Published online Feb 7, 2017. doi: 10.3748/wjg.v23.i5.891
Peer-review started: August 15, 2016
First decision: October 28, 2016
Revised: November 11, 2016
Accepted: November 28, 2016
Article in press: November 28, 2016
Published online: February 7, 2017
Processing time: 161 Days and 12.6 Hours
To investigate the efficacy of thrombomodulin (TM)-α for treatment of disseminated intravascular coagulopathy (DIC) in the field of gastrointestinal surgery.
Thirty-six peri-operative DIC patients in the field of gastrointestinal surgery who were treated with TM-α were retrospectively investigated. The relationships between patient demographics and the efficacy of TM-α were examined. Analysis of survival at 28 d was also performed on some parameters by means of the Kaplan-Meier method. Relationships between the initiation of TM-α and patient demographics were also evaluated.
Abscess formation or bacteremia was the most frequent cause of DIC (33%), followed by digestive tract perforation (31%). Twenty-six patients developed DIC after surgery, frequently within 1 wk (81%). TM-α was most often administered within 1 d of the DIC diagnosis (72%) and was continued for more than 3 d (64%). Although bleeding tendency was observed in 7 patients (19%), a hemostatic procedure was not needed. DIC scores, systemic inflammatory response syndrome (SIRS) scores, quick-sequential organ failure assessment (qSOFA) scores, platelet counts, and prothrombin time ratios significantly improved after 1 wk (P < 0.05, for all). The overall survival rate at 28 d was 71%. The duration of TM-α administration (≥ 4 , ≤ 6) and improvements in DIC-associated scores (DIC, SIRS and qSOFA) at 1 wk were significantly better prognostic factors for 28-d survival (P < 0.05, for all). TM-α was administered significantly earlier to patients with severe clinical symptoms, such as high qSOFA scores, sepsis, shock or high lactate values (P < 0.05, for all).
Early administration of TM-α and improvements in each parameter were essential for treatment of DIC. The diagnosis of patients with mild symptoms requires further study.
Core tip: The present study investigated the efficacy of thrombomodulin (TM)-α for treatment of disseminated intravascular coagulopathy (DIC) in the field of gastrointestinal surgery. DIC frequently developed within 1 wk of surgery. TM-α was frequently administered within 1 d of the DIC diagnosis and was continued for more than 3 d. The duration of TM-α administration and improvements in DIC-associated parameters at 1 wk were better prognostic factors for 28-d survival. TM-α was administered significantly earlier to patients with severe clinical symptoms. The early administration of TM-α and improvements in DIC parameters were essential for the treatment of DIC.
- Citation: Konishi H, Okamoto K, Shoda K, Arita T, Kosuga T, Morimura R, Komatsu S, Murayama Y, Shiozaki A, Kuriu Y, Ikoma H, Nakanishi M, Ichikawa D, Fujiwara H, Otsuji E. Early thrombomodulin-α administration outcome for acute disseminated intravascular coagulopathy in gastrointestinal surgery. World J Gastroenterol 2017; 23(5): 891-898
- URL: https://www.wjgnet.com/1007-9327/full/v23/i5/891.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i5.891
Disseminated intravascular coagulopathy (DIC) is characterized by the systemic activation of blood coagulation and continuous generation of intravascular fibrin, which contribute to multiple organ dysfunction syndrome or other life-threating conditions[1,2]. In the field of gastrointestinal surgery, DIC has been reported as a consequence of emergency surgery, severe complications or some types of malignancies[3-5], and as a frequent complicating factor for conditions leading to sepsis or a shock status. The acute DIC scoring system put forth by the Japanese Association for Acute Medicine (JAAM) is in widespread use for diagnosis of acute DIC[6,7]. In order to effectively treat DIC, the underlying causes need to be improved and appropriate drugs need to be administered intensively in the earliest stage possible. However, survival rates are never high, as has been previously reported[1,2].
In DIC, the activation of coagulation and inhibition of fibrinolysis lead to a hypercoagulable state and the deposition of fibrin in micro-vessels. Thrombomodulin-α (TM-α) is a recombinant human soluble thrombomodulin, which is a thrombin receptor on endothelial cell surfaces[8-10]. Thrombin binds to TM-α, and the thrombin-TM-α complex inactivates intravascular coagulation by activating the protein C pathway. The subsequent formation of thrombin and triggering of inflammatory reactions are regulated by TM-α, after which hypercoagulable DIC states become improved. Therefore, TM-α was approved as a curative medicine for the treatment of DIC in 2008, and its effects on DIC have been investigated in multicenter randomized clinical trials in Japan[8,11]. Resolution rates for DIC and bleeding symptoms were found to be significantly better for patients treated with TM-α than those treated with heparin[12,13].
Previous studies have determined the efficacy of TM-α treatments for DIC associated with gastroenterological surgery[4,5]; however, the data are still insufficient to establish the optimal therapeutic strategies for hematological malignancies or infections such as sepsis. The optimal initiation time or duration of the administration of TM-α and the predictive factors for therapeutic efficacy remain unclear for actual clinical practice. In the present study, the treatment of DIC by TM-α in the field of gastrointestinal surgery was retrospectively summarized, and outcomes were investigated.
Thirty-six patients were retrospectively investigated. Each had been diagnosed with DIC in the peri-operative period and treated with TM-α between January 2012 and December 2015 at the Division of Digestive Surgery in Kyoto Prefectural University of Medicine (Japan). The JAAM DIC scoring system was applied as the diagnostic criteria for DIC (DIC score ≥ 4)[6,7]. The baseline demographics and characteristics of patients are summarized in Tables 1 and 2. Some types of digestive cancers were the cause of DIC in 13 patients (cancer-associated).
| Subjects | n = 36 |
| Sex, F/M | 12/24 |
| Age, median (range) | 71 (48-86) |
| Underlying disease | |
| Perforation | |
| Gastric | 1 |
| Small intestine | 2 |
| Colo/rectal | 8 |
| Abscess/bacteremia | 12 |
| Ileus | 3 |
| Pancreatitis | 2 |
| Pneumonia | 5 |
| Drug-induced | 3 |
| Peri-operative, no/yes | 10/26 |
| Cancer-associated, no/yes | 23/13 |
| Post-operative day, ≤ 7/> 7 | 21/5 |
| Combination treatment for DIC | |
| Unfractionated heparins | 4 |
| Anti-thrombin concentrates | 28 |
| γ-globulin agents | 29 |
| Vasopressors | 26 |
| Protease inhibitors | 4 |
| Sivelestat sodium hydrates | 4 |
| Steroid preparations | 7 |
| Dialysis | 5 |
| Blood transfusion | 4 |
| Subjects | n = 36 | |
| DIC scores | 4 | 15 |
| Before the treatment | 5 | 9 |
| (JAAM criteria) | 6 | 8 |
| 7 | 1 | |
| 8 | 3 | |
| SIRS scores | 0/1 | 5 |
| Before the treatment | 2 | 9 |
| 3 | 15 | |
| 4 | 7 | |
| qSOFA scores | 0 | 10 |
| Before the treatment | 1 | 4 |
| 2 | 17 | |
| 3 | 5 | |
| Duration of DIC | -2/-1 | 4 |
| Before the administration of TM-α (d) | 0 | 16 |
| 1 | 10 | |
| 2 | 1 | |
| 3 | 4 | |
| ≥ 4 | 1 | |
| Duration of administration | 1 | 5 |
| 2 | 5 | |
| 3 | 3 | |
| 4 | 3 | |
| 5 | 9 | |
| 6 | 4 | |
| ≥ 7 | 7 | |
TM-α (Recomodulin® injection; Asahi Kasei Pharma Corporation, Tokyo, Japan) was administered intravenously at a dose of 380 U/kg per day and continued as necessary[8-10]. This dose was decreased to 130 U/kg in a patient with severe renal failure, according to attending physicians’ decision and the manufacturer’s instructions. Particularly, the patients who needed dialysis or were considered to have increased creatinine and decreased eGFR were given a reduced dose of TM-α. The duration of the administration of TM-α, and its combined usage with other treatment drugs, were also decided by the attending physicians. Clinicopathological and laboratory data obtained at 1 wk and 2 wk after the initiation of TM-α administration were investigated, and the mortality rate at 28 d was determined.
This study was conducted in accordance with the principles of the Declaration of Helsinki, and written informed consent for the treatment and data collection was obtained from all patients. We did not seek individual ethical approval by the Facility of Science Committee at Kyoto Prefectural University of Medicine because this was a retrospective observational study without interpositions and with the medical practice necessary for therapeutic purposes.
In the present study, the following criteria were employed to diagnose DIC and compare treatment efficacies. Systemic inflammatory response syndrome (SIRS) scores were evaluated according to a previous study[14] and a SIRS score of ≥ 3 was converted to 1 point for the JAAM DIC score[7]. Quick sequential organ failure assessment (qSOFA) scores were determined by more than 1 point of altered mentation, systolic blood pressure of ≤ 100 mmHg, and respiratory rate of ≥ 22/min[15]. With respect to updates to the definitions for sepsis and septic shock criteria, sepsis was determined by more than 1 point of the qSOFA score in the present study[15], while traditional sepsis was defined by the existence of infection and SIRS. Shock was defined by a serum lactate level of > 2 mmol/L and a requirement for vasopressors to maintain mean arterial pressure despite adequate fluid resuscitation[15].
Statistical analyses were performed using the JMP 12 software program. The Wilcoxon signed-rank test was used to analyze the relationships between various biochemical measurements. A survival curve for overall survival was derived using the Kaplan-Meier method and compared by the stratified log-rank test. A P value less than 0.05 was considered significant.
Clinical data of the 36 patients in this study are summarized in Tables 1 and 2. DIC was caused by a wide variety of diseases, with abscess formation or bacteremia after surgery being the most frequent cause (12/36, 33%), followed by perforation of the digestive tract (11/36, 31%). Twenty-six patients (72%) developed DIC after surgery, frequently within 1 wk of surgery (21/26, 81%). TM-α was frequently used in conjunction with other drugs and treatments, such as combined administration with anti-thrombin concentrates, γ-globulin agents, and vasopressors. Unfractionated heparins were administered to 4 patients (11%) as an alternative to TM-α.
A number of patients were diagnosed as having DIC with JAAM score of 4 or 5 (24/36, 67%). At the time of the DIC diagnosis, 5 (14%) and 14 (39%) patients did not fulfill the criteria of SIRS (≥ 2) and qSOFA (≥ 2), respectively. For most patients, TM-α was administered within 1 d of the DIC diagnosis (26/36, 72%) and was continued for more than 3 d (23/36, 64%). However, 5 patients (14%) were administered TM-α for only 1 d; the reasons for the discontinuation of its administration are listed in Table 3. Although bleeding tendency was observed in 7 patients (19%), severe bleeding was not observed and a hemostatic procedure was not required.
| Duration (d) | Total number | Cases | Reasons |
| 1 | 5 | 2 | Dialysis |
| 3 | Bleeding tendency | ||
| 2 | 5 | 3 | Death |
| 2 | Bleeding tendency | ||
| 3 | 3 | 1 | Resolved |
| 2 | Bleeding tendency | ||
| 4 | 3 | 3 | Resolved |
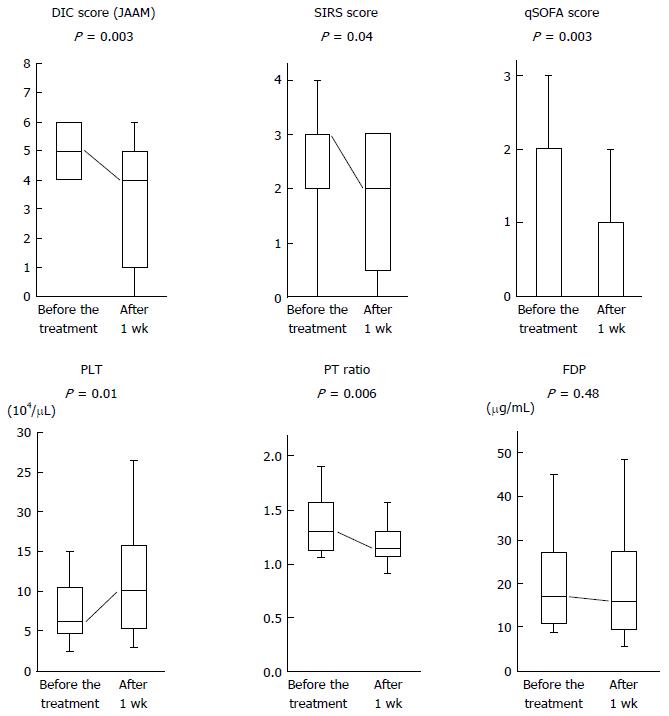
Figure 1 shows alterations in each DIC-associated parameter between before and after 1 wk of the treatment in patients administered TM-α for more than 1 d. DIC scores (P = 0.003), SIRS scores (P = 0.04), qSOFA scores (P = 0.003), platelet counts (P = 0.01) and prothrombin time ratios (P = 0.006) were significantly improved after 1 wk of the treatment. C-reactive protein and creatinine values were also improved (data not shown).
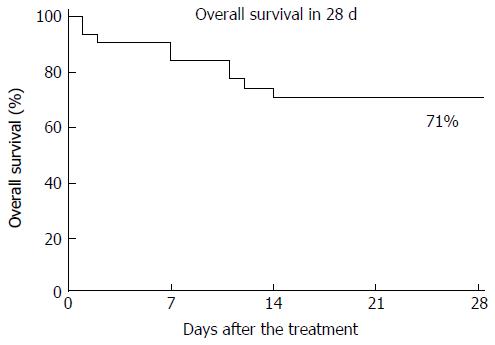
The overall survival at 28 d for all patients administered TM-α for more than 1 d is shown in Figure 2, and the overall survival rate was 71%. A survival analysis on some parameters is shown in Table 4. The duration of administration (≥ 4, ≤ 6; P = 0.03) and improvements in DIC scores (P = 0.01), SIRS scores (P = 0.09) and qSOFA scores (P = 0.001) at 1 wk were significant prognostic factors for 28-d survival.
| Factor | n = 31 | 28-d survival rate | P value | |
| Sex | Male | 22 | 73% | 0.83 |
| Female | 9 | 67% | ||
| Age | ≤ 70 | 14 | 64% | 0.54 |
| > 70 | 17 | 76% | ||
| Duration of administration | ≥ 4, ≤ 6 | 16 | 88% | 0.03 |
| ≤ 3, ≥ 7 | 15 | 53% | ||
| Initiation of administration after DIC (d) | ≤ 1 | 27 | 74% | 0.43 |
| ≥ 2 | 4 | 50% | ||
| DIC scores before the treatment | ≤ 5 | 21 | 67% | 0.52 |
| ≥ 6 | 10 | 80% | ||
| Improvement in DIC scores at 1 wk | ≤ 3 | 14 | 93% | 0.01 |
| ≥ 4 | 17 | 53% | ||
| SIRS scores before the treatment | ≤ 2 | 12 | 58% | 0.2 |
| ≥ 3 | 19 | 79% | ||
| Improvement in SIRS scores at 1 wk | ≤ 2 | 21 | 86% | 0.09 |
| ≥ 3 | 7 | 57% | ||
| qSOFA scores before the treatment | ≤ 1 | 11 | 73% | 0.8 |
| ≥ 2 | 20 | 70% | ||
| Improvement in qSOFA scores at 1 wk | ≤ 1 | 22 | 91% | 0.001 |
| ≥ 2 | 6 | 33% | ||
| Sepsis | Present | 20 | 70% | 0.8 |
| Absent | 11 | 73% | ||
| Shock | Present | 16 | 81% | 0.24 |
| Absent | 15 | 60% | ||
| Lactate values before the treatment | ≥ 2 | 8 | 75% | 0.69 |
| < 2 | 18 | 83% |
In the survival analysis, patients administered TM-α within 1 d of the DIC diagnosis had slightly better prognoses than those administered it after 2 d (74% vs 50%; Table 4). TM-α was administered significantly earlier for patients with severe clinical symptoms at the time of DIC diagnosis, such as high qSOFA scores (P = 0.001), sepsis (P = 0.001), shock (P = 0.02) or high lactate values (P = 0.02) (Table 5).
| Factor | Treatment initiation after DIC | P value | ||
| ≤ 0 d | ≥ 1 d | |||
| Duration of administration | ≥ 4, ≤ 6 | 9 | 7 | 0.94 |
| ≤ 3, ≥ 7 | 11 | 9 | ||
| DIC scores before the treatment | ≤ 5 | 12 | 12 | 0.34 |
| ≥ 6 | 8 | 4 | ||
| SIRS scores before the treatment | ≤ 2 | 6 | 8 | 0.22 |
| ≥ 3 | 14 | 8 | ||
| qSOFA scores before the treatment | ≤ 1 | 3 | 11 | 0.001 |
| ≥ 2 | 17 | 5 | ||
| Sepsis | Present | 17 | 5 | 0.001 |
| Absent | 3 | 11 | ||
| Shock | Present | 13 | 4 | 0.02 |
| Absent | 7 | 12 | ||
| Lactate values before the treatment | ≤ 3 | 6 | 10 | 0.02 |
| > 3 | 11 | 3 | ||
DIC is not prevalent in the field of gastrointestinal surgery, but it is life-threating once it develops[4]. Early intensive care, including the administration of anti-thrombin concentrates and γ-globulin agents, has been shown to effectively improve the prognosis of patients with DIC[13,16]. The early administration of TM-α has also been reported to improve severe DIC and prognoses in the field of gastrointestinal surgery[3-6].
In the present study, peri-operative DIC patients in the field of gastrointestinal surgery who were treated with TM-α were retrospectively investigated. Some DIC-associated parameters, such as DIC (JAAM), SIRS and qSOFA scores, were significantly improved at 1 wk after the initiation of the TM-α treatment, and these improvements correlated with the better 28-d survival rate. On the other hand, in spite of the diagnosis of DIC, the administration of TM-α was significantly delayed in patients with mild symptoms, such as low SIRS or qSOFA scores, and the absence of sepsis or shock.
In order to achieve an early and accurate diagnosis of DIC, not only the counts of each parameter but also the changes in platelet counts are important. In the present study, the DIC scores of 4 patients were decided by changes in platelet counts measured in a 24-h period (data not shown). DIC scores were found to increase due to decrease in platelet counts of > 30% or 50% in a 24-h period, and further reductions of platelet counts were observed on the next day. Therefore, the rate of decreases in platelet counts is also important for reaching an early decision on the DIC score.
In the survival analysis, the overall survival rate at 28 d (71%) was similar to previous findings[4]. On the other hand, the survival rate of patients administered TM-α at 2 d after the DIC diagnosis was slightly worse (74% vs 50%). Early treatments, including TM-α, are generally considered to be advantageous for improving the prognosis of DIC patients[5,6,10]. In the present study, the administration of TM-α was significantly delayed (by more than 1 d after the DIC diagnosis) in patients who were less symptomatic (i.e., not meeting the criteria of qSOFA, sepsis or shock and having low lactate values). Therefore, early and accurate diagnosis of DIC and initiation of treatments will be needed for all patients with mild symptoms who are suspected of having DIC.
The definitions of sepsis and shock were recently revised[15]. Previously, traditional sepsis had been defined as the presence of infection and SIRS, but it is now defined by an increase in the SOFA score (≥ 2). Moreover, shock is defined by a requirement for vasopressors and enhanced serum lactate levels (> 2 mmol/L). In the present study, we used qSOFA scores exclusively because we were unable to confirm all data to provide an accurate SOFA score.
The indication of TM-α administration is decided by the JAAM DIC score (≥ 4) only, and these DIC patients frequently present with accompanying severe complications such as shock or sepsis[2,13-16]. It remains controversial whether these severe conditions can influence the therapeutic effects of DIC. In the present study, the parameters showing severe conditions were also investigated, but were found to not significantly affect the efficacy of TM-α or prognosis of patients.
The present study had some limitations. The number of patients examined was small because DIC is not prevalent in the field of gastrointestinal surgery. Furthermore, DIC scores were retrospectively evaluated by only the JAAM acute DIC scoring system, and we did not confirm that the attending physicians gave accurate DIC scores at diagnosis. In the future, the comparisons with other criteria, such as the International Society for Thrombosis and Haemostasis DIC score, will be needed. In previous studies, DIC patients treated with TM-α for approximately 6 d have been commonly evaluated[4,10], while patients in the present study were treated for shorter or longer durations. Some of our patients who were administered TM-α for a shorter duration showed amelioration of the DIC, whereas many patients with a shorter or longer duration of administration had worse prognoses. A 6-d administration is needed if patient conditions permit it, and the advantages and disadvantages of the early discontinuation of administration due to improvements in DIC require further investigations. Another limitation is that all patients in this study were treated with TM-α and other drugs, and comparisons of the efficacy of the treatments, prognosis of patients or development of side effects between TM-α and the other drugs were not performed.
In conclusion, although the number of patients examined in the present study was small, we herein demonstrated that the early diagnosis of DIC and initiation of the TM-α administration are effective for achieving improvements in DIC in the field of gastrointestinal surgery. The diagnosis of patients with mild symptoms requires further study.
Disseminated intravascular coagulopathy (DIC) has been reported in the field of gastrointestinal surgery as a consequence of emergency surgery, severe complications or some types of malignancies, and has been shown to frequently complicate conditions leading to sepsis or a shock status. In order to effectively treat DIC, the underlying causes need to be resolved, or at least improved, and appropriate drugs need to be administered intensively at earlier stage. However, survival rates are never high, as has been reported consistently.
Thrombomodulin-α (TM-α) was approved as a curative medicine for the treatment of DIC, and previous studies have reported its efficacy for DIC associated with gastrointestinal surgery. However, the data are still insufficient to establish optimal therapeutic strategies. The research hotspot is the introduction of an optimized treatment with TM-α and identification and clinical application of predictive factors to improve therapeutic efficacy of DIC in the field of gastrointestinal surgery.
DIC is not prevalent in the field of gastrointestinal surgery, but is life-threating once it develops. In the present study, some DIC-associated parameters, such as DIC (Japanese Association for Acute Medicine), systemic inflammatory response syndrome (SIRS) and quick-sequential organ failure assessment (qSOFA) scores, were significantly improved at 1 wk after the initiation of TM-α treatment, and these improvements correlated with better 28-d survival. Early diagnosis of DIC and initiation of the TM-α administration are effective for achieving improvements in DIC in the field of gastrointestinal surgery. On the other hand, in spite of DIC diagnosis, administration of TM-α was significantly delayed in patients with mild symptoms, such as low SIRS or qSOFA scores, and the absence of sepsis or shock.
The data in this study suggested that early diagnosis of DIC and initiation of the TM-α administration are clinically effective for DIC treatment in the field of gastrointestinal surgery. Furthermore, this study also provided readers with important information regarding the delay of TM-α administration for less symptomatic DIC patients.
DIC leads to a hypercoagulable state and the deposition of fibrin in micro-vessels. TM-α is a recombinant human soluble thrombomodulin, which is a thrombin receptor on endothelial cell surfaces. Thrombin binds to TM-α, and the thrombin-TM-α complex inactivates intravascular coagulation by activating the protein C pathway. Therefore, the further formation of thrombin and triggering of inflammatory reactions are regulated by TM-α, and hypercoagulable DIC states are improved.
This manuscript reports on the early diagnosis and earlier initiation of recombinant thrombomodulin for DIC patients in the field of digestive surgery. Identification of an optimized treatment with TM-α and clinical application of predictive factors will be very useful for DIC treatment, improving the therapeutic efficacy.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Fareed J, Garcia-Olmo D S- Editor: Yu J L- Editor: A E- Editor: Zhang FF
| 1. | Levi M. Disseminated intravascular coagulation. Crit Care Med. 2007;35:2191-2195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 2. | Okamoto K, Tamura T, Sawatsubashi Y. Sepsis and disseminated intravascular coagulation. J Intensive Care. 2016;4:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 3. | Tamura K, Saito H, Asakura H, Okamoto K, Tagawa J, Hayakawa T, Aoki N. Recombinant human soluble thrombomodulin (thrombomodulin alfa) to treat disseminated intravascular coagulation in solid tumors: results of a one-arm prospective trial. Int J Clin Oncol. 2015;20:821-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Hashimoto D, Chikamoto A, Miyanari N, Ohara C, Kuramoto M, Horino K, Ohshima H, Baba H. Recombinant soluble thrombomodulin for postoperative disseminated intravascular coagulation. J Surg Res. 2015;197:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Ito T, Nagahara A, Osada T, Kato J, Ueyama H, Saito H, Taniki N, Kanazawa R, Shimizu R, Sai J. Efficacy of recombinant human soluble thrombomodulin in patients with sepsis and disseminated intravascular coagulation in the gastroenterology field. Biomed Rep. 2015;3:457-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Mimuro J, Takahashi H, Kitajima I, Tsuji H, Eguchi Y, Matsushita T, Kuroda T, Sakata Y. Impact of recombinant soluble thrombomodulin (thrombomodulin alfa) on disseminated intravascular coagulation. Thromb Res. 2013;131:436-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Gando S, Saitoh D, Ogura H, Fujishima S, Mayumi T, Araki T, Ikeda H, Kotani J, Kushimoto S, Miki Y. A multicenter, prospective validation study of the Japanese Association for Acute Medicine disseminated intravascular coagulation scoring system in patients with severe sepsis. Crit Care. 2013;17:R111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 8. | Saito H, Maruyama I, Shimazaki S, Yamamoto Y, Aikawa N, Ohno R, Hirayama A, Matsuda T, Asakura H, Nakashima M. Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: results of a phase III, randomized, double-blind clinical trial. J Thromb Haemost. 2007;5:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 434] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 9. | Tsuruta K, Yamada Y, Serada M, Tanigawara Y. Model-based analysis of covariate effects on population pharmacokinetics of thrombomodulin alfa in patients with disseminated intravascular coagulation and normal subjects. J Clin Pharmacol. 2011;51:1276-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Eguchi Y, Gando S, Ishikura H, Saitoh D, Mimuro J, Takahashi H, Kitajima I, Tsuji H, Matsushita T, Tsujita R. Post-marketing surveillance data of thrombomodulin alfa: sub-analysis in patients with sepsis-induced disseminated intravascular coagulation. J Intensive Care. 2014;2:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Aikawa N, Shimazaki S, Yamamoto Y, Saito H, Maruyama I, Ohno R, Hirayama A, Aoki Y, Aoki N. Thrombomodulin alfa in the treatment of infectious patients complicated by disseminated intravascular coagulation: subanalysis from the phase 3 trial. Shock. 2011;35:349-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 12. | Yamakawa K, Ogura H, Fujimi S, Morikawa M, Ogawa Y, Mohri T, Nakamori Y, Inoue Y, Kuwagata Y, Tanaka H. Recombinant human soluble thrombomodulin in sepsis-induced disseminated intravascular coagulation: a multicenter propensity score analysis. Intensive Care Med. 2013;39:644-652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Iba T, Thachil J. Present and future of anticoagulant therapy using antithrombin and thrombomodulin for sepsis-associated disseminated intravascular coagulation: a perspective from Japan. Int J Hematol. 2016;103:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864-874. [PubMed] |
| 15. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15803] [Cited by in RCA: 17171] [Article Influence: 1907.9] [Reference Citation Analysis (2)] |
| 16. | Yoshimura J, Yamakawa K, Ogura H, Umemura Y, Takahashi H, Morikawa M, Inoue Y, Fujimi S, Tanaka H, Hamasaki T. Benefit profile of recombinant human soluble thrombomodulin in sepsis-induced disseminated intravascular coagulation: a multicenter propensity score analysis. Crit Care. 2015;19:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |