Published online Sep 28, 2017. doi: 10.3748/wjg.v23.i36.6685
Peer-review started: June 21, 2017
First decision: July 13, 2017
Revised: July 28, 2017
Accepted: August 25, 2017
Article in press: August 25, 2017
Published online: September 28, 2017
Processing time: 99 Days and 8 Hours
To assess the prognostic value of lymphovascular invasion (LVI) in Bismuth-Corlette type IV hilar cholangiocarcinoma (HC) patients.
A retrospective analysis was performed on 142 consecutively recruited type IV HC patients undergoing radical resection with at least 5 years of follow-up. Survival analysis was performed by the Kaplan-Meier method, and the association between the clinicopathologic variables and survival was evaluated by log-rank test. Multivariate analysis was adopted to identify the independent prognostic factors for overall survival (OS) and disease-free survival (DFS). Multiple logistic regression analysis was performed to determine the association between LVI and potential variables.
LVI was confirmed histopathologically in 29 (20.4%) patients. Multivariate analysis showed that positive resection margin (HR = 6.255, 95%CI: 3.485-11.229, P < 0.001), N1 stage (HR = 2.902, 95%CI: 1.132-7.439, P = 0.027), tumor size > 30 mm (HR = 1.942, 95%CI: 1.176-3.209, P = 0.010) and LVI positivity (HR = 2.799, 95%CI: 1.588-4.935, P < 0.001) were adverse prognostic factors for DFS. The independent risk factors for OS were positive resection margin (HR = 6.776, 95%CI: 3.988-11.479, P < 0.001), N1 stage (HR = 2.827, 95%CI: 1.243-6.429, P = 0.013), tumor size > 30 mm (HR = 1.739, 95%CI: 1.101-2.745, P = 0.018) and LVI positivity (HR = 2.908, 95%CI: 1.712-4.938, P < 0.001). LVI was associated with N1 stage and tumor size > 30 mm. Multiple logistic regression analysis indicated that N1 stage (HR = 3.312, 95%CI: 1.338-8.198, P = 0.026) and tumor size > 30 mm (HR = 3.258, 95%CI: 1.288-8.236, P = 0.013) were associated with LVI.
LVI is associated with N1 stage and tumor size > 30 mm and adversely influences DFS and OS in type IV HC patients.
Core tip: Previous studies have reported that lymphovascular invasion (LVI) provokes an adverse impact on the long-term survival of several malignances, including breast, gastric, and esophageal carcinoma, among many others. However, the correlation between LVI and hilar cholangiocarcinoma remains unclear. In our study, LVI was found to be an independent risk factor for overall survival and disease-free survival in Bismuth-Corlette type IV hilar cholangiocarcinoma patients. To our knowledge, this report indicates for the first time that LVI is an adverse predictor of long-term survival in the setting of type IV hilar cholangiocarcinoma.
- Citation: Li B, Xiong XZ, Zhou Y, Wu SJ, You Z, Lu J, Cheng NS. Prognostic value of lymphovascular invasion in Bismuth-Corlette type IV hilar cholangiocarcinoma. World J Gastroenterol 2017; 23(36): 6685-6693
- URL: https://www.wjgnet.com/1007-9327/full/v23/i36/6685.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i36.6685
Hilar cholangiocarcinoma (HC), also known as Klatskin tumor, is a neoplasia arising from the biliary epithelium at the common hepatic duct bifurcation and may extend to the intrahepatic biliary tree and liver[1,2]. The only clinical approach that is considered to provide patients with an opportunity for a curative outcome and importantly long-term survival is radical surgical resection[2-6]. The Bismuth-Corlette classification is the most widely used preoperative system of evaluation that can predict resectability of the lesion and assist in the design of an appropriate surgical approach. A Bismuth type IV lesion is defined as a tumor that can invade the secondary biliary radicals of both hepatic ducts. During the past decades, accompanied liver resection has been increasingly but gradually recognized as the mainstay of surgical approaches for targeting a Bismuth type IV tumor[7-9], however, there are relatively few studies that have reported in any detail the factors that might affect long-term survival of type IV HC patients. The over-arching aim of the current study was to identify prognostic factors for long-term survival of patients following radical surgery for type IV HC patients, especially in the setting of lymphovascular invasion (LVI).
One hundred and forty-two consecutive patients who underwent radical resection for a pathological diagnosis of type IV HC at the West China Hospital between January 2000 and February 2012 were enrolled in this study and then reviewed retrospectively. The inclusion criteria included the following: (1) patients who were confirmed with Bismuth type IV HC by pathological examination; and (2) patients who had undergone radical resection (R0 and R1 resection). The exclusion criteria included the following: (1) patients with gallbladder or intrahepatic cholangiocarcinoma extending to the hilum; (2) presence of a recurrent or metastatic tumor; and (3) R2 resection.
Preoperative assessment consisted of acquiring a medical history, physical examination, laboratory tests and radiographic analyses. All patients were evaluated by contrast-enhanced ultrasound, contrast-enhanced computed tomography or magnetic resonance cholangiography with magnetic resonance cholangiopancreatography with the intention of determining the Bismuth type, the location and extent of the tumor. Biliary drainage, including endoscopic retrograde cholangiopancreatography (ENBD) and percutaneous transhepatic cholangiodrainage (PTCD), was applied in the setting of patients presenting with obstructive jaundice that exceeded 85 μmol/L total bilirubin.
Based on the relative location and extent of the tumor, different types of resection were performed, which included extrahepatic bile duct resection and en bloc resection of the caudate lobe combined with left hemihepatectomy, right hemihepatectomy and trisectionectomy. In addition, standard regional lymph node dissection should be performed. However, under conditions where tumor metastases to the distant lymph nodes was confirmed during surgery, the surgical intervention was abandoned. According to the American Joint Committee on Cancer (AJCC, 7th edition), the location of regional lymph nodes was defined as follows: along the common bile duct, cystic duct, portal vein and proper hepatic artery[10]. Vascular resection and reconstruction was only performed when vessels could not be detached from the tumor.
The pathological evidence of cancer was determined by examination of paraffin sections. All included Bismuth type IV HC cases were histopathologically confirmed by an experienced pathologist. The presence of tumor emboli within peritumoural endothelial lined spaces was defined as LVI. Resection margin was defined as ductal (i.e., proximal and distal ducts) and with evidence of radial margins. The radial margin was defined as the vertical margin between the tumor edge and dissected periductal structures (e.g., liver parenchyma, blood vessels and adjacent fat tissues). An R0 resection was defined as the presence of a microscopically tumor-free resection margin. An R1 resection was defined as microscopic evidence of tumor tissue at the resection margin, and an R2 resection was defined as macroscopic evidence of tumor tissue at the resection margin. In this study, radical resection was defined as an R0 and R1 resection, a negative resection margin indicated R0 resection, and a positive resection margin indicated an R1 resection.
Whether or not chemotherapy and radiotherapy can benefit HC patients was controversial. None of the patients received postoperative routine chemotherapy or radiotherapy. All enrolled patients had routine follow-ups every 3 mo in the first year and every 6 mo subsequently until at least 5 years after the surgery. The tumor markers [serum levels of carbohydrate antigen 19-9 (CA19-9) and carcinoembryonic antigen], liver functions and ultrasonography were conducted. If there was a suspicion of recurrence, contrast-enhanced computed tomography or magnetic resonance imaging was further performed. Tumor recurrence was diagnosed on the basis of the combined findings of typical radiological appearance, quantification of CA19-9 levels, and clinical presentation. The date of the first suspicious radiological finding was recorded as the date of initial disease recurrence.
Patient data were retrospectively collected and statistical analyses were performed with SPSS version 19.0 (SPSS Inc. Chicago, IL, United States). Survival was described using the Kaplan-Meier method and differences between subgroups were reviewed by the log-rank test. Multivariate analysis for prognostic factors was performed using a Cox proportional hazards model to analyze variables whose P-value was less than 0.1 in the univariate analysis. Multiple logistical regression analysis was performed to determine the association between LVI and potential variables. Two-sided P values < 0.05 were considered statistically significant.
Patients’ characteristics and operative outcomes are shown in Table 1. Altogether 142 patients had a radical resection for type IV HC, including 75 men and 67 women with a median age of 59 years (range: 23-78). Pre-operative biliary drainage was carried out in 105 of the 123 obstructive jaundice (total bilirubin > 85 μmol/L) patients, wherein 71 patients underwent PCTD and 34 patients underwent ENBD. Preoperative portal vein embolization was performed in 6 patients.
| Characteristic | n (%) |
| Age (yr)1 | 59 (23-78) |
| Gender | |
| Male | 75 (52.8) |
| Female | 67 (47.2) |
| Albumin level (g/L)2 | 36.74 ± 4.93 |
| CA19-9 | |
| > 200 | 82 (57.7) |
| < 200 | 60 (42.3) |
| Radiological examination | |
| Contrast-enhanced US | 8 (5.6) |
| Contrast-enhanced CT | 43 (30.3) |
| MRI + MRCP | 91 (64.1) |
| Operative time (min)2 | 429.47 ± 134.19 |
| Blood loss (ml)1 | 600 (180-4000) |
| Transfusion | 76 (53.5) |
| R0 resection | 107 (75.4) |
| Differentiation degree | |
| Well differentiated | 14 (9.9) |
| Moderately differentiated | 90 (63.4) |
| Poorly differentiated | 38 (26.8) |
| Perineutral invasion positive | 69 (48.6) |
| N stage | |
| N0 | 89 (62.7) |
| N1 | 53 (37.3) |
| Tumor diameter (mm)1 | 30 (12-55 |
| Hospital stay1 | 19 (5-115) |
Radical resection included extrahepatic bile duct resection and en bloc resection of the caudate lobe combined with left hemihepatectomy (n = 73, 51.4%), extended left hemihepatectomy (n = 5, 3.5%), left trisectionectomy (n = 6, 4.2%), right hemihepatectomy (n = 51, 35.9%), extended right hemihepatectomy (n = 5, 3.5%), and right trisectionectomy (n = 2, 1.4%). Regional lymph node dissection was conventionally performed. The R0 resection rate was 75.6%.
As shown in Table 2, potential factors that might influence the DFS and OS were analyzed. Univariate analysis demonstrated that age (P = 0.039), preoperative ALB (P = 0.005), resection margin (P < 0.001), histologic grade (P = 0.023), T stage (P = 0.004), N stage (P < 0.001), AJCC stage (P < 0.001), LVI (P < 0.001), tumor size (P < 0.001), portal vein invasion (P = 0.003) and hepatic artery invasion (P = 0.008) significantly influenced DFS. By contrast, patient gender, preoperative CA19-9, surgical method, perineural invasion and transfusion did not significantly influence DFS. Preoperative ALB (P = 0.009), resection margin (P < 0.001), histologic grade (P = 0.026), T stage (P = 0.001), N stage (P < 0.001), AJCC stage (P < 0.001), LVI (P < 0.001), tumor size (P < 0.001), portal vein invasion (P = 0.002) and hepatic artery invasion (P = 0.013), but not patient age, gender, preoperative CA19-9, surgical methods, perineural invasion or transfusion, significantly influenced the OS. Multivariate analysis indicated that positive resection margin, higher N stage, tumor size > 30 mm and LVI were adverse factors that influenced DFS and OS.
| Variable | Disease-free survival | Overall survival | ||||||
| 5-yr survival | Univariate analysis | Multivariate analysis | 5-yr survival | Univariate analysis | Multivariate analysis | |||
| P value | HR (95%CI) | P value | P value | HR (95%CI) | P value | |||
| Gender | 0.682 | 0.965 | ||||||
| Male | 13 (18.8) | 14 (20.3) | ||||||
| Female | 10 (16.4) | 10 (16.4) | ||||||
| Age (yr) | 0.039 | 1.291 (0.680-2.453) | 0.435 | 0.230 | ||||
| < 65 | 19 (19.2) | 20 (20.2) | ||||||
| > 65 | 4 (12.9) | 4 (12.9) | ||||||
| CA19-9 | 0.287 | 0.301 | ||||||
| < 200 | 13 (23.2) | 14 (25) | ||||||
| > 200 | 10 (13.5) | 10 (13.5) | ||||||
| ALB (g/L) | 0.005 | 0.669 (0.413-1.085) | 0.103 | 0.009 | 0.760 (0.492-1.173) | 0.215 | ||
| < 35 | 3 (7.1) | 3 (7.1) | ||||||
| > 35 | 21 (23.9) | 20 (22.7) | ||||||
| Surgical method | 0.847 | 0.684 | ||||||
| Left-sided hepatectomy | 13 (15.1) | 13 (15.1) | ||||||
| Right-sided hepatectomy | 10 (17.2) | 11 (19.0) | ||||||
| Resection margin | < 0.001 | 6.255 (3.485-11.229) | < 0.001 | < 0.001 | 6.776 (3.988-11.479) | < 0.001 | ||
| Positive | 0 | 0 | ||||||
| Negative | 23 (23.7) | 24 (24.7) | ||||||
| Histologic grade | 0.023 | 1.594 (0.994-2.554) | 0.053 | 0.026 | 1.294 (0.830-2.017) | 0.256 | ||
| Well/moderate | 20 (21.5) | 21 (22.6) | ||||||
| poor | 3 (8.1) | |||||||
| Perineural invasion | 0.211 | 0.417 | ||||||
| Present | 14 (22.6) | 15 (24.2) | ||||||
| Absent | 9 (13.2) | 9 (13.2) | ||||||
| T stage | 0.004 | 1.582 (0.390-6.415) | 0.521 | 0.001 | 2.399 (0.734-7.836) | 0.147 | ||
| T1/2 | 22 (22) | 21 (21) | ||||||
| T3/4 | 2 (6.7) | 2 (6.7) | ||||||
| N stage | < 0.001 | 2.902 (1.132-7.439) | 0.027 | < 0.001 | 2.827 (1.243-6.429) | 0.013 | ||
| N0 | 22 (27.2) | 23 (28.4) | ||||||
| N1 | 1 (2.0) | 1 (2.0) | ||||||
| AJCC stage | < 0.001 | 0.673 (0.289-1.567) | 0.358 | < 0.001 | 0.844 (0.351-2.028) | 0.704 | ||
| Stage I/II | 20 (32.8) | 21 (34.4) | ||||||
| Stage III/IV | 3 (4.9) | 3 (4.9) | ||||||
| Lymphovascular invasion | < 0.001 | 2.799 (1.588-4.935) | < 0.001 | < 0.001 | 2.908 (1.712-4.938) | < 0.001 | ||
| Present | 0 | 0.000 | ||||||
| Absent | 23 (22.8) | 24 (24.8) | ||||||
| Tumor size (mm) | < 0.001 | 1.942 (1.176-3.209) | 0.010 | < 0.001 | 1.739 (1.101-2.745) | 0.018 | ||
| ≤ 30 | 15 (20) | 16 (21.3) | ||||||
| > 30 | 8 (14.5) | 8 (14.5) | ||||||
| Portal vein invasion | 0.003 | 1.759 (0.534-5.800) | 0.353 | 0.002 | 1.130 (0.408-3.127) | 0.815 | ||
| Present | 1 (4.2) | 1 (4.2) | ||||||
| Absent | 22 (20.8) | 23 (21.7) | ||||||
| Hepatic invasion | 0.008 | 1.499 (0.612-3.668) | 0.376 | 0.013 | 1.196 (0.526-2.719) | 0.669 | ||
| Present | 1 (9.1) | 1 (9.1) | 1 (9.1) | |||||
| Absent | 22 (18.5) | 22 (18.5) | 23 (19.3) | |||||
| Transfusion | 0.445 | 0.199 | ||||||
| Yes | 11 (16.4) | 11 (16.4) | ||||||
| No | 12 (19.0) | 13 (20.6) | ||||||
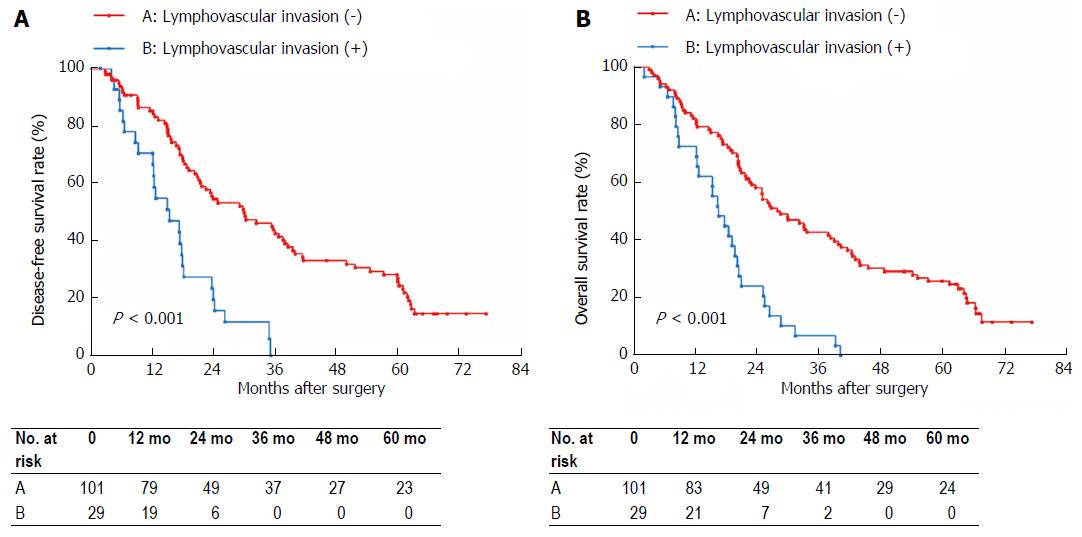
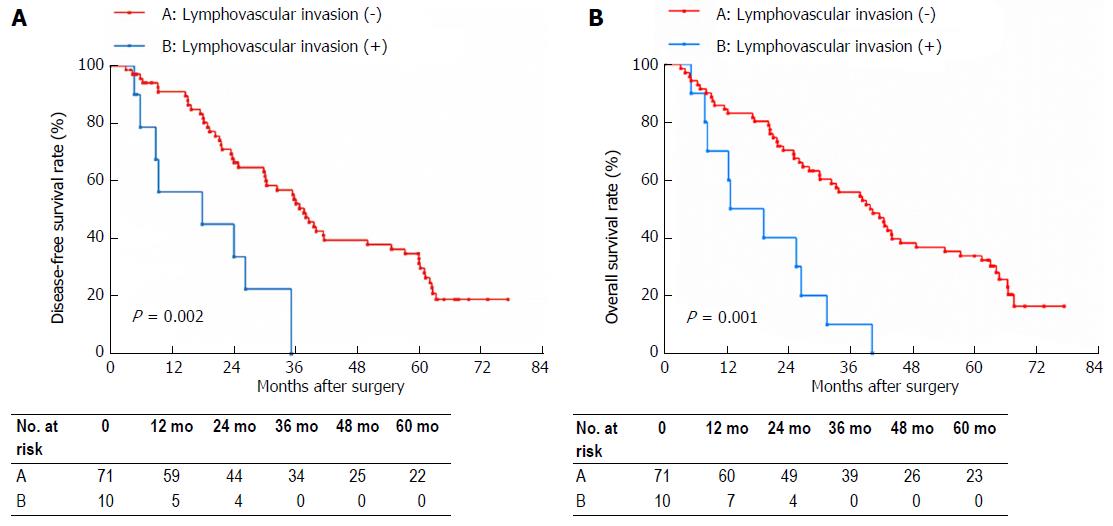
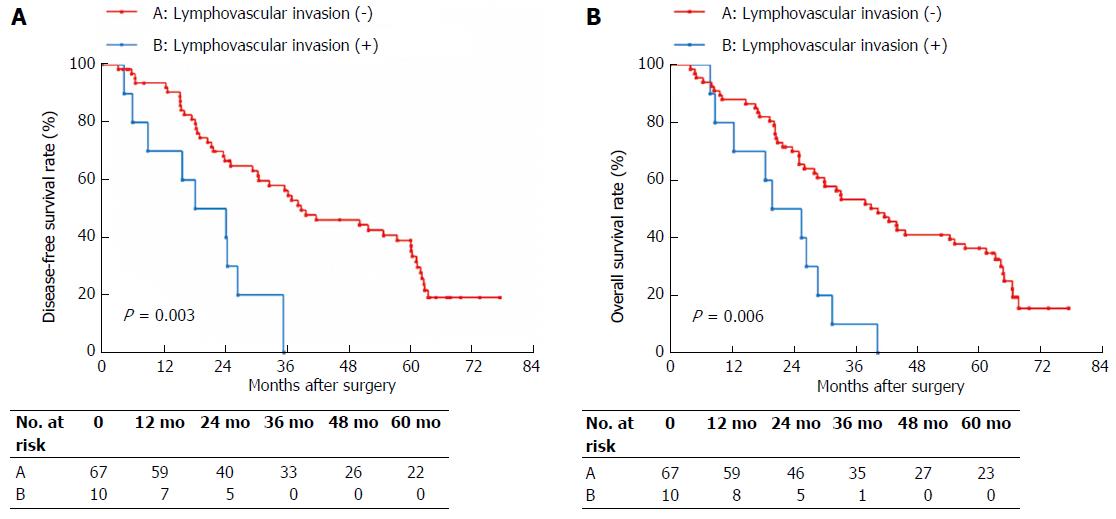
LVI was confirmed histologically in 29 (20.4%) of the 142 patients. Table 3 shows the association of LVI with tumor size, N stage and AJCC stage. On univariate analysis, N stage (P < 0.001) and tumor size (P = 0.001), but not AJCC stage, were significantly associated with LVI. Multivariate analysis using a logistic regression model indicated that N1 stage (P = 0.026) and tumor size > 30 mm were significant factors that were associated with LVI in Bismuth type IV HC. DFS and OS based on LVI status are shown in Figure 1. The 5-year DFS rate in the LVI negative group was significantly higher than that in the LVI positive group (22.8% vs 0%, P < 0.001) as was the OS (23.8% vs 0%, P < 0.001). Based on the LVI status, N0 stage patients and patients with tumor size ≤ 30 mm were divided into two subgroups, respectively. In the N0 stage subgroups, the 5-year DFS rate in the LVI negative group was significantly higher than that in the LVI positive group (31.0% vs 0%, P = 0.002), and the 5-year OS rate in the LVI negative group was also significantly higher than that in the LVI positive group (32.4% vs 0%, P = 0.001). In the tumor size ≤ 30 mm subgroups, the 5-year DFS rate in the LVI negative group was significantly higher than that in the LVI positive group (31.8% vs 0%, P = 0.003), and the 5-year OS rate in the LVI negative group was significantly higher than that in the LVI positive group (34.3% vs 0%, P = 0.006).
| Variable | Lymphovascualr invasion (+), n = 29 | Lymphovascualr invasion (-), n = 113 | Univariate analysis (P value) | OR (95%CI) | Multivariate analysis (P value) |
| AJCC stage | 0.063 | 0.223 (0.026-1.927) | 0.173 | ||
| I/II | 8 | 27 | |||
| III/IV | 21 | 86 | |||
| Tumor diameter (mm) | 0.001 | 3.258 (1.288-8.236) | 0.013 | ||
| ≤ 30 | 10 | 77 | |||
| > 30 | 19 | 36 | |||
| N stage | < 0.001 | 3.312 (1.338-8.198) | 0.026 | ||
| N0 | 10 | 79 | |||
| N1 | 19 | 34 |
LVI is recognized as a dismal prognostic factor for OS in patients with breast cancer, colorectal cancer, and esophageal cancer[11-14]. However, no studies have yet been published on whether LVI affects the prognosis of Bismuth type IV HC. Thus, the current study was undertaken to clarify the significance of LVI in type IV HC patients who had a radical resection. LVI is defined as the involvement of arterial vessels, venules and lymphatic channels[11], but it is histologically difficult to distinguish, and the American Joint Committee on Cancer/Union Internationale Contre Cancer staging guidelines use the term lymphovascular to refer to those structures[11]. Furthermore, LVI can be confirmed specifically on HE-stained specimens[13]. In our series, LVI was confirmed histopathologically in 29 patients.
It is well recognized that resection margin status is the most important factor affecting long-term survival outcomes of HC patients[14,15], and positive resection margin remains a major dismal prognostic factor for type IV HC patients. Furthermore, since type IV HC had been considered unresectable[16], radical resection performed in such patients is technically challenging. Bracingly, in our center the R0 resection rate is 75.4%. Regional lymph node involvement represents another important dismal prognostic factor in HC patients who had undergone radical resection[17,18]. Concordantly, the multivariate analysis showed that lymph node metastasis was an adverse factor affecting DFS and OS in type IV HC. Furthermore, DeOliveira et al[19] proposed a new staging system, in which tumor size > 30 mm was defined as the T3 stage. The choice of 30 mm as a cutoff value for T3 is based on increasing evidence that the smaller the tumor, the better the prognosis[20,21]. Our results indicated that tumor size > 30 mm had an unfavorable impact on both DFS and OS. More importantly, we found that LVI is a significant adverse prognostic factor influencing DFS and OS in multivariate analysis. To our knowledge, no other reports have shown the correlation between LVI and the prognosis of type IV HC.
Previous studies suggested that LVI may interact with other adverse risk factors, which then have a dismal impact on the OS of esophageal cancer patients[22,23]. Lee et al[24] found that the presence of LVI correlated with the presence of lymph node metastasis in patients with gastric carcinoma. For different types of cholangiocarcinoma, the effect of LVI on prognosis was also different. Kim et al[25] reported that LVI did not influence the survival of patients with distal cholangiocarcinoma. Fisher et al[26] reported that LVI had an adverse influence on survival of patients with intrahepatic cholangiocarcinoma. Both of studies found that LVI was associated with lymph node metastasis[25,26]. In our analysis, N1 stage was a significant factor that was associated with LVI in type IV HC patients. It is generally known that tumor cells and tumor stromal cells (such as macrophages and thrombocytes) can produce pro-lymphangiogenic factors, which increase lymphovascular density in and around the tumors[27,28], mainly peritumoral regions. Lymph node metastases often occurred in tumors lacking intratumoral functional lymphatics, suggesting that functional lymphatics at the peritumoral regions are the route of lymphatic dissemination. Increased peritumoral lymphovascular density is considered to increase the flow of lymphatic fluid and provide an opportunity for invasive tumor cells to access the lymphatic vessels[29]. This may elucidate our results that LVI is closely related to lymph node metastasis. Moreover, we speculated that LVI may be the precursor of lymph node metastasis. Thus, we divided patients without lymph nodes metastases into LVI positive and LVI negative subgroups, and found that the 5-year DFS and OS rates in the LVI negative group were significantly higher than those in the LVI positive group (Figure 2A and B). This result indicates that LVI is an admirable prognostic predictor for patients with type IV HC when lymph node metastasis is absent.
Additionally, tumor size > 30 mm was another significant factor correlated with LVI in our series. Tumor size is recognized as a staging basis for many malignant tumors, including thyroid carcinoma, breast carcinoma, and liver carcinoma among others. Gurleyik et al[30] reported that LVI correlated with tumor size, and the rate of LVI positive increased with tumor size in patients with breast carcinoma. The significant correlation between LVI and tumor size could be explained through two potential aspects: (1) as the tumor size increases, the peritumoral areas increase. Thus, the tumor is endowed with the potential to make contact with an increasing lymphovasculature, and thus the possibility of LVI increases; and (2) tumor size is proportional to the time of growth: the larger the tumor, the greater the duration of time that the tumor can continue developing and growing in size. During a relatively longer growth time, a tumor has increasing opportunities to develop LVI. In our study, patients with tumor size ≤ 30 mm were also divided into LVI positive and LVI negative subgroups. Here, the 5-year DFS and OS rates in the LVI negative group were significantly higher than those found in the LVI positive group (Figure 3A and B). This result indicates that LVI is also an excellent prognostic predictor in type IV HC patients with smaller tumor size.
Some limitations of the study should also be taken into account when interpreting the results. First, our study was retrospective with inherent limitations in its design. Thus, some clinical bias was inevitable. Next, LVI was confirmed by hematoxylin and eosin (HE) staining alone without application of an immunohistochemical staining with D2-40 antibody, which may improve the detection rate of LVI[31]. Third, N1 stage and tumor size > 30 mm might serve as potential confounding factors that could affect the association between LVI and the eventual prognosis. Finally, we did not carry out preclinical medical experiments to elaborate the specific molecular mechanism that could play a key role in the capacity of LVI to affect the prognosis of type IV HC patients. Future research should take into account this topic with a greater sample size. Prospective studies, even randomized controlled trials, are also urgently needed. Of course, the specific molecular mechanism responsible for LVI affecting the prognosis of type IV HC patients will be determined by empirical research.
In conclusion, the presence of LVI may be regarded as an indicator of biologically aggressive behavior, metastatic ability, and regional and systemic risk of metastasizing the primary malignancy. LVI is associated with N1 stage and tumor size > 30 mm and imparts an adverse influence on OS and DFS in type IV HC patients who received radical resection.
Despite advances in surgical techniques and that resection rates for Bismuth type IV hilar cholangiocarcinoma (HC) continue to increase, the prognosis of patients with type IV HC remains unsatisfactory. The reasons for this remain unclear and seem to be complex and multifactorial. Lymphovascular invasion (LVI) is associated with a poorer prognosis in patients with various malignances. This study sought to investigate whether LVI could predict type IV HC prognosis.
This study estimates prognostic factors that might be associated with overall survival (OS) and disease-free survival (DFS) after radical resection in type IV HC patients. To our knowledge, this study represents the first clinical insight indicating that LVI is associated with the prognosis of type IV HC.
This findings confirmed that R1 resection, N1 stage, presence of LVI and tumor size > 30 mm were adverse prognostic predictors for type IV HC patients after radical resection. Further, LVI was found to be associated with N1 stage and tumor size > 30 mm and adversely influence OS and DFS.
Observations from the present study might formally indicate novel factors for predicting post-surgical survival in HC. Moreover, LVI might present a potentially novel target for developing anti-cancer strategies.
HC is a neoplasia arising from the biliary epithelium at the common hepatic duct bifurcation that might extend to the intrahepatic biliary tree and liver. Bismuth-Corlette classification is the most commonly used HC typing system, which is often used by surgeons to develop preliminary surgical protocols.
This is a retrospective study evaluating the effect of LVI on the prognosis of Bismuth-Corlette type IV HC. The authors concluded that LVI had an adverse influence on the prognosis of patients with Bismuth-Corlette type IV HC. This manuscript is very interesting and well written.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Hashimoto T, Kawakubo K S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Huang Y
| 1. | Xiong J, Nunes QM, Huang W, Wei A, Ke N, Mai G, Liu X, Hu W. Major hepatectomy in Bismuth types I and II hilar cholangiocarcinoma. J Surg Res. 2015;194:194-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, Choti MA, Yeo CJ, Schulick RD. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 882] [Cited by in RCA: 1015] [Article Influence: 56.4] [Reference Citation Analysis (1)] |
| 3. | Ji GW, Zhu FP, Wang K, Jiao CY, Shao ZC, Li XC. Clinical Implications of Biliary Confluence Pattern for Bismuth-Corlette Type IV Hilar Cholangiocarcinoma Applied to Hemihepatectomy. J Gastrointest Surg. 2017;21:666-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Kow AW, Wook CD, Song SC, Kim WS, Kim MJ, Park HJ, Heo JS, Choi SH. Role of caudate lobectomy in type III A and III B hilar cholangiocarcinoma: a 15-year experience in a tertiary institution. World J Surg. 2012;36:1112-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Furusawa N, Kobayashi A, Yokoyama T, Shimizu A, Motoyama H, Miyagawa S. Surgical treatment of 144 cases of hilar cholangiocarcinoma without liver-related mortality. World J Surg. 2014;38:1164-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Nagino M, Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y, Nimura Y. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg. 2013;258:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 497] [Article Influence: 41.4] [Reference Citation Analysis (1)] |
| 7. | Govil S, Reddy MS, Rela M. Surgical resection techniques for locally advanced hilar cholangiocarcinoma. Langenbecks Arch Surg. 2014;399:707-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Ito F, Cho CS, Rikkers LF, Weber SM. Hilar cholangiocarcinoma: current management. Ann Surg. 2009;250:210-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 169] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 9. | Xiang S, Lau WY, Chen XP. Hilar cholangiocarcinoma: controversies on the extent of surgical resection aiming at cure. Int J Colorectal Dis. 2015;30:159-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Groot Koerkamp B, Wiggers JK, Allen PJ, Busch OR, D’Angelica MI, DeMatteo RP, Fong Y, Gonen M, Gouma DJ, Kingham TP. American Joint Committee on Cancer staging for resected perihilar cholangiocarcinoma: a comparison of the 6th and 7th editions. HPB (Oxford). 2014;16:1074-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Hoda SA, Hoda RS, Merlin S, Shamonki J, Rivera M. Issues relating to lymphovascular invasion in breast carcinoma. Adv Anat Pathol. 2006;13:308-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Lee JH, Jang HS, Kim JG, Cho HM, Shim BY, Oh ST, Yoon SC, Kim YS, Choi BO, Kim SH. Lymphovascular invasion is a significant prognosticator in rectal cancer patients who receive preoperative chemoradiotherapy followed by total mesorectal excision. Ann Surg Oncol. 2012;19:1213-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Lagarde SM, Phillips AW, Navidi M, Disep B, Immanuel A, Griffin SM. The presence of lymphovascular and perineural infiltration after neoadjuvant therapy and oesophagectomy identifies patients at high risk for recurrence. Br J Cancer. 2015;113:1427-1433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Natsume S, Ebata T, Yokoyama Y, Igami T, Sugawara G, Shimoyama Y, Nagino M. Clinical significance of left trisectionectomy for perihilar cholangiocarcinoma: an appraisal and comparison with left hepatectomy. Ann Surg. 2012;255:754-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | Nuzzo G, Giuliante F, Ardito F, Giovannini I, Aldrighetti L, Belli G, Bresadola F, Calise F, Dalla Valle R, D’Amico DF. Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: results of an Italian multicenter analysis of 440 patients. Arch Surg. 2012;147:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 205] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 16. | Croome KP, Rosen CB, Heimbach JK, Nagorney DM. Is Liver Transplantation Appropriate for Patients with Potentially Resectable De Novo Hilar Cholangiocarcinoma? J Am Coll Surg. 2015;221:130-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Guglielmi A, Ruzzenente A, Campagnaro T, Pachera S, Conci S, Valdegamberi A, Sandri M, Iacono C. Prognostic significance of lymph node ratio after resection of peri-hilar cholangiocarcinoma. HPB (Oxford). 2011;13:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Giuliante F, Ardito F, Guglielmi A, Aldrighetti L, Ferrero A, Calise F, Giulini SM, Jovine E, Breccia C, De Rose AM. Association of Lymph Node Status With Survival in Patients After Liver Resection for Hilar Cholangiocarcinoma in an Italian Multicenter Analysis. JAMA Surg. 2016;151:916-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 19. | Deoliveira ML, Schulick RD, Nimura Y, Rosen C, Gores G, Neuhaus P, Clavien PA. New staging system and a registry for perihilar cholangiocarcinoma. Hepatology. 2011;53:1363-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 208] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 20. | Wang ST, Shen SL, Peng BG, Hua YP, Chen B, Kuang M, Li SQ, He Q, Liang LJ. Combined vascular resection and analysis of prognostic factors for hilar cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2015;14:626-632. [PubMed] |
| 21. | Hu HJ, Mao H, Shrestha A, Tan YQ, Ma WJ, Yang Q, Wang JK, Cheng NS, Li FY. Prognostic factors and long-term outcomes of hilar cholangiocarcinoma: A single-institution experience in China. World J Gastroenterol. 2016;22:2601-2610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Wang S, Chen X, Fan J, Lu L. Prognostic Significance of Lymphovascular Invasion for Thoracic Esophageal Squamous Cell Carcinoma. Ann Surg Oncol. 2016;23:4101-4109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Chen WH, Huang YL, Chao YK, Yeh CJ, Chang HK, Tseng CK, Liu YH. Prognostic significance of lymphovascular invasion in patients with esophageal squamous cell carcinoma treated with neoadjuvant chemoradiotherapy. Ann Surg Oncol. 2015;22:338-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Lee K, Park DJ, Choe G, Kim HH, Kim WH, Lee HS. Increased intratumoral lymphatic vessel density correlates with lymph node metastasis in early gastric carcinoma. Ann Surg Oncol. 2010;17:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Kim HJ, Kim CY, Hur YH, Koh YS, Kim JC, Kim HJ, Cho CK. Prognostic factors for survival after curative resection of distal cholangiocarcinoma: perineural invasion and lymphovascular invasion. Surg Today. 2014;44:1879-1886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Fisher SB, Patel SH, Kooby DA, Weber S, Bloomston M, Cho C, Hatzaras I, Schmidt C, Winslow E, Staley CA 3rd, Maithel SK. Lymphovascular and perineural invasion as selection criteria for adjuvant therapy in intrahepatic cholangiocarcinoma: a multi-institution analysis. HPB (Oxford). 2012;14:514-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Schlereth SL, Refaian N, Iden S, Cursiefen C, Heindl LM. Impact of the prolymphangiogenic crosstalk in the tumor microenvironment on lymphatic cancer metastasis. Biomed Res Int. 2014;2014:639058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Rofstad EK, Huang R, Galappathi K, Andersen LM, Wegner CS, Hauge A, Gaustad JV, Simonsen TG. Functional intratumoral lymphatics in patient-derived xenograft models of squamous cell carcinoma of the uterine cervix: implications for lymph node metastasis. Oncotarget. 2016;7:56986-56997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Harrell MI, Iritani BM, Ruddell A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am J Pathol. 2007;170:774-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 297] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 30. | Gurleyik G, Gurleyik E, Aker F, Aktekin A, Emir S, Gungor O, Saglam A. Lymphovascular invasion, as a prognostic marker in patients with invasive breast cancer. Acta Chir Belg. 2007;107:284-287. [PubMed] |
| 31. | Weber SK, Sauerwald A, Pölcher M, Braun M, Debald M, Serce NB, Kuhn W, Brunagel-Walgenbach G, Rudlowski C. Detection of lymphovascular invasion by D2-40 (podoplanin) immunoexpression in endometrial cancer. Int J Gynecol Cancer. 2012;22:1442-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |