Published online Nov 21, 2017. doi: 10.3748/wjg.v23.i43.7678
Peer-review started: June 30, 2017
First decision: July 25, 2017
Revised: August 15, 2017
Accepted: September 5, 2017
Article in press: September 5, 2017
Published online: November 21, 2017
Processing time: 143 Days and 12.2 Hours
To investigate the effect of resveratrol on biliary secretion of cholephilic compounds in healthy and bile duct-obstructed rats.
Resveratrol (RSV) or saline were administered to rats by daily oral gavage for 28 d after sham operation or reversible bile duct obstruction (BDO). Bile was collected 24 h after the last gavage during an intravenous bolus dose of the Mdr1/Mrp2 substrate azithromycin. Bile acids, glutathione and azithromycin were measured in bile to quantify their level of biliary secretion. Liver expression of enzymes and transporters relevant for bile production and biliary secretion of major bile constituents and drugs were analyzed at the mRNA and protein levels using qRT-PCR and Western blot analysis, respectively. The TR-FRET PXR Competitive Binding Assay kit was used to determine the agonism of RSV at the pregnane X receptor.
RSV increased bile flow in sham-operated rats due to increased biliary secretion of bile acids (BA) and glutathione. This effect was accompanied by the induction of the hepatic rate-limiting transporters for bile acids and glutathione, Bsep and Mrp2, respectively. RSV also induced Cyp7a1, an enzyme that is crucial for bile acid synthesis; Mrp4, a transporter important for BA secretion from hepatocytes to blood; and Mdr1, the major apical transporter for xenobiotics. The findings were supported by increased biliary secretion of azithromycin. The TR-FRET PXR competitive binding assay confirmed RSV as a weak agonist of the human nuclear receptor PXR, which is a transcriptional regulator of Mdr1/Mrp2. RSV demonstrated significant hepatoprotective properties against BDO-induced cirrhosis. RSV also reduced bile flow in BDO rats without any corresponding change in the levels of the transporters and enzymes involved in RSV-mediated hepatoprotection.
Resveratrol administration for 28 d has a distinct effect on bile flow and biliary secretion of cholephilic compounds in healthy and bile duct-obstructed rats.
Core tip: For the first time, our results provide information about the ability of resveratrol to increase bile flow in healthy rats by increasing the biliary excretion of bile acids and glutathione via posttranscriptional induction of their rate-limiting transporters, Bsep and Mrp2, respectively, and via the up-regulation of Cyp7a1, an enzyme that is crucial for bile acid synthesis. Resveratrol simultaneously induced hepatic expression of Mdr1, which was verified by increased biliary excretion of its substrate, azithromycin. Our findings were consistent with an agonistic effect of resveratrol on PXR. Our data therefore imply that oral administration of resveratrol may modify the kinetics of endo- and xenobiotics.
- Citation: Dolezelova E, Prasnicka A, Cermanova J, Carazo A, Hyrsova L, Hroch M, Mokry J, Adamcova M, Mrkvicova A, Pavek P, Micuda S. Resveratrol modifies biliary secretion of cholephilic compounds in sham-operated and cholestatic rats. World J Gastroenterol 2017; 23(43): 7678-7692
- URL: https://www.wjgnet.com/1007-9327/full/v23/i43/7678.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i43.7678
Bile is the major excretory route for potentially harmful endogenous substrates, such as bilirubin and bile acids (BA), as well as for numerous exogenous lipophilic compounds. The secretion of these substrates into bile is mediated by an interacting network of transporter proteins at the basolateral and apical membranes of hepatocytes. The apical Bsep (Bile salt export pump) and Mrp2 (Multidrug resistance-associated protein 2) are the key rate-limiting transporters for biliary secretion of BA and glutathione that create an osmotic driving force that attracts water to biliary lumen and ensures bile flow. The potential toxicity of BA concentration in the biliary lumen is prevented by the formation of micelles with phospholipids and cholesterol, which are then secreted into the bile by apical Mdr2 (Multidrug resistance protein 2) and Abcg5/g8 (ATP-binding cassette sub-family g5/g8) proteins, respectively[1-3]. Additionally, the apical membrane transporters Mdr1 and Bcrp mainly mediate the excretion of xenobiotics. All these pathways are sensitively regulated and modified by numerous stimuli, including diseases, drugs and food ingredients[4], which may either directly affect the activity of individual transporters or indirectly act by changing their expression. Especially in the second mechanism, transcriptional regulation of the nuclear farnesoid X receptor (FXR - bile acid sensor) and pregnane X receptor (PXR - xenobiotics sensor) plays a key role in modulating transporter activity. Thus, such events may not only significantly alter bile production but also the kinetics of other substrates, including drugs.
Resveratrol (trans-3,4´,5-trihydroxystilbene) is a natural polyphenol found in grape skin, red wine, peanuts, and now, in food supplements. Numerous reports have documented that this agent mitigates the progression of a wide variety of illnesses, such as malignances, cardiovascular diseases and various ischemic, toxic and inflammatory tissue injuries[5-9]. Further, resveratrol (RSV) showed marked hepatoprotective potential in different situations, such as non-alcoholic fatty liver disease, extrahepatic cholestasis, and α-naphthylisothiocyanate-, acetaminophen-, or carbon tetrachloride-induced hepatotoxicity[10-12]. The majority of these benefits are related to the significant anti-inflammatory and antioxidant properties of resveratrol, as well as to the reduction of hepatic lipid accumulation[10,13]. The molecular mechanism behind these effects originates from the stimulation of the adenosine monophosphate-activated protein kinase/Sirtuin 1 (AMPK-SIRT1) pathway in association with the inhibition of NF-κB-mediated proinflammatory cytokine production, the suppression of p53 with reduction of apoptosis, and the induction of autophagy[11,14,15].
Recent results have also suggested that resveratrol may prevent the impairment of bile formation during α-naphthylisothiocyanate (ANIT)-induced intrahepatic cholestasis[16]. Herein, intraperitoneal administration of RSV (15 and 30 mg/kg BW) to ANIT-induced rats partially recovered bile flow (BF). In accordance, RSV administration to ANIT-induced rats attenuated the impaired biliary excretion (BE) of both BA and glutathione. The authors analyzed the expression of four transporter proteins and attributed this effect to the preserved expression of the Mrp2 transporter as a part of the anti-inflammatory activity of RSV in association with the reduction of BA concentrations in serum[16]. In this study[16], compared to the commonly used oral administration, intraperitoneal administration of RSV overcame its low bioavailability and markedly increased its exposure to the organism. Moreover, the effect of RSV on BF has not been determined in healthy rats or in the general population without cholestatic liver impairment, despite its wide use as a food ingredient.
The aim of the present study was therefore to evaluate the influence of orally administered RSV on multiple mechanisms involved in bile production as well as the biliary secretion of cholephilic compounds in rats with intact liver. The influence of RSV on bile flow and the associated mechanisms was also studied in rats with reversible long-term obstructive cholestasis associated with initiated liver fibrosis.
Trans-resveratrol was purchased from Sigma-Aldrich (St. Louis, MO, United States). Azithromycin was used in its original formulation for parenteral administration - Sumamed (Pliva-Lachema a.s., Brno, Czech Republic). All other reagents and supplies were obtained from Sigma (St. Louis, MO, United States) and Bio-Rad Laboratories (Hercules, CA, United States), respectively, and were of the highest available purity.
Male Wistar rats (n = 6 in each group) weighing 280 to 320 g (Konarovice, Czech Republic) were used throughout the study. The animals received human care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” published by the United States National Institutes of Health (NIH publication, 1996). The study protocol (reg. No. 3163/2008-30) was approved by the animal welfare committee of Charles University, Faculty of Medicine in Hradec Kralove. The rats were randomly divided into four groups - two sham-operated (Sham) and two bile duct-obstructed (BDO) groups. Surgery was performed under short pentobarbital anesthesia [50 mg/kg body weight (BW)]. Briefly, sham-operated animals underwent incision of the abdominal wall, manipulation of the bile duct without obstruction and two-layer suturing of the incision. BDO groups underwent bile duct cannulation, where the free end of the cannula was ligated and stitched to the abdominal wall just under the skin. Animals recovered from anesthesia and then received the first dose of either saline (Sham or BDO groups; 0.3 mL/kg BW) or resveratrol (Sham-R and BDO-R groups; 10 mg/kg BW in saline) two hours after surgery by gastric gavage. Next, animals received treatment once a day by gastric gavage for 28 consecutive days in order to initiate biliary cirrhosis in the BDO groups. All animals underwent a clearance study (see below) 24 h after the last gavage of saline or RSV to evaluate bile flow and biliary secretion of cholephilic substrates (bile acids, glutathione, and azithromycin).
The dose of 10 mg/kg resveratrol p.o. was selected on the basis of the minimal effective dose in similar studies in animals[12,17] and according to the recommended dose for resveratrol supplementation in humans (250-2000 mg/d). The dose was tested in a preliminary study.
Under anesthesia induced by pentobarbital (50 mg/kg), the bile duct was either cannulated (sham-operated animals) or its obstruction was released (BDO animals) by cutting the free tip of the biliary cannula. In addition, all rats were cannulated with a polyethylene tube in the right jugular vein for drug administration and continuous infusion of physiological saline (2 mL/h, to replace fluid loss) and the left carotid artery (for blood sampling). Thereafter, the rats received a single intravenous bolus of azithromycin (20 mg/kg), a substrate for Mdr1/Mrp2 transporters. Bile samples were collected in pre-weighed tubes at 30-min intervals for 120 min. The body temperature of the animals was maintained at 37 °C by keeping the animals on a heated platform. At the end of the experiment, all rats were sacrificed by exsanguination from the aorta, and the livers were removed and immediately frozen in liquid nitrogen. Plasma samples were obtained from whole blood by centrifugation at 2000 × g for 5 min at 4 °C. Samples were stored at -80 °C until analysis. The median lobe of the liver from each animal was used for histological and molecular analyses.
Bilirubin concentrations in the plasma and ALT, as well as AST activities in the plasma, were measured by routine laboratory methods on a Cobas Integra ® 800 (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instructions. Bile acids in the plasma and bile were assayed using a commercial kit (Diazyme, CA, United States). Concentrations of reduced (GSH) and oxidized (GSSG) glutathione were analyzed separately using an HPLC method on a Shimadzu system with fluorescence detection[18]. The total glutathione amount in the bile was calculated as the sum of the reduced and oxidized forms of glutathione. The concentration of azithromycin in bile was determined by a previously described HPLC method[19].
Gene expression was examined as previously described[18]. The total RNA from the liver tissue was isolated using TRI reagent (Sigma-Aldrich, St. Louis, MO, United States). TaqMan Fast Universal PCR Master Mix and predesigned TaqMan Gene Expression Assay kits (Table 1) were obtained from Life Technologies (Prague, Czech Republic). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a reference for normalizing the data.
| Gene symbol | Transporter/Receptor | Life technologies cat. number |
| Abcc2 | Mrp2 | Rn00563231_m1 |
| Abcb11 | Bsep | Rn00582179_m1 |
| Abcg2 | Bcrp | Rn00710585_m1 |
| Abcb1a | Mdr1a | Rn00591394_ml |
| Abcb1b | Mdr1b | Rn00561753_ml |
| Abcb4 | Mdr2 | Rn00562185_ml |
| Abcc3 | Mrp3 | Rn01452854_m1 |
| Abcc4 | Mrp4 | Rn01465702_m1 |
| Slc10a1 | Ntcp | Rn00566894_ml |
| Slco1a1 | Oatp1a1 | Rn00755148_m1 |
| Slco1a4 | Oatp1a4 | Rn00756233_ml |
| Slc22a7 | Oat2 | Rn00585513_m1 |
| Slc10a2 | Asbt | Rn00691576_m1 |
| Cyp7a1 | Rn00564065_m1 | |
| Cyp8b1 | Rn00579921_m1 | |
| NrOb2 | Shp | Rn00589173_m1 |
| TNF-α | Rn99999017_m1 | |
| IL-6 | Rn99999011_ml | |
| Acta2 | Rn01759928_g1 | |
| TGF-β1 | Rn00572010_m1 | |
| Col1a1 | Rn01463848_m1 | |
| Pdgfrβ | Rn00709573_m1 | |
| Timp-2 | Rn00573232_m1 | |
| Nqo1 | Rn00566528_m1 | |
| GAPDH | 4352338E |
Crude plasma membranes were prepared from rat liver homogenates as previously described[18]. Proteins (100 mg) were separated by SDS-PAGE, then transferred to a PVDF membrane (Millipore, NY, United States) and incubated with the appropriate antibodies (Table 2). The immunoreactive bands on the autoradiography films were quantified using Quantity One imaging software (Bio-Rad Laboratories, Hercules, CA). The equal loading of proteins onto the gel was confirmed by the immunodetection of β-actin.
| Protein | Source | Dilution | Secondary antibody dilution |
| Mrp2 | Signet Laboratories | 1:500 | 1:1000 |
| Bsep | Santa Cruz | 1:300 | 1:2000 |
| Bcrp | Signet Laboratories | 1:500 | 1:500 |
| P-gp | Signet Laboratories | 1:500 | 1:1000 |
| Mdr2 | Abcam | 1:500 | 1:500 |
| Mrp3 | Alexis | 1:500 | 1:500 |
| Mrp4 | Abcam | 1:1000 | 1:1000 |
| Ntcp | Santa Cruz | 1:300 | 1:3000 |
| Oatp1a4 | Millipore | 1:5000 | 1:5000 |
| Cyp7a1 | Thermo Fisher Scientific | 1:1000 | 1:2000 |
| Cyp8b1 | Thermo Fisher Scientific | 1:500 | 1:1000 |
| Sirt1 | Cell Signaling | 1:1000 | 1:2000 |
| p-AMPKα | Cell Signaling | 1:1000 | 1:2000 |
| PCNA | Sigma-Aldrich | 1:800 | 1:2000 |
| ZO-1 | Invitrogen | 1:500 | 1:1000 |
| β-actin | Sigma | 1:5000 | 1:5000 |
The LanthaScreen® TR-FRET PXR Competitive Binding Assay (Invitrogen/Life Technologies, Carlsbad, CA, United States) was performed to evaluate whether RSV is a potential PXR ligand. This cell-free assay was used since RSV interferes with classical luciferase reporter gene assays. The experiment utilizes human PXR-LBD tagged with glutathione-S-transferase (GST), which was labeled upon binding to a terbium (Tb)-labeled anti-GST antibody. The assay measures the ability of the evaluated compound to replace the fluorescent PXR ligand (Fluormone™ PXR (SXR) Green or “tracer”) from the receptor. The competition between the evaluated compound and tracer results in a loss in the fluorescence resonance energy transfer (FRET) signal between the Tb-anti-GST antibody and the tracer, whereby the difference can be measured. The assay was performed according to the manufacturer’s instructions. The fluorescence was measured using a Synergy 2 Multi-Mode Microplate Reader (BioTek, Winooski, VT, United States) at the recommended settings, except for measuring the emission signal at 528 nm instead of 520 nm. The calculation of TR-FRET ratio was performed by dividing the emission at 528 nm by emission at 495 nm.
All paraffin-embedded liver tissue sections were stained with Masson’s trichrome. For the semi-quantitative analysis, six images were taken of each liver sample, and the area occupied by the biliary ducts and connective tissue was evaluated from each image using ImageJ software (National Institutes of Health, Bethesda, MD; http://rsweb.nih.gov/ij/). The final result for each rat was the mean of the 6 evaluations and was expressed as the percentage of the whole field of view for the images.
Experiments were carried out on 6 animals per group. All experimental data are expressed as the mean ± SE. Statistical significance was examined in the groups of control, cirrhotic, and resveratrol-treated animals with one-way ANOVA followed by Newman-Keuls post hoc test. For two-group comparisons, Student’s t-test was employed. All analyses were performed using GraphPad Prism 6.0 software (San Diego, CA, United States). A difference of P < 0.05 was considered statistically significant.
The administration of RSV to sham rats did not change any of the biochemical parameters of the evaluated plasma samples (Figure 1). Similarly, light microscopy revealed normal liver architecture without pathological processes after sham operation and resveratrol administration, all of which support good tolerance of the compound (Figure 1). In contrast, untreated BDO for 28 d led to a significant increase in plasma indicators of cholestasis, namely, the concentrations of bile acids and cholesterol, as well as indicators of liver cell membrane injury, i.e., in activities of both ALT and AST. Liver histology confirmed changes that were typical for chronic cholestasis, i.e., significant proliferation of bile ducts and fibrosis in the expanded portal areas of the liver acinus. Increased expression of ZO-1 protein (Figure 1), an integral protein of zonula occludens, confirmed the impairment of the blood-biliary barrier integrity during BDO as previously described[20]. The administration of RSV to BDO animals produced significant attenuation of biochemical and histopathological alterations (Figure 1). RSV reduced bile duct proliferation and prevented fibrotic changes as visualized by trichrome staining in histological sections from cholestatic liver. Consistently, RSV markedly reduced protein content of PCNA (proliferating cell nuclear antigen), an indicator of cell proliferation, as well as collagen type 1 alpha 1 (Col1a1) expression, as both were significantly increased in BDO rats (Figure 1). BDO rats treated with RSV also demonstrated a reduced expression of ZO-1, indicating the improved integrity of the blood-biliary barrier.
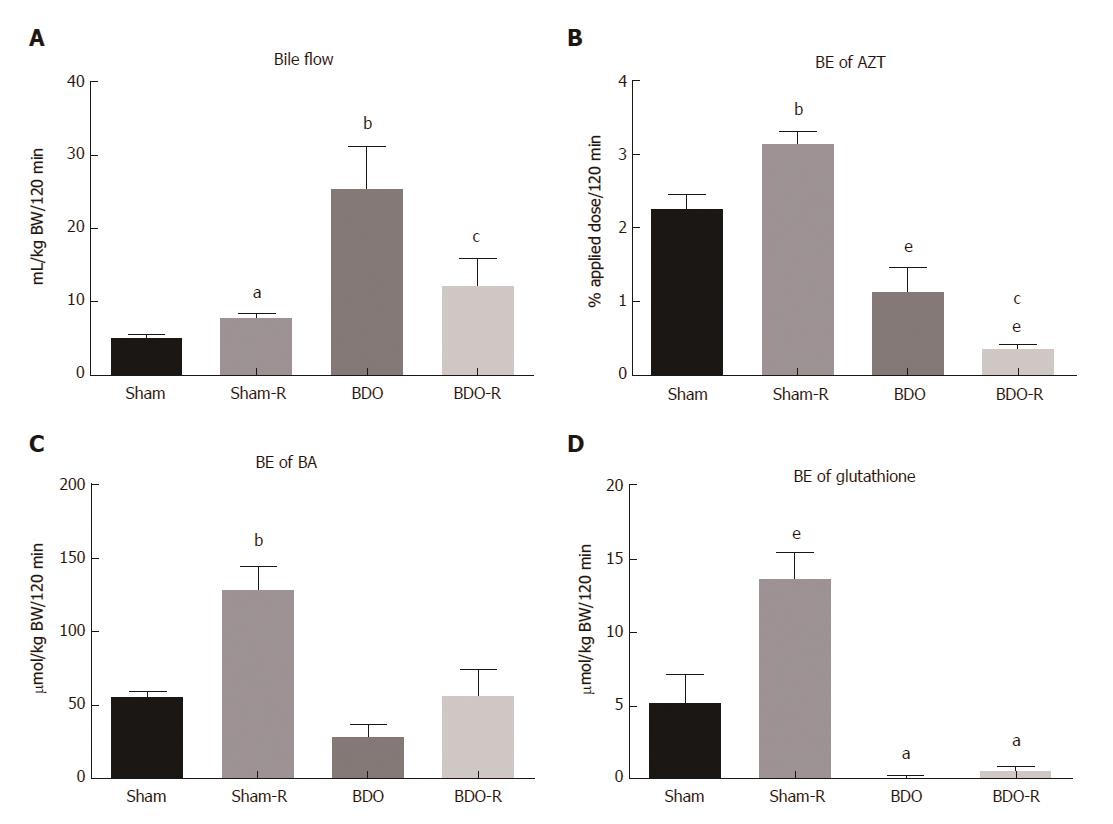
Bile flow was evaluated in rats 24 h after the last oral administration of resveratrol or saline. Resveratrol induced a moderate, but significant, increase in the bile flow of sham-operated rats (Figure 2A). This effect was accompanied by stimulated biliary secretion of bile acids and glutathione (Figure 2C and D), indicating that the choleretic action of long-term RSV administration is both BA-dependent and BA-independent. Moreover, RSV treatment increased biliary secretion of azithromycin, a drug transported by apical multidrug resistance proteins, suggesting the possibility of interaction of RSV with co-administered Mdr1/Mrp2 substrates (Figure 2B). BDO in saline-administered animals decreased biliary excretion of bile acids, glutathione and azithromycin to 49%, 2% and 47%, respectively (Figure 2). In contrast, the same animals demonstrated markedly increased bile flow compared to sham-operated healthy rats, which signified the dominating effect of increased hepatic paracellular permeability (altered function of the blood-biliary barrier) during extrahepatic cholestasis[21]. Oral gavage of RSV significantly reversed the massive choleresis in BDO animals. It accompanied reduced biliary secretion of azithromycin but left biliary excretion of both BA and glutathione unchanged (Figure 2). This discrepancy indicates the protective effect of RSV on the integrity of the blood-biliary barrier.
Interestingly, the liver concentrations of GSH and GSSG did not show any change in the experimental groups (Figure 3). This suggested that the increase in the BE of glutathione in RSV-treated Sham rats was the consequence of enhanced activity of the related Mrp2 transporter but not the increased concentration of glutathione. Additionally, the unchanged GSH/GSSG ratio indicated the absence of marked oxidative stress in cholestatic livers. This result was consistent with unmodified mRNA expression of NAD(P)H dehydrogenase quinone 1 (Nqo1), a gene activated by oxidative stress (Figure 3).
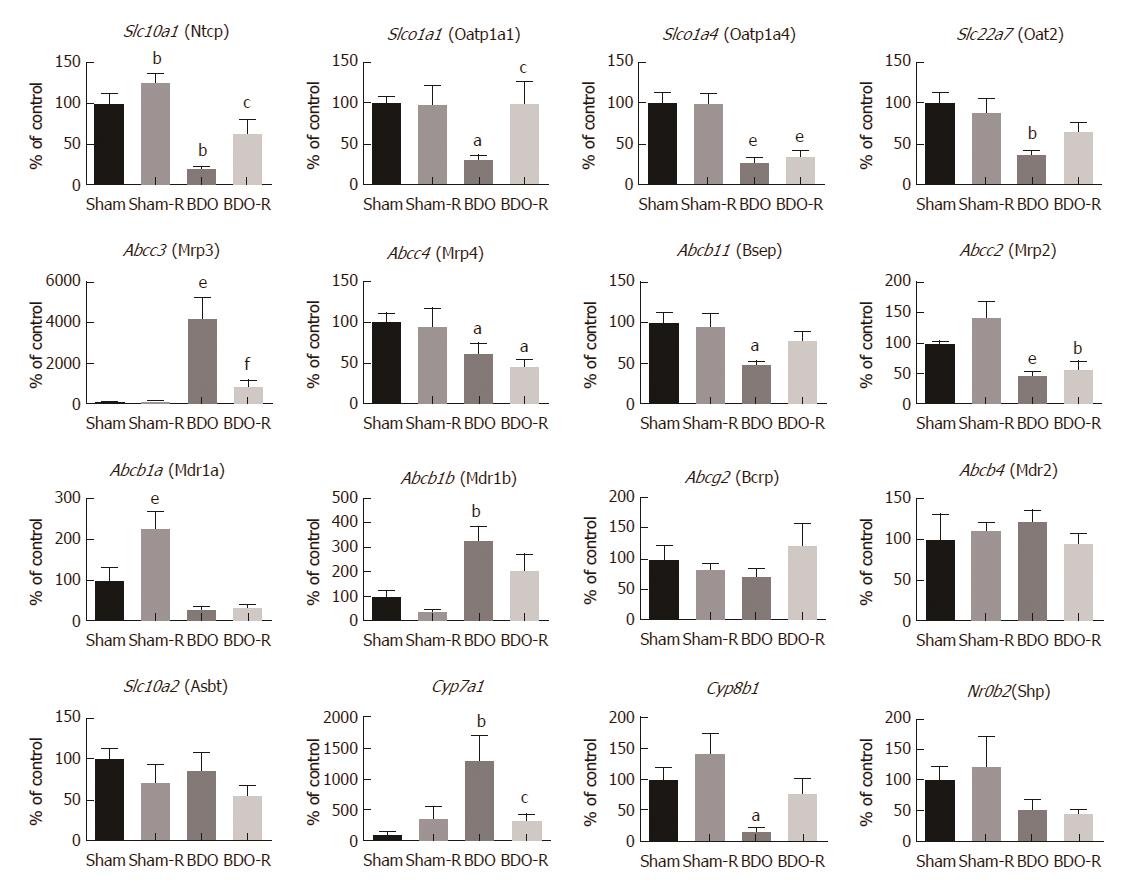
To describe the mechanisms responsible for the observed effects of RSV on bile production and secretion, we analyzed the mRNA levels of 16 genes involved in these processes. However, RSV-treated sham animals demonstrated only the increased mRNA of Abcb1a (one of two genes encoding Mdr1 protein) (Figure 4). This indicated a weak influence of RSV on the transcription of these pathways at the given dosage. Untreated long-term obstructive cholestasis down-regulated the expression of most of the uptake transporters localized at the basolateral membrane of the hepatocyte such as Slc10a1 (Ntcp), Slco1a1 (Oatp1a1), and Slc22a7 (Oat2). Similarly, the expression of major apical efflux transporters, such as Abcb11 (Bsep), Abcc2 (Mrp2), as well as Cyp8b1 enzyme, were also down-regulated. In contrast, BDO led to a marked induction of Abcc3 (Mrp3), Abcb1b (Mdr1b), and Cyp7a1 expression. Resveratrol gavage to BDO animals reversed the changes in the expression of the Slc10a1 (Ntcp), Slco1a1 (Oatp1a1), and Abcc3 (Mrp3) transporters, as well as the Cyp7a1 enzyme.
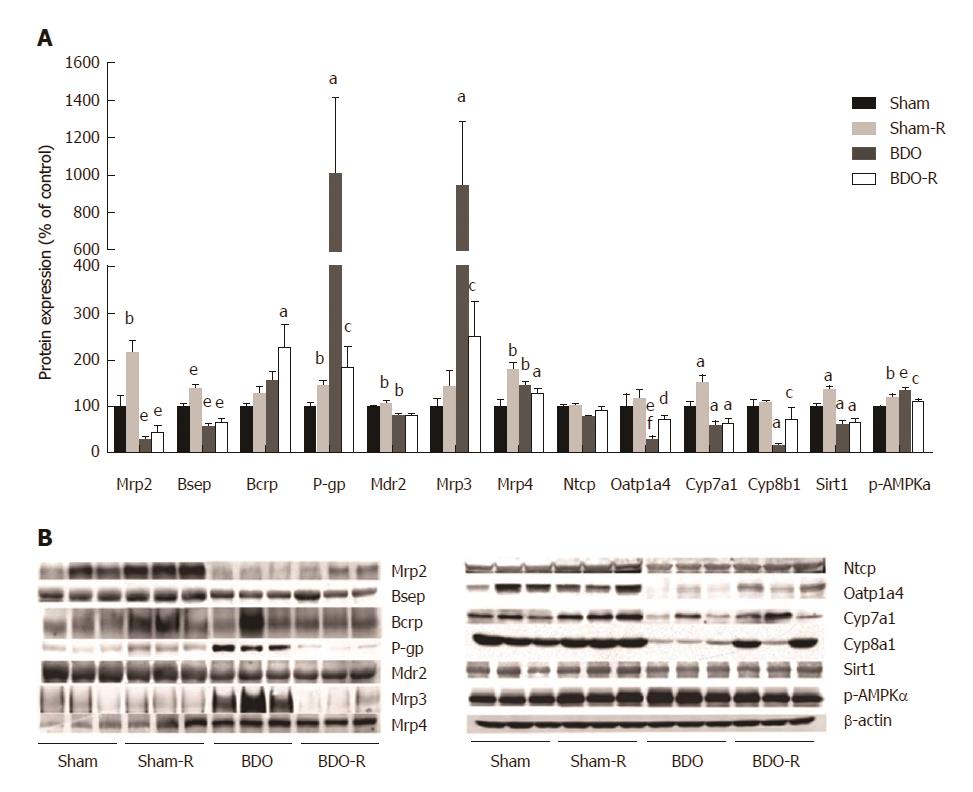
Resveratrol administration to healthy animals caused a significant increase in the expression of the liver transporter proteins responsible for BA (Bsep, Mrp4), glutathione (Mrp2), and drug (Mdr1/P-gp) export from hepatocytes as well as the increased expression of Cyp7a1, the rate-limiting enzyme for bile acid synthesis from cholesterol (Figure 5). Similarly, to previous reports[21-23], untreated BDO significantly reduced the protein content of Bsep, Mrp2, Mdr2, and Oatp1a4, and increased the protein content of Mrp3, Mrp4, and Mdr1 as a part of the anticholestatic defense response in the liver. Administration of RSV to BDO animals reversed the induction of Mdr1 and Mrp3 expression and the reduction in the expression of the Oatp1a4 transporter (Figure 5).
We analyzed Sirt1 and p-AMPKα protein expression to verify the predicted[15] modulation of this pathway by RSV as the major mechanism of its hepatic effects. We found that RSV significantly up-regulated both Sirt1 and p-AMPKα in sham rats but not in BDO animals (Figure 5). Bile duct obstruction alone did not affect p-AMPKα expression. In contrast, the Sirt1 protein level was significantly suppressed in the BDO group. These data indicated that resveratrol activated the Sirt1/AMPK pathway in healthy rats.
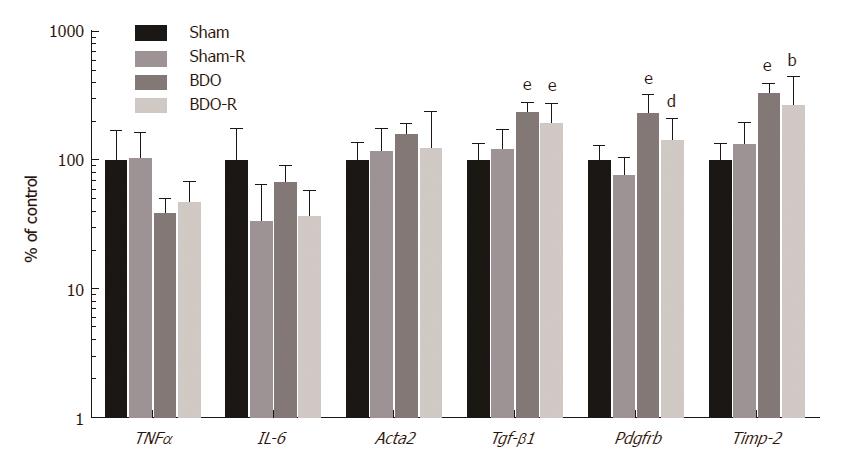
Due to the significant influence of RSV on the production of major inflammatory cytokines in the liver during various pathologies, we also analyzed these pathways at the level of mRNA (Figure 6). Quantitative RT-PCR analysis revealed no significant changes in the expression of either the acute phase cytokines such as tumor necrosis factor α (TNF-α) or IL-6 in any of the experimental groups. Similarly, RSV did not change the expression of any of the indicators in sham rats. All the obtained data suggested that the modulation of cytokine production is not involved in either the effect of RSV on bile production or in the secretory mechanisms in sham rats. On the other hand, the saline treated BDO group showed the induction of mRNA expression of transforming growth factor β1 (TGF-β1), platelet-derived growth factor receptor β (Pdgfrβ), and tissue inhibitor of metalloproteinase-2 (Timp-2). RSV administration to the BDO group led to a significant decline in the gene expression of Pdgfrβ (Figure 6).
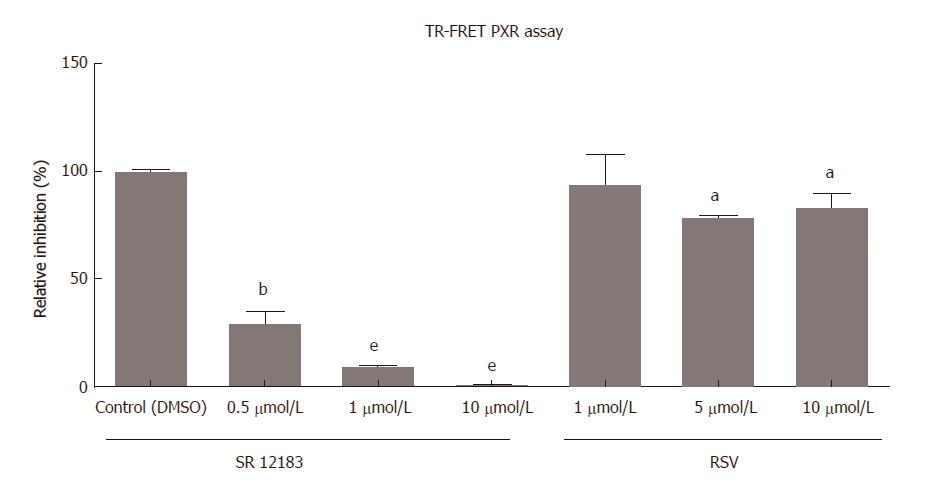
The LanthaScreen® TR-FRET PXR Competitive Binding assay was performed as a cell-free method to determine the ability of PXR to bind to the PXR ligand binding domain (LBD) and replace a fluorescent PXR ligand. We observed a weak, but statistically significant, decrease in fluorescence after administering RSV treatment (5 and 10 μmol/L) (Figure 7). Treatment with SR12813, a model high-affinity PXR agonist, completely suppressed TR-FRET fluorescence in the experiments, indicating the replacement of fluorescent PXR tracer from the PXR cavity. The results demonstrated that RSV is a human PXR activator with lower potency and intrinsic activity.
A major finding of our study is the identification of the inducing effect of orally administering RSV at a clinically relevant dosage on bile production and secretion in rats with intact biliary tracts. This effect was based on the increased biliary secretion of the major osmotic constituents of bile, BA and glutathione. The molecular background of RSV-induced choleresis was the induced protein expression of Bsep and Mrp2, the respective rate-limiting transporters for the BA-dependent and BA-independent bile flow at the apical membranes of hepatocytes. These mechanisms provide further support for the reported up-regulation of Mrp2 by RSV in ANIT-induced intrahepatic cholestasis in rats[16]. The increased transport of BA through the bile to the intestine also supports the observed increase in fecal excretion of BA in RSV-administered mice[24], as well as in tumor-bearing rats[25]. In addition, RSV administration to sham rats up-regulated hepatic Mrp4, a basolateral efflux transporter for BA. The possibility of increased export of BA back to the blood through Mrp4 is consistent with the unchanged plasma concentrations of BA in RSV-treated sham rats despite the increased BA biliary secretion. Unchanged BA plasma concentrations were also previously reported in RSV-treated mice despite reduced reabsorption of BA in the ileum[24]. These data indicated that RSV induces complex changes in BA transport in the liver of sham rats with intact bile flow.
RSV treatment in our sham rats also increased the protein expression of Cyp7a1, a rate-limiting enzyme for BA synthesis. This was consistent with the recently reported up-regulation of Cyp7a1 by a 0.4% resveratrol diet in mice. Therein, the effect was ascribed to the RSV-mediated reduction of BA reabsorption in the ileum and, consequently, the decreased activation of FXR in the ileum, which was followed by reduced Fgf15 production and disinhibition of Cyp7a1 hepatic mRNA and protein expression[24]. In addition, experiments with RSV-treated HepG2 cells demonstrated a direct induction of ABCB11 (BSEP) and CYP7A1 mRNA levels without mediators released from the ileum[26]. We therefore regarded the activation of FXR by RSV as a pivotal transcriptional effect regulating BA metabolism[27]. Importantly, we excluded the direct activation of the human FXR by RSV, as analyzed by a reporter gene assay (data not shown). This result corresponded to the absence of changes in mRNA levels of FXR target genes, such as Bsep, Mrp2, Mrp4, Shp or Cyp7a1 in our RSV-treated sham rats. This indicated the posttranscriptional effect of RSV as one of the major pathways of bile formation. One such mechanism has been described for Bsep and Mrp2 proteins[28]. It is based on the increased positioning and stabilization of Bsep and Mrp2 at the canalicular membrane of hepatocytes, by agents that increase hepatic cyclic adenosine monophosphate (cAMP), with the consequent activation of protein kinase A[29]. RSV increases cAMP by competitive inhibition of degrading phosphodiesterases. The subsequent activation of the cAMP-activated protein kinase A (AMPK)/SIRT signaling pathway is currently considered to be one of the major hepatoprotective mechanisms of RSV[15]. Indeed, we verified an increase in the expression of p-AMPK and Sirt1 in resveratrol-administered sham animals. It is therefore possible that the activation of this cascade is also responsible for the inducing effect of RSV on Bsep, Mrp2, and Mrp4 in healthy sham rats. The exact posttranscriptional mechanism of RSV in the liver should be further studied.
Choleretic agents stimulate bile production either via direct osmotic activity when they are concentrated in the bile, such as bile acids or penicillin G[30], or by transcriptional induction of Bsep or Mrp2 through the stimulation of nuclear receptors, namely, either FXR or PXR[31,32]. The possibility of a direct osmotic effect of resveratrol in our setting may be excluded because bile collection was performed in rats 24 h after receiving the last dose of RSV. Considering the very short half-life of the parent compound, that is approximately 1.31 h[33], the concentrations of unchanged resveratrol in bile should be negligible at the time of the evaluation. Our results also argued for an intensive transcriptional regulation through nuclear receptors. Detailed in vitro and in vivo experimentation excluded the activation of FXR by RSV. RSV administration to sham rats induced only liver Abcb1a, a PXR/Pxr target gene encoding Mdr1, the major canalicular transporter for biliary excretion of numerous xenobiotics, including drugs[34]. The effect paralleled the induction of Mdr1 protein and increased the BE of the well-known Mdr1 and Mrp2 substrate, azithromycin[34]. This was consistent with the recently reported inducing effect of RSV on the excretion of another Mdr1 substrate, cyclosporine[35]. Thus, we have performed TR-FRET PXR coactivator assay and confirmed that RSV activates PXR (Figure 7). This result indicated a low potency of RSV at the receptor[36]. However, the activation of the PXR-Abcb1a axis in RSV-administered sham rats suggests a sufficient concentration of RSV within the liver[37] at clinically relevant dosage. Together with the reported ability of RSV to inhibit the main drug metabolizing enzymes such as CYP3A4 or CYP2C9 with an IC50 of 1.1-4.5 μmol/L[38], and the recently described inhibition of Mdr1, Mrp2 and Oat1/3[39], our data suggested the potential of RSV to induce significant variability in the pharmacokinetics of simultaneously administered drugs.
The induction of BA-dependent choleresis during extrahepatic cholestasis is known for the worsening of ongoing liver impairment by raising the intrabiliary pressure and by biliary infarcts with concentrated BA[40]. However, RSV has repeatedly showed hepatoprotection in bile duct-ligated rats[12,17,41]. Therefore, we also examined both the bile production and the liver status in rats with BDO for 28 d. Untreated BDO rats developed typical histological, and biochemical signs upon initiated biliary cirrhosis[42] that were accompanied by increased hepatic gene expression of fibrotic markers such as TGF-β1, Col1a1, Pdgfrβ and Timp-2. Untreated BDO rats also developed changes in the expression of transporters, which was typical for a spontaneous cholestatic defense response (Figure 2)[22]. Interestingly, BDO rats did not show the mRNA induction of acute inflammatory markers, TNFα or IL-6, or significant oxidative stress, as measured by glutathione content, GSH to GSSG ratio, and Nqo1 expression. This discrepancy was produced by the free radical scavenging effect of accumulating bilirubin[43] and impaired biliary secretion of GSH before the release of obstruction. Similar to our previous findings, the release of biliary obstruction resulted in massive choleresis due to the impairment of the blood-biliary barrier[21].
Administration of RSV to BDO rats indeed attenuated histological and biochemical signs of the initiated biliary cirrhosis, including the reduction of BA concentrations in plasma and fibrotic markers in the liver. Hepatoprotection by RSV paralleled the decrease in bile flow, without the corresponding reduction in BE of BA or glutathione. This was consistent with the unchanged hepatic expression of Bsep or Mrp2 in BDO-R animals. In this situation, RSV reversed the BDO-induced up-regulation of ZO-1, an integral protein of tight-junctions, which indicates the protective effect of RSV on the alteration of the permeability of the blood-biliary barrier during obstructive cholestasis. Together with the unchanged expression of the rate-limiting Cyp7a1 enzyme for BA synthesis and the unchanged urinary excretion of bile acids (unpublished observation), these data suggested that the reduced plasma concentrations of BA in BDO-R rats may result from RSV-mediated reduction of intestinal reabsorption as shown in healthy mice[24]. This effect deserves further analysis. However, the marked differences between RSV-mediated changes in BA homeostasis in sham and BDO rats indicated a different regulatory mechanism. As such, we did not detect activation of AMPKα-Sirt1 following RSV treatment in BDO rats. The expression of proinflammatory cytokines was also not modified by RSV treatment in bile duct-obstructed rats. This result was consistent with the previously observed disappearance of the inhibitory effect of RSV on the expression of proinflammatory mediators the seventh day after bile duct ligation[41]. We detected only a reduction in Pdgfrβ mRNA expression (Figure 6), which was consistent with the reduced bile duct proliferation in RSV-treated BDO animals. On the other hand, accumulating BA levels are among the main mediators of hepatic damage during obstructive cholestasis that promotes inflammation and fibrosis[44]. Thus, our data indicated that the BA-lowering effect is the primary hepatoprotective mechanism of RSV during BDO in rats.
We noticed the down-regulation of hepatic Mdr1 by RSV in cholestatic animals is supported by the reduced biliary secretion of AZT. This effect resulted from decreased Mdr1b gene expression by RSV in BDO rats, perhaps as a consequence of reduced BA levels, which contributes to Mdr1 activation during cholestasis[45]. Although the exact mechanism requires further analysis, our data so far suggest the potential of RSV to alter the elimination of Mdr1 substrates during obstructive cholestasis.
The present study provides insight into the choleretic activity of RSV oral administration in rats with intact liver and biliary tract. This effect was caused by the induction of Bsep, Mrp2 and Cyp7a1 protein expression in the liver. Together with the induction of the expression of the Mdr1 and Mrp4 transporters, the results also indicated the potential of RSV to increase biliary secretion of co-administered substrates, including drugs from the organism. Moreover, a significant hepatoprotective effect of RSV in rats with extrahepatic cholestasis was related to a reduction in the plasma concentrations of BA as well as the prevention of blood-biliary barrier damage. RSV did not induce obvious changes in the expression of BA transporters in BDO rats, which can explain such an observation. Extrahepatic mechanisms of RSV-mediated reduction of BA plasma concentrations during obstructive cholestasis therefore must be a focus of further studies.
All pathways responsible for bile secretion, the major excretory route for potentially harmful endogenous substrates such as bilirubin and bile acids (BA) as well as for numerous exogenous lipophilic compounds, are sensitively regulated and modified by numerous stimuli, including diseases, drugs and food ingredients, which may either directly affect the activity of individual transporters or indirectly act by changing their expression. Resveratrol, a natural polyphenol, was shown to have a beneficial effect on a variety of diseases, such as malignancies and toxic and inflammatory tissue injuries, and it was shown to have marked hepatoprotective potential in situations such as non-alcoholic fatty liver disease, extrahepatic cholestasis, and α-naphthylisothiocyanate-, acetaminophen-, or carbon tetrachloride-induced hepatotoxicity.
Recent results have suggested that intraperitoneally administered resveratrol may prevent the impairment of bile formation during α-naphthylisothiocyanate (ANIT)-induced intrahepatic cholestasis. However, the effect of resveratrol on bile formation and secretion has not been determined in healthy rats or in the general population without cholestatic liver impairment, despite its wide use as a nutraceutical. Moreover, in comparison to the commonly used oral administration, intraperitoneal administration of RSV overcame its low bioavailability and markedly increased its exposure to the organism.
This is the first study to show the ability of orally administered RSV to increase bile flow in healthy rats as a consequence of increased biliary excretion of bile acids and glutathione via posttranscriptional induction of their rate-limiting transporters, Bsep and Mrp2, respectively, and by the up-regulation of Cyp7a1, an enzyme crucial for bile acid synthesis. Resveratrol simultaneously induced hepatic expression of Mdr1, which was verified by the increased biliary excretion of its substrate, azithromycin. Our findings were consistent with the agonistic effect of resveratrol on PXR. Moreover, RSV showed significant hepatoprotective potential in rats with long-term extrahepatic cholestasis, which was related to the reduction of plasma concentrations of BA and to the prevention of blood-biliary barrier damage.
Our data indicated the potential of orally administered resveratrol to increase biliary secretion of co-administered substrates, including drugs from the organism. Moreover, new mechanisms of RSV-mediated hepatoprotection by the reduction of bile acid plasma concentrations during extrahepatic cholestasis supports the safe use of resveratrol in such disorders.
Bile is the major excretory route for potentially harmful endogenous substrates (e.g., bilirubin and bile acids), and for numerous exogenous lipophilic compounds. Biliary secretion is an active process mediated by transporting proteins secreting their substrates into the bile canaliculi in the liver. Cholestasis is a pathological condition in which bile cannot flow from the liver to the duodenum.
The authors presented a carefully executed scientific study on the effect of resveratrol on biliary excretion in sham-operated and bile duct-obstructed rats. This study revealed that administration of resveratrol results in an increase in bile flow and the activation of transporters of bile acids and lipophilic compounds in normal rats. The authors showed that resveratrol improves the morphology of the bile duct and disturbed the molecular and biochemical indicators in bile duct-obstructed rats.
We gratefully acknowledge the skillful technical assistance of Jitka Hájková and Hana Laštůvková.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Czech Republic
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Liaskou E, Reshetnyak VI, Smith RC S- Editor: Ma YJ L- Editor: A E- Editor: Huang Y
| 1. | Boyer JL. Bile formation and secretion. Compr Physiol. 2013;3:1035-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 514] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 2. | Brandoni A, Hazelhoff MH, Bulacio RP, Torres AM. Expression and function of renal and hepatic organic anion transporters in extrahepatic cholestasis. World J Gastroenterol. 2012;18:6387-6397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Roma MG, Crocenzi FA, Mottino AD. Dynamic localization of hepatocellular transporters in health and disease. World J Gastroenterol. 2008;14:6786-6801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Geier A, Wagner M, Dietrich CG, Trauner M. Principles of hepatic organic anion transporter regulation during cholestasis, inflammation and liver regeneration. Biochim Biophys Acta. 2007;1773:283-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 229] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 5. | Amiot MJ, Riva C, Vinet A. Effects of dietary polyphenols on metabolic syndrome features in humans: a systematic review. Obes Rev. 2016;17:573-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 288] [Article Influence: 32.0] [Reference Citation Analysis (1)] |
| 6. | Bunaciu RP, Yen A. Resveratrol and Malignancies. Curr Pharmacol Rep. 2015;1:266-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Udenigwe CC, Ramprasath VR, Aluko RE, Jones PJ. Potential of resveratrol in anticancer and anti-inflammatory therapy. Nutr Rev. 2008;66:445-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 204] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 8. | Heebøll S, Thomsen KL, Pedersen SB, Vilstrup H, George J, Grønbæk H. Effects of resveratrol in experimental and clinical non-alcoholic fatty liver disease. World J Hepatol. 2014;6:188-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Ferramosca A, Di Giacomo M, Zara V. Antioxidant dietary approach in treatment of fatty liver: New insights and updates. World J Gastroenterol. 2017;23:4146-4157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 112] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (1)] |
| 10. | Muriel P, Rivera-Espinoza Y. Beneficial drugs for liver diseases. J Appl Toxicol. 2008;28:93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Wang Y, Jiang Y, Fan X, Tan H, Zeng H, Wang Y, Chen P, Huang M, Bi H. Hepato-protective effect of resveratrol against acetaminophen-induced liver injury is associated with inhibition of CYP-mediated bioactivation and regulation of SIRT1-p53 signaling pathways. Toxicol Lett. 2015;236:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 12. | Ara C, Kirimlioglu H, Karabulut AB, Coban S, Ay S, Harputluoglu M, Kirimlioglu V, Yilmaz S. Protective effect of resveratrol against oxidative stress in cholestasis. J Surg Res. 2005;127:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Kirimlioglu V, Ara C, Yilmaz M, Ozgor D, Isik B, Sogutlu G, Kirimlioglu H, Karabulut AB, Yilmaz S, Kayaalp C. Resveratrol, a red wine constituent polyphenol, protects gastric tissue against the oxidative stress in cholestatic rats. Dig Dis Sci. 2006;51:298-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Tian Y, Ma J, Wang W, Zhang L, Xu J, Wang K, Li D. Resveratrol supplement inhibited the NF-κB inflammation pathway through activating AMPKα-SIRT1 pathway in mice with fatty liver. Mol Cell Biochem. 2016;422:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 125] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 15. | Zhang Y, Chen ML, Zhou Y, Yi L, Gao YX, Ran L, Chen SH, Zhang T, Zhou X, Zou D. Resveratrol improves hepatic steatosis by inducing autophagy through the cAMP signaling pathway. Mol Nutr Food Res. 2015;59:1443-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 16. | Wang T, Zhou ZX, Sun LX, Li X, Xu ZM, Chen M, Zhao GL, Jiang ZZ, Zhang LY. Resveratrol effectively attenuates alpha-naphthylisothiocyanate-induced acute cholestasis and liver injury through choleretic and anti-inflammatory mechanisms. Acta Pharmacologica Sinica. 2014;35:1527-1536. [RCA] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Cenesiz S, Yarim GF, Karabulut AB, Ara C. Changing of antioxidant enzyme activity on the biliary obstructed rats treated with resveratrol. Dtsch Tierarztl Wochenschr. 2007;114:345-348. [PubMed] |
| 18. | Hirsova P, Karlasova G, Dolezelova E, Cermanova J, Zagorova M, Kadova Z, Hroch M, Sispera L, Tomsik P, Lenicek M. Cholestatic effect of epigallocatechin gallate in rats is mediated via decreased expression of Mrp2. Toxicology. 2013;303:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Fuksa L, Brcakova E, Cermanova J, Hroch M, Chladek J, Kolouchova G, Malakova J, Martinkova J, Staud F, Micuda S. Amiodarone modulates pharmacokinetics of low-dose methotrexate in rats. Biopharm Drug Dispos. 2008;29:289-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Maly IP, Landmann L. Bile duct ligation in the rat causes upregulation of ZO-2 and decreased colocalization of claudins with ZO-1 and occludin. Histochem Cell Biol. 2008;129:289-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Kolouchova G, Brcakova E, Hirsova P, Sispera L, Tomsik P, Cermanova J, Hyspler R, Slanarova M, Fuksa L, Lotkova H. Pravastatin modulates liver bile acid and cholesterol homeostasis in rats with chronic cholestasis. J Gastroenterol Hepatol. 2011;26:1544-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Wagner M, Zollner G, Trauner M. New molecular insights into the mechanisms of cholestasis. J Hepatol. 2009;51:565-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 190] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 23. | Brcakova E, Fuksa L, Cermanova J, Kolouchova G, Hroch M, Hirsova P, Martinkova J, Staud F, Micuda S. Alteration of methotrexate biliary and renal elimination during extrahepatic and intrahepatic cholestasis in rats. Biol Pharm Bull. 2009;32:1978-1985. [PubMed] |
| 24. | Chen ML, Yi L, Zhang Y, Zhou X, Ran L, Yang J, Zhu JD, Zhang QY, Mi MT. Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. MBio. 2016;7:e02210-e02215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 332] [Cited by in RCA: 546] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 25. | Miura D, Miura Y, Yagasaki K. Hypolipidemic action of dietary resveratrol, a phytoalexin in grapes and red wine, in hepatoma-bearing rats. Life Sci. 2003;73:1393-1400. [PubMed] |
| 26. | Shao D, Wang Y, Huang Q, Shi J, Yang H, Pan Z, Jin M, Zhao H, Xu X. Cholesterol-Lowering Effects and Mechanisms in View of Bile Acid Pathway of Resveratrol and Resveratrol Glucuronides. J Food Sci. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Halilbasic E, Baghdasaryan A, Trauner M. Nuclear receptors as drug targets in cholestatic liver diseases. Clin Liver Dis. 2013;17:161-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | Shoda J, Miura T, Utsunomiya H, Oda K, Yamamoto M, Kano M, Ikegami T, Tanaka N, Akita H, Ito K. Genipin enhances Mrp2 (Abcc2)-mediated bile formation and organic anion transport in rat liver. Hepatology. 2004;39:167-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Misra S, Varticovski L, Arias IM. Mechanisms by which cAMP increases bile acid secretion in rat liver and canalicular membrane vesicles. Am J Physiol Gastrointest Liver Physiol. 2003;285:G316-G324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Fukami M, Tanka A, Takikawa H. Effect of penicillin G on the biliary excretion of cholephilic compounds in rats. J Hepatobiliary Pancreat Sci. 2011;18:684-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Baghdasaryan A, Chiba P, Trauner M. Clinical application of transcriptional activators of bile salt transporters. Mol Aspects Med. 2014;37:57-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Cermanova J, Kadova Z, Zagorova M, Hroch M, Tomsik P, Nachtigal P, Kudlackova Z, Pavek P, Dubecka M, Ceckova M. Boldine enhances bile production in rats via osmotic and farnesoid X receptor dependent mechanisms. Toxicol Appl Pharmacol. 2015;285:12-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Marier JF, Vachon P, Gritsas A, Zhang J, Moreau JP, Ducharme MP. Metabolism and disposition of resveratrol in rats: extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model. J Pharmacol Exp Ther. 2002;302:369-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 346] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 34. | Sugie M, Asakura E, Zhao YL, Torita S, Nadai M, Baba K, Kitaichi K, Takagi K, Takagi K, Hasegawa T. Possible involvement of the drug transporters P glycoprotein and multidrug resistance-associated protein Mrp2 in disposition of azithromycin. Antimicrob Agents Chemother. 2004;48:809-814. [PubMed] |
| 35. | Yang S-Y, Tsai S-Y, Hou Y-C, Chao P-DL. Inductive modulation on P-glycoprotein and cytochrome 3A by resveratrol, a constituent of grapes. Food Chem. 2012;133:683-688. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Smutny T, Pavek P. Resveratrol as an inhibitor of pregnane X receptor (PXR): another lesson in PXR antagonism. J Pharmacol Sci. 2014;126:177-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Vitrac X, Desmoulière A, Brouillaud B, Krisa S, Deffieux G, Barthe N, Rosenbaum J, Mérillon JM. Distribution of [14C]-trans-resveratrol, a cancer chemopreventive polyphenol, in mouse tissues after oral administration. Life Sci. 2003;72:2219-2233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 192] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 38. | Detampel P, Beck M, Krähenbühl S, Huwyler J. Drug interaction potential of resveratrol. Drug Metab Rev. 2012;44:253-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 39. | Jia Y, Liu Z, Wang C, Meng Q, Huo X, Liu Q, Sun H, Sun P, Yang X, Ma X. P-gp, MRP2 and OAT1/OAT3 mediate the drug-drug interaction between resveratrol and methotrexate. Toxicol Appl Pharmacol. 2016;306:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 40. | Fickert P, Zollner G, Fuchsbichler A, Stumptner C, Weiglein AH, Lammert F, Marschall HU, Tsybrovskyy O, Zatloukal K, Denk H. Ursodeoxycholic acid aggravates bile infarcts in bile duct-ligated and Mdr2 knockout mice via disruption of cholangioles. Gastroenterology. 2002;123:1238-1251. [PubMed] |
| 41. | Chan CC, Cheng LY, Lin CL, Huang YH, Lin HC, Lee FY. The protective role of natural phytoalexin resveratrol on inflammation, fibrosis and regeneration in cholestatic liver injury. Mol Nutr Food Res. 2011;55:1841-1849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 42. | Trauner M, Boyer JL. Cholestatic syndromes. Curr Opin Gastroenterol. 2004;20:220-230. [PubMed] |
| 43. | Zelenka J, Muchova L, Zelenkova M, Vanova K, Vreman HJ, Wong RJ, Vitek L. Intracellular accumulation of bilirubin as a defense mechanism against increased oxidative stress. Biochimie. 2012;94:1821-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Allen K, Jaeschke H, Copple BL. Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis. Am J Pathol. 2011;178:175-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 401] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 45. | Micuda S, Brcakova E, Fuksa L, Cermanova J, Osterreicher J, Hroch M, Mokry J, Pejchal J, Martinkova J, Staud F. P-glycoprotein function and expression during obstructive cholestasis in rats. Eur J Gastroenterol Hepatol. 2008;20:404-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |