment guidelines consider PVTT a contraindication for transplantation, HR and TACE[44,45]. The hilar position of PVTT (Vp3-Vp4), in contrast with peripheral PVTT (Vp1-Vp2), further worsen the prognosis, also because represents a controindication to loco-regional therapies. When feasible, surgical resection of the tumor and associated PVTT might be the best possible treatment, but only a small percentage of patients can benefit of this approach[9]. Mild-moderate improvement in survival of patients with Vp1-Vp2 PVTT have been reported with TACE[10,11] and TARE[12]. However, both TACE and TARE demonstrated only minimal or absent advantages in patients with Vp3-Vp4 PVTT[11,12]. Ablation techniques such as PEI and RFA are usually not indicated in the management of intermediate and advanced HCC[44,45]. In 1990, Livraghi et al[15] reported complete ablation and absence of recurrence at 4-12 mo follow-up in 4 patients with segmental (V1p-V2p PVTT) treated with PEI. Several papers reported synchronous ablation of segmental PVTT and associated HCC nodule by RFA, MW ablation or ILT[14-18]. However, most authors excluded patients with involvement of main branches or main portal trunk (Vp3-Vp4 PVTT) because ablation of nodules next to hepatic hilum is considered unsafe. The high risk of damage to main bile ducts (stricture, rupture, obstruction) and to the hepatic artery (bleeding, pseudoaneurysm, arterio-portal fistula) after ablation of tumors in the center of the liver has been well described and demonstrated both in vivo experimental studies and in patients series[19,23]. Several measures have been suggested to avoid complications from damage to hepatic hilum structures during RFA and MW ablation[46], however none of these approaches have been extensively applicated in large series. In 2009 and in 2014 Giorgio et al[16,17] published their long-term results of RFA in 35 HCC patients with Vp3 and/or Vp4 PVTT. They reported absence of major complications, complete recanalization of main portal trunk in 26/35 (74%) patients and cumulative survival rates at 1, 3, and 5 years of 63%, 30% and 20%, respectively. Lu et al[18] demonstrated similar results in 108 patients treated with ILT for Vp3-Vp4 PVTT, reporting a 3-year survival rate of 22.4%. Both authors did not describe, in their procedures, any safety measure in order to avoid damage to hepatic hilum structures. In our knowledge, no other Author followed this high risk strategy.
A potential ideal ablation technique for Vp3-Vp4 PVTT should be able to kill tumor cells in the portal vessels without heat generation and without affecting patency of main bile ducts, arterial vessels and even without any damage to PV wall. The electroporation is a process in which electric impulses can cause structural changes in biological membranes[24,47,48]. Depending on pulse amplitude, duration, and the number of pulses, two possible results can be achieved. At subcritical electric fields, electroporation leads to transient pore formation with increase of membranes permeability to macromolecules that hardly could penetrate the cells in absence of electroporation. The average pore size is stationary and very small and, subsequently, a complete membranes’ recovery occur (reversible electroporation)[47]. At supercritical field strengths, the pore radius increases reaching a critical pore size. Therefore, the membrane disgregates without any possibility of recovery [irreversible electroporation (IRE)][49]. In the last 20 years, two electroporation-based therapeutic techniques have been introduced: IRE and ECT. IRE uses high intensity electric pulses to obtain death of all cells in the electric field through irreversible permeabilization of cell membranes[49-52]. ECT is a local tumor ablation modality that, through reversible electroporation, enhances cell membrane permeability, and enables non-permeant or poorly permeant chemotherapeutic agents to enter cells, greatly enhancing their efficacy in killing tumor cells[24,31].
ECT and IRE can be used to treat tumours surrounded by vital structures such as larger blood vessels, nerves, and viscera without subsequent damage to these structures[24,47,48]. The safety and efficacy of their use around vascular, hollow viscera and ductile structures in liver and pancreas have been already demonstrated in many published papers[29-33]. However, few papers have evaluated the feasibility and effectiveness of percutaneous ECT on deep tumours[31-33] and to our knowledge we are the first to evaluate the safety and effectiveness of percutaneous ECT in the treatment of PVTT from HCC.
The analysis of pathologic findings in specimens obtained with biopsies of PVTT performed before and after ECT procedures is particularly interesting. Pretreatment biopsies of thrombosis gave adequate material for definitive diagnosis of PVTT from HCC in 5/6 patients. Post-treatment biopsies of the treated thrombus were performed in all patients. Necrosis and apoptotic aspects of tumor cells were detected in post-treatment biopsies in all specimens. In some patients the post-treatment biopsy material was particularly adequate to describe what actually happens when tumor tissue and normal cells are exposed to ECT. Infact, three out of six post-treatment biopsy specimens included PV wall and periportal tissues around the tumor thrombus. In these cases, the damage of tumor cells (necrosis and apoptosis) was clearly showed, while no involutive aspect was detected in perivascular tissue and even in the portal endothelium. This selective effectiveness of ECT technique to damage only tumor cells is based on the intrisic mechanism of the chemotherapeutic agent used in the procedure. In particular, bleomicyn cytotoxicity is specific for cell cycle G2 phase[53]. As a result, only rapidly growing tissues, with cells in mitosis, are the target of the drug. However, when bleomycin is administered in a peripheral vein, only a low antitumoral efficacy is expected because of a poor diffusion through cells membranes and, therefore, only slight availability of the drug in the site of action (intracellular DNA)[53]. The electroporation process, obtained by the electric field through electrodes insertion in the target tissue, allows the bleomicyn to concentrate in the cells and operate the cell cycle block with subsequent cell death[24]. ECT ablation is not a physical process but a biochemical therapy, physically induced and amplified, highly selective for tumor cells[25]. ECT does not destroy nor modify the stromal architecture of the target tissue and damages normal cells only partially or not at all[25]. As a result, ECT treatment can be safely indicated in tumor next to, or even inside, the hepatic hilum, a condition that represent a contraindication to all the other locoregional therapies. In our opinion, Vp3-Vp4 PVTT represents the ideal condition to evaluate the ECT safety and efficacy. On this assumption, we started this prospective study on a series of consecutive cirrhotic patients affected from HCC and Vp3-Vp4 PVTT.
Our results showed that ECT is highly effective and safe for Vp3-Vp4 PVTT. In five patients, we observed the complete necrosis of the PVTT. In cases with an associated HCC nodule we also achieved the complete necrosis of the tumor. In two patients we observed at 2-3 mo follow-up the complete recanalization of the treated portal vessel. In no case, during a mediane follow-up of 12 mo, we observed a local recurrence. ECT of PVTT can be performed safely. No intraoperative and early postoperative complication occurred. A late complication occurred in one patient who underwent ECT for a complete tumor thrombosis of terminal part of main portal trunk and both right and left PV. Postoperative CEUS showed complete necrosis of all portions of PVTT. He left the hospital the day after treatment. In two weeks, he developed moderate ascites and Color Doppler US showed bland complete thrombosis of the whole axis of main trunk of PV. To treat the progression of bland thrombosis, dosage of LMW heparin was increased (from 4.000 UI to 8000 UI daily). At weekly Color Doppler US examination, the thrombus seemed to remain stable. However, five weeks after ECT treatment, the patient died for a severe hemorrage from rupture of esophageal varices. Another patient died for rupture of esophageal varices at 14 mo follow-up. In this patient, all imaging studies performed during follow-up excluded local o distant recurrence of HCC.
Four patients are alive and no local recurrence of the treated PVTT or the treated HCC nodule have been detected at 9-20 mo follow-up. In two patients distant recurrence occurred and were treated with percutaneous ablation in all cases.
This study has some limitations, represented by the small number of treated patients and the short-term follow-up. However, the preliminary results of our case series might represent the proof of concept for a future prospective study on consecutive patients and a long-term follow-up. Actually, our study is a prospective series, still going on. In this initial experience, we selected advanced HCC patients not eligible to systemic therapy with Sorafenib. The efficacy and safety profile of ECT procedure could encourage a combination treatment of ECT plus Sorafenib even in naive patients, in which systemic therapy is indicated. The main concern with the results in this study is the occurrence of a bland thrombosis of main portal trunk and fatal late hemorrhage from esophageal varices. In our short series (6 patients) a single fatal complication account for 16%-17% of cases. This rate, if confirmed on larger series, would be inacceptable, and patients with gastric varices or with esophageal varices even < F2 staging, should be excluded from treatment, or alternatively, advised of the high risk of the occurrence of this complication.
In conclusion, results of our prospective series suggest that ECT is a feasible effective and safe therapy for Vp3-Vp4 PVTT from HCC in cirrhotic patients not eligible to other therapeutic approaches. A high risk of hemorrhage from gastroesophageal varices after ECT treatment of main, right or left PV must be considered in the pre-treatment evaluation of the patients.
In patients with hepatocellular carcinoma (HCC) on liver cirrhosis and portal vein tumor thrombus (PVTT) at hepatic hilum, Current guidelines only recommend systemic chemotherapy with Sorafenib. Thermal ablation techniques are harmful when applied near the hepatic hilum because of the high risk of irreversible damage to main bile ducts and other structures. A potential ideal ablation technique in these cases should be able to kill tumor cells in the portal vessels without heat generation and without affecting patency of main bile ducts, arterial and venous vessels.
Electrochemotherapy (ECT) is a non-thermal local tumor ablation that enhances cell membrane permeability, and enables non-permeant or poorly permeant chemotherapeutic agents to enter cells, greatly enhancing their efficacy. Safety and efficacy of the use of ECT in proximity of vascular and ductile structures of liver and pancreas have been already demonstrated in several studies.
ECT can be used to treat tumours surrounded by vital structures such as larger blood vessels, nerves, and viscera without subsequent damage to these structures. The safety and efficacy of their use around vascular, hollow viscera and ductile structures in liver and pancreas have been already demonstrated in many published papers. However, few papers have evaluated the feasibility and effectiveness of percutaneous ECT on deep tumours and to our knowledge we are the first to evaluate the safety and effectiveness of percutaneous ECT in the treatment of PVTT from HCC.
If this experience will be confirmed, many tumors at hepatic hilum, not susceptible of surgical resection or systemic therapy, could be effectively treated in the future, even percutaneously.
HCC patients with PVTT usually have poor prognosis for absence of effective satisfying treatment measures. This article entitled by Tarantino et al showed that “In patients with cirrhosis, ECT seems effective and safe for curative treatment of Vp3-Vp4 PVTT from HCC”. This paper provides new method of the PVTT treatment in patients with advanced HCC.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Moris D, Ximenes RO, Zheng SJ S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
| 1. | Bosch FX, Ribes J, Cléries R, Díaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005;9:191-211, v. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 605] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 2. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3591] [Article Influence: 276.2] [Reference Citation Analysis (4)] |
| 3. | Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 905] [Article Influence: 34.8] [Reference Citation Analysis (1)] |
| 4. | Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol. 2006;12:7561-7567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 209] [Cited by in RCA: 229] [Article Influence: 12.1] [Reference Citation Analysis (1)] |
| 5. | Schöniger-Hekele M, Müller C, Kutilek M, Oesterreicher C, Ferenci P, Gangl A. Hepatocellular carcinoma in Central Europe: prognostic features and survival. Gut. 2001;48:103-109. [PubMed] |
| 6. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4517] [Article Influence: 347.5] [Reference Citation Analysis (2)] |
| 7. | Katagiri S, Yamamoto M. Multidisciplinary treatments for hepatocellular carcinoma with major portal vein tumor thrombus. Surg Today. 2014;44:219-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Shi J, Lai EC, Li N, Guo WX, Xue J, Lau WY, Wu MC, Cheng SQ. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol. 2010;17:2073-2080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 9. | Chen XP, Qiu FZ, Wu ZD, Zhang ZW, Huang ZY, Chen YF, Zhang BX, He SQ, Zhang WG. Effects of location and extension of portal vein tumor thrombus on long-term outcomes of surgical treatment for hepatocellular carcinoma. Ann Surg Oncol. 2006;13:940-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Chung GE, Lee JH, Kim HY, Hwang SY, Kim JS, Chung JW, Yoon JH, Lee HS, Kim YJ. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology. 2011;258:627-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 250] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 11. | Xue TC, Xie XY, Zhang L, Yin X, Zhang BH, Ren ZG. Transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: a meta-analysis. BMC Gastroenterol. 2013;13:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 12. | Kulik LM, Carr BI, Mulcahy MF, Lewandowski RJ, Atassi B, Ryu RK, Sato KT, Benson A, Nemcek AA, Gates VL. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47:71-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 456] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 13. | Kim DY, Park W, Lim DH, Lee JH, Yoo BC, Paik SW, Kho KC, Kim TH, Ahn YC, Huh SJ. Three-dimensional conformal radiotherapy for portal vein thrombosis of hepatocellular carcinoma. Cancer. 2005;103:2419-2426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Neeman Z, Libutti SK, Patti JW, Wood BJ. Percutaneous radiofrequency ablation of hepatocellular carcinoma in the presence of portal vein thrombosis. Clin Imaging. 2003;27:417-420. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Livraghi T, Grigioni W, Mazziotti A, Sangalli G, Vettori C. Percutaneous alcohol injection of portal thrombosis in hepatocellular carcinoma: a new possible treatment. Tumori. 1990;76:394-397. [PubMed] |
| 16. | Giorgio A, Di Sarno A, de Stefano G, Farella N, Scognamiglio U, de Stefano M, Giorgio V. Hepatocellular carcinoma with cirrhosis: are patients with neoplastic main portal vein invasion eligible for percutaneous radiofrequency ablation of both the nodule and the portal venous tumor thrombus? AJR Am J Roentgenol. 2009;193:948-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Giorgio A, Calisti G, Montesarchio L, Scognamiglio U, Matteucci P, Coppola C, Scarano F, Amendola F, Giorgio V. Hepatocellular carcinoma invading portal venous system in cirrhosis: long term results of percutaneous radiofrequency ablation of both the nodule and portal vein tumor thrombus. A case control study. Anticancer Res. 2014;34:6785-6790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Lu ZH, Shen F, Yan ZL, Li J, Yang JH, Zong M, Shi LH, Wu MC. Treatment of portal vein tumor thrombus of hepatocellular carcinoma with percutaneous laser ablation. J Cancer Res Clin Oncol. 2009;135:783-789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Rhim H, Yoon KH, Lee JM, Cho Y, Cho JS, Kim SH, Lee WJ, Lim HK, Nam GJ, Han SS. Major complications after radio-frequency thermal ablation of hepatic tumors: spectrum of imaging findings. Radiographics. 2003;23:123-134; discussion 134-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 256] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 20. | Liu N, Gao J, Liu Y, Li T, Feng K, Ma K, Dong J, Li X, Wang S, Bie P. Determining a minimal safe distance to prevent thermal injury to intrahepatic bile ducts in radiofrequency ablation of the liver: a study in dogs. Int J Hyperthermia. 2012;28:210-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | McGhana JP, Dodd GD. Radiofrequency ablation of the liver: current status. AJR Am J Roentgenol. 2001;176:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 406] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 22. | Tamai F, Furuse J, Maru Y, Yoshino M. Intrahepatic pseudoaneurysm: a complication following radio-frequency ablation therapy for hepatocellular carcinoma. Eur J Radiol. 2002;44:40-43. [PubMed] |
| 23. | Poggi G, Teragni C, Gazzaruso C, Bernado G. Massive hepatic infarction complicating ultrasound-guided percutaneous radiofrequency thermal ablation. Liver Int. 2004;24:704-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Mir LM, Morsli N, Garbay JR, Billard V, Robert C, Marty M. Electrochemotherapy: a new treatment of solid tumors. J Exp Clin Cancer Res. 2003;22:145-148. [PubMed] |
| 25. | Marty M, Sersa G, Garbay J, Collinsd CG, Snojb M, Billarda V, Geertsenc PF, Larkind JO, Miklavcice D, Pavlovice I. Electrochemotherapy—an easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. EJC. 2006;4:3-13. [RCA] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 548] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 26. | Ricotti F, Giuliodori K, Cataldi I, Campanati A, Ganzetti G, Ricotti G, Offidani A. Electrochemotherapy: an effective local treatment of cutaneous and subcutaneous melanoma metastases. Dermatol Ther. 2014;27:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Curatolo P, Quaglino P, Marenco F, Mancini M, Nardò T, Mortera C, Rotunno R, Calvieri S, Bernengo MG. Electrochemotherapy in the treatment of Kaposi sarcoma cutaneous lesions: a two-center prospective phase II trial. Ann Surg Oncol. 2012;19:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Campana LG, Valpione S, Falci C, Mocellin S, Basso M, Corti L, Balestrieri N, Marchet A, Rossi CR. The activity and safety of electrochemotherapy in persistent chest wall recurrence from breast cancer after mastectomy: a phase-II study. Breast Cancer Res Treat. 2012;134:1169-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 29. | Tafuto S, von Arx C, De Divitiis C, Maura CT, Palaia R, Albino V, Fusco R, Membrini M, Petrillo A, Granata V. Electrochemotherapy as a new approach on pancreatic cancer and on liver metastases. Int J Surg. 2015;21 Suppl 1:S78-S82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Kos B, Voigt P, Miklavcic D, Moche M. Careful treatment planning enables safe ablation of liver tumors adjacent to major blood vessels by percutaneous irreversible electroporation (IRE). Radiol Oncol. 2015;49:234-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 31. | Granata V, Fusco R, Piccirillo M, Palaia R, Petrillo A, Lastoria S, Izzo F. Electrochemotherapy in locally advanced pancreatic cancer: Preliminary results. Int J Surg. 2015;18:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 32. | Mali B, Gorjup V, Edhemovic I, Brecelj E, Cemazar M, Sersa G, Strazisar B, Miklavcic D, Jarm T. Electrochemotherapy of colorectal liver metastases--an observational study of its effects on the electrocardiogram. Biomed Eng Online. 2015;14 Suppl 3:S5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Miklavcic D, Davalos RV. Electrochemotherapy (ECT) and irreversible electroporation (IRE) -advanced techniques for treating deep-seated tumors based on electroporation. Biomed Eng Online. 2015;14 Suppl 3:I1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 34. | Cantisani V, David E, Meloni FM, Dietrich CF, Badea R, Messineo D, D’Ambrosio F, Pisgalia F. Recall strategies for patients found to have a nodule in cirrhosis: is there still a role for CEUS? Med Ultrason. 2015;17:515-520. [PubMed] |
| 35. | Bota S, Piscaglia F, Marinelli S, Pecorelli A, Terzi E, Bolondi L. Comparison of international guidelines for noninvasive diagnosis of hepatocellular carcinoma. Liver Cancer. 2012;1:190-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 36. | Tarantino L, Ambrosino P, Di Minno MN. Contrast-enhanced ultrasound in differentiating malignant from benign portal vein thrombosis in hepatocellular carcinoma. World J Gastroenterol. 2015;21:9457-9460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 37. | Sorrentino P, Tarantino L, D’Angelo S, Terracciano L, Ferbo U, Bracigliano A, Panico L, De Chiara G, Lepore M, De Stefano N. Validation of an extension of the international non-invasive criteria for the diagnosis of hepatocellular carcinoma to the characterization of macroscopic portal vein thrombosis. J Gastroenterol Hepatol. 2011;26:669-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Sorrentino P, D’Angelo S, Tarantino L, Ferbo U, Bracigliano A, Vecchione R. Contrast-enhanced sonography versus biopsy for the differential diagnosis of thrombosis in hepatocellular carcinoma patients. World J Gastroenterol. 2009;15:2245-2251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Tarantino L, Francica G, Sordelli I, Esposito F, Giorgio A, Sorrentino P, de Stefano G, Di Sarno A, Ferraioli G, Sperlongano P. Diagnosis of benign and malignant portal vein thrombosis in cirrhotic patients with hepatocellular carcinoma: color Doppler US, contrast-enhanced US, and fine-needle biopsy. Abdom Imaging. 2006;31:537-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 40. | Pirisi M, Avellini C, Fabris C, Scott C, Bardus P, Soardo G, Beltrami CA, Bartoli E. Portal vein thrombosis in hepatocellular carcinoma: age and sex distribution in an autopsy study. J Cancer Res Clin Oncol. 1998;124:397-400. [PubMed] |
| 41. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10244] [Article Influence: 602.6] [Reference Citation Analysis (2)] |
| 42. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4644] [Article Influence: 273.2] [Reference Citation Analysis (0)] |
| 43. | Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, Galle PR, Santoro A, Beaugrand M, Sangiovanni A. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57:821-829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 664] [Cited by in RCA: 653] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 44. | Jelic S, Sotiropoulos GC. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v59-v64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 45. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6566] [Article Influence: 469.0] [Reference Citation Analysis (1)] |
| 46. | Ogawa T, Kawamoto H, Kobayashi Y, Nakamura S, Miyatake H, Harada R, Tsutsumi K, Fujii M, Kurihara N, Kato H. Prevention of biliary complication in radiofrequency ablation for hepatocellular carcinoma-Cooling effect by endoscopic nasobiliary drainage tube. Eur J Radiol. 2010;73:385-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Mir LM, Orlowski S. Mechanisms of electrochemotherapy. Adv Drug Deliv Rev. 1999;35:107-118. [PubMed] |
| 48. | Gehl J. Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol Scand. 2003;177:437-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 592] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 49. | Appelbaum L, Ben-David E, Faroja M, Nissenbaum Y, Sosna J, Goldberg SN. Irreversible electroporation ablation: creation of large-volume ablation zones in in vivo porcine liver with four-electrode arrays. Radiology. 2014;270:416-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 50. | Thomson KR, Cheung W, Ellis SJ, Federman D, Kavnoudias H, Loader-Oliver D, Roberts S, Evans P, Ball C, Haydon A. Investigation of the safety of irreversible electroporation in humans. J Vasc Interv Radiol. 2011;22:611-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 328] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 51. | Philips P, Hays D, Martin RC. Irreversible electroporation ablation (IRE) of unresectable soft tissue tumors: learning curve evaluation in the first 150 patients treated. PLoS One. 2013;8:e76260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 52. | Deodhar A, Dickfeld T, Single GW, Hamilton WC, Thornton RH, Sofocleous CT, Maybody M, Gónen M, Rubinsky B, Solomon SB. Irreversible electroporation near the heart: ventricular arrhythmias can be prevented with ECG synchronization. AJR Am J Roentgenol. 2011;196:W330-W335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 53. | Dorr RT. Bleomycin pharmacology: mechanism of action and resistance, and clinical pharmacokinetics. Semin Oncol. 1992;19:3-8. [PubMed] |