Published online Sep 14, 2017. doi: 10.3748/wjg.v23.i34.6287
Peer-review started: February 24, 2017
First decision: April 7, 2017
Revised: May 5, 2017
Accepted: July 12, 2017
Article in press: July 12, 2017
Published online: September 14, 2017
Processing time: 203 Days and 16 Hours
To evaluate the association of body mass index (BMI) with the overall survival of pancreatic ductal adenocarcinoma (PDAC) patients.
A retrospective analysis of PDAC patients diagnosed in the National Cancer Center of China between January 1999 and December 2014 was performed. These patients were categorized into four BMI groups (< 18.5, 18.5-22.9, 23-27.4 and ≥ 27.5 kg/m2). χ2 tests for comparison of the proportions of categorical variables, and Student’s t-test or Mann-Whitney test for continuous variables were employed. Survival analysis was performed with the Kaplan-Meyer method. Their HRs of mortality and 95%CIs were estimated using the Cox proportional hazards model.
With a median age of 59.6 years (range: 22.5-84.6 years), in total 1783 PDAC patients were enrolled in this study. Their mean usual BMI was 24.19 ± 3.53 for the whole cohort. More than half of the patients (59.3%) experienced weight loss during the disease onset and progression. Compared with healthy-weight individuals, newly diagnosed patients who were overweight or obese had more severe weight loss during their disease onset and progression (P < 0.001). Individuals who were overweight or obese were associated with positive smoking history (P < 0.001). A significant difference in comorbidity of diabetes (P = 0.044) and coronary artery disease (P < 0.001) was identified between high BMI and normal-weight patients. After a median follow-up of 8 mo, the survival analysis showed no association between BMI and the overall survival (P = 0.90, n = 1783). When we stratified the whole cohort by pancreatic cancer stage, no statistically significant association between BMI and overall survival was found for resectable (P = 0.99, n = 217), unresectable locally advanced (P = 0.90, n = 316) and metastatic patients (P = 0.88, n = 1250), respectively. The results did not change when we used the BMI at diagnosis.
Our results showed no significance of BMI for the overall survival of PDAC patients.
Core tip: It remains controversial whether body mass index (BMI) influences the prognosis of pancreatic cancer. The strengths of this study included a large quantity of patients and the accurate BMI categorization according the Asian criterion. To the best of our knowledge, it is the largest study in Asia to evaluate the prognostic role of BMI on the overall survival of pancreatic cancer patients. Our results showed no significant influence of BMI on the overall survival of pancreatic ductal adenocarcinoma patients and are consistent with those of many other studies.
- Citation: Jiang QL, Wang CF, Tian YT, Huang H, Zhang SS, Zhao DB, Ma J, Yuan W, Sun YM, Che X, Zhang JW, Chu YM, Zhang YW, Chen YT. Body mass index does not affect the survival of pancreatic cancer patients. World J Gastroenterol 2017; 23(34): 6287-6293
- URL: https://www.wjgnet.com/1007-9327/full/v23/i34/6287.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i34.6287
Pancreatic cancer is a devastating malignant cancer, with a 5-year survival rate of < 6%[1]. The survival length of patients is greatly influenced by disease stage at diagnosis, but few other survival markers have been well characterized[2-4].
Excessive adipose can impair both humoral and cellular immunity, which may be important in the development of pancreatic cancer. Although many studies have demonstrated that a high body mass index (BMI), an indirect measure of adiposity, is associated with increased risk of developing pancreatic cancer, it remains controversial whether BMI influences the prognosis of pancreatic cancer. Several studies have reported that a higher BMI is associated with decreased survival of pancreatic cancer patients[5-10], whereas other results informed that BMI was not a statistically significant prognostic factor[11-15]. Tsai et al[16] even expressed improved long-term survival of pancreatic cancer patients with higher BMIs. As such, we conducted a single-center, large-scale retrospective study to determine the associations between BMI and overall survival of Chinese pancreatic ductal adenocarcinoma (PDAC) patients.
The medical records of all patients diagnosed with PDAC from January 1999 to December 2014 in the Cancer Hospital, Chinese Academy of Medical Science and Peking Union Medical College were studied by retrospective chart review. All patients had histological diagnosis of PDAC confirmed by pathology. Clinical information was systematically abstracted from these patients’ medical records using an established abstraction form by trained individuals with a medical background. For the whole cohort, the extracted information included age, sex, weight, height, smoking history, alcohol use history, previous history of cancer, and previous abdominal surgery. The patients’ height and weight of 1 year prior to diagnosis and at diagnosis were all recorded. Comorbidities were also recorded, such as diabetes, coronary artery disease and hypertension. Clinical disease stage was defined on the basis of the patients’ initial computed tomographic images and endoscopic ultrasound reports, as stated in the American Joint Committee on Cancer Staging Manual using the TNM staging system. Follow-up information was obtained through telephone-based interviews, as well as from the records of outpatient follow-up, if any. The primary outcome variable was overall survival, defined for patients as the period from date of diagnosis to date of death. Informed consent was obtained from all individual participants included in the study.
BMI was calculated as weight (kg) divided by the square of height (m2), using self-reported usual adult height and weight. According to the Asian criterion, BMI was classified as underweight (< 18.5 kg/m2), healthy weight (18.5-22.9 kg/m2), overweight (23.0-27.4 kg/m2), and obese (≥ 27.5 kg/m2). χ2 tests for comparison of the proportions of categorical variables, and Student’s t-test or Mann-Whitney test for continuous variables were employed. The unconditional logistic regression model was used to estimate the ORs and 95%CIs for the associations among BMI, clinical characteristics and overall survival of PDAC patients, and for the PDAC subtypes in different clinical stages. Survival analysis was performed by the Kaplan-Meyer method. The group with BMI 18.5-22.9 kg/m2 was the reference group. All P-values presented were two-sided, and all analyses were carried out using SAS Software Version 9.2 (SAS Institute, Cary, NC, United States). Statistical significance was defined as a P value of < 0.05.
Data were extracted from a total of 1783 subjects with PDAC who had complete medical history. Histological or cytological diagnosis was obtained from the primary pancreatic lesion or metastases in all cases. The pathology was confirmed by two pathologists. Baseline characteristics of 1783 patients with pancreatic cancer are listed in Table 1. Of those subjects, 1009 (56.6%) were male, with a median age of 59.6 years. Among the total, 29.6% patients had a positive smoking history, 22.3% had a drinking history, and 22.3%, 13.9% and 15.0% patients had diabetes, coronary artery disease and hypertension at diagnosis of pancreatic cancer as concomitant comorbidities, respectively.
| Characteristic | All patients | BMI category, kg/m2 | P value | |||
| < 18.5 | 18.5-22.9 | 23-27.4 | ≥ 27.5 | |||
| Patients | 1783 | 98 (5.5) | 588 (33.0) | 791 (44.4) | 306 (17.2) | |
| Sex | 0.97 | |||||
| Male | 1009 (56.6) | 54 (3.0) | 334 (18.7) | 445 (25.0) | 176 (9.9) | |
| Female | 774 (43.4) | 44 (2.5) | 254 (14.2) | 346 (19.4) | 130 (7.3) | |
| Male/female ratio | 1.30 | 1.23 | 1.31 | 1.29 | 1.35 | |
| BMI as kg/m2, mean ± SD | 24.19 ± 3.53 | 17.60 ± 0.84 | 21.16 ± 1.23 | 25.07 ± 1.29 | 29.68 ± 2.11 | |
| Age at diagnosis in yr | ||||||
| Median | 59.6 | 62.3 | 59.0 | 59.6 | 59.7 | |
| Range | 22.5-84.6 | 28.7-81.7 | 22.5-80.2 | 30.7-84.6 | 26.0-84.6 | |
| Weight loss as % of usual adult weight | ||||||
| None | 725 (40.7) | 61 (3.4) | 333 (18.7) | 299 (16.8) | 32 (1.8) | |
| > 0, ≤ 10% | 652 (36.6) | 23 (1.3) | 190 (10.7) | 321 (18.0) | 118 (6.6) | |
| > 10% | 406 (22.8) | 14 (0.79) | 65 (3.6) | 171 (9.6) | 156 (8.7) | < 0.0001 |
| Any weight loss | 1058 (59.3) | 37 (2.1) | 255 (14.3) | 492 (27.6) | 274 (15.4) | < 0.0001 |
| Smoking | 0.0003 | |||||
| Never | 1243 (69.7) | 72 (4.1) | 408 (22.9) | 568 (31.9) | 195 (10.9) | |
| Ever | 527 (29.6) | 22 (1.2) | 176 (9.9) | 220 (12.3) | 109 (6.1) | |
| Alcohol | 0.42 | |||||
| Never | 1377 (77.2) | 76 (4.3) | 465 (26.1) | 614 (34.4) | 222 (12.5) | |
| Ever | 398 (22.3) | 21 (1.2) | 121 (6.8) | 174 (9.8) | 82 (4.6) | |
| Comorbidity | ||||||
| Diabetes | 398 (22.3) | 13 (0.7) | 125 (7.0) | 196 (11.0) | 64 (3.6) | 0.044 |
| Coronary artery disease | 247 (13.9) | 8 (0.4) | 46 (2.6) | 166 (9.3) | 27 (1.5) | < 0.0001 |
| Hypertension | 267 (15.0) | 9 (0.5) | 88 (4.9) | 111 (6.2) | 59 (3.3) | 0.055 |
| Previous history of cancer | 14 (0.8) | 1 (0.06) | 6 (0.34) | 6 (0.34) | 1 (0.06) | 0.72 |
| Previous abdominal surgery | 87 (4.9) | 7 (0.39) | 34 (1.9) | 34 (1.9) | 12 (0.67) | 0.35 |
| Stage group | 0.89 | |||||
| Resectable | 217 (12.2) | 15 (0.84) | 73 (4.1) | 93 (5.2) | 36 (2.0) | |
| Unresectable locally advanced | 316 (17.7) | 17 (0.95) | 108 (6.1) | 143 (8.0) | 48 (2.7) | |
| Metastatic | 1250 (70.1) | 66 (3.7) | 407 (22.8) | 555 (31.1) | 222 (12.5) | |
Among those with known disease stage, 217 had potentially resectable tumors at the time of diagnosis, 316 had locally advanced disease, and 1250 had distant metastasis. Their mean baseline BMI was 24.19 kg/m2, and 44.4% of the patients were classified as overweight and 17.2% as obese. More than half of the patients (59.3%) experienced weight loss during their disease onset progression. Compared with healthy-weight individuals, the newly diagnosed patients who were overweight or obese had more severe weight loss during the disease onset progression (P < 0.001). Individuals who were overweight or obese are associated with a positive smoking history (P = 0.0003). A significant difference in comorbidity of diabetes (P = 0.044) and coronary artery disease (P < 0.001) was identified between high BMI values and normal-weight patients.
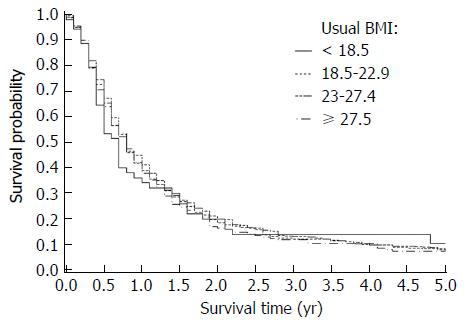
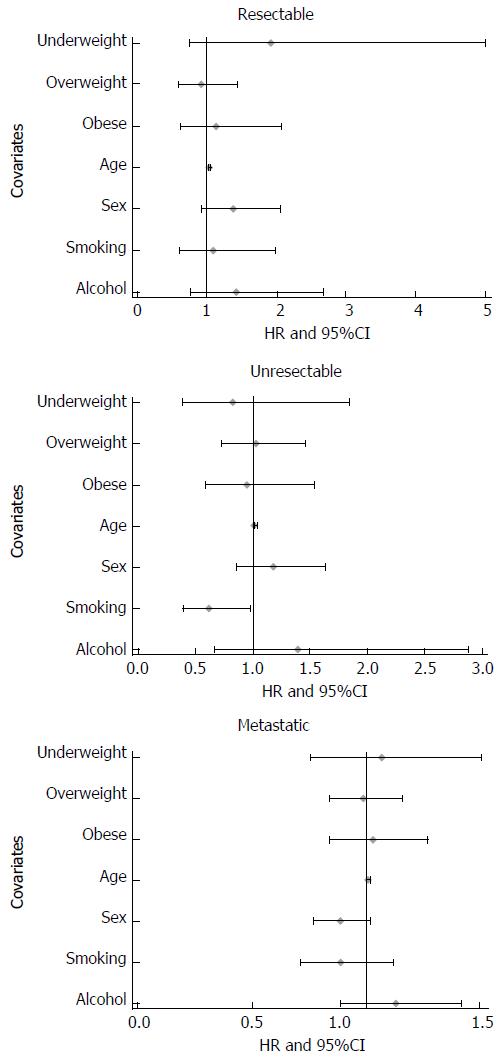
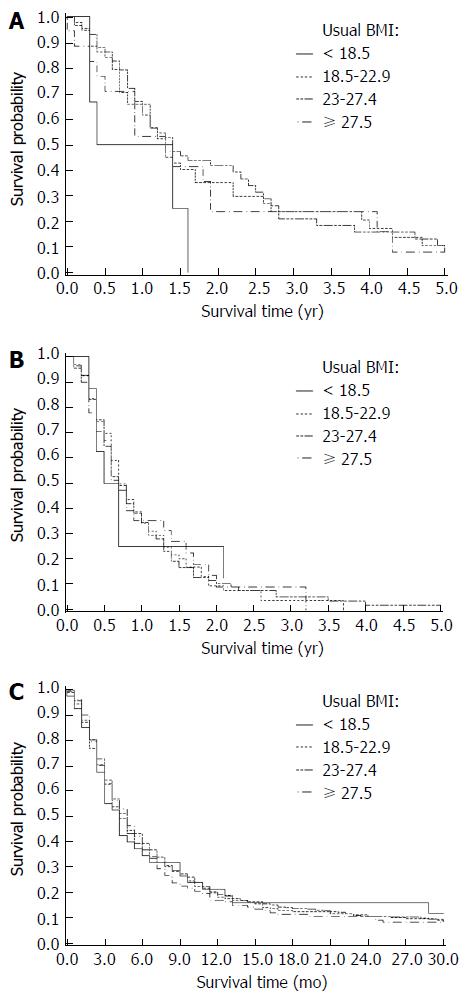
The median follow-up duration was 8 mo for all cases. Median overall survival for each BMI category (kg/m2) was as follows: < 18.5: 8 mo; 18.5-22.9: 9.7 mo; 23.0-27.4: 9.5 mo; and ≥ 27.5: 9.8 mo (Table 2). Kaplan-Meier survival comparisons (Figure 1) showed no association between BMI and the overall survival of pancreatic cancer patients (P > 0.05). When we stratified the whole cohort by pancreatic cancer stage, no statistically significant association between BMI and overall survival was found for resectable (P = 0.99, n = 217), unresectable locally advanced (P = 0.90, n = 316) and metastatic (P = 0.88, n = 1250) patients. The multivariate Cox regression survival analysis showed the same results (Figures 2 and 3). Considering the effect of cancer-related weight loss among patients, the results did not change when we used the BMI at diagnosis.
| Stage group | BMI category | n | Median overall survival, mo (95%CI) | HR | 95%CI | P value | Ptrend value |
| Overall | < 18.5 | 98 | 8.0 (5.8-11.3) | 1.12 | 0.83-1.52 | 0.45 | |
| 18.5-22.9 | 588 | 9.7 (8.7-11.1) | 1.00 | Ref. | Ref. | ||
| 23-27.4 | 791 | 9.5 (8.6-10.5) | 0.99 | 0.87-1.14 | 0.92 | ||
| ≥ 27.5 | 306 | 9.8 (7.6-11.2) | 1.05 | 0.88-1.25 | 0.61 | 0.90 | |
| Resectable | < 18.5 | 15 | 11.1 (3.3-19.3) | 1.92 | 0.74-5.01 | 0.18 | |
| 18.5-22.9 | 73 | 15.6 (10.1-21.3) | 1.00 | Ref. | Ref. | ||
| 23-27.4 | 93 | 16.7 (11.9-27.4) | 0.92 | 0.59-1.44 | 0.71 | ||
| ≥ 27.5 | 36 | 16.2 (4.5-23.7) | 1.13 | 0.61-2.08 | 0.70 | 0.99 | |
| Unresectable locally advanced | < 18.5 | 17 | 7.0 (3.4-26.1) | 0.83 | 0.38-1.84 | 0.65 | |
| 18.5-22.9 | 108 | 8.7 (7.3-11.7) | 1.00 | Ref. | Ref. | ||
| 23-27.4 | 143 | 8.6 (6.8-11.5) | 1.03 | 0.73-1.45 | 0.86 | ||
| ≥ 27.5 | 48 | 8.6 (4.9-15.6) | 0.95 | 0.59-1.54 | 0.84 | 0.90 | |
| Metastatic | < 18.5 | 66 | 4.9 (2.8-8.0) | 1.07 | 0.76-1.51 | 0.70 | |
| 18.5-22.9 | 407 | 5.5 (4.0-6.4) | 1.00 | Ref. | Ref. | ||
| 23-27.4 | 555 | 5.7 (3.7-6.8) | 0.99 | 0.84-1.16 | 0.89 | ||
| ≥ 27.5 | 222 | 5.6 (3.3-7.7) | 1.02 | 0.84-1.27 | 0.76 | 0.88 |
Overweight/obesity and its related morbidities represent a growing health problem, claiming 2.8 million lives annually according to the WHO 2010 Global Report[17]. Obesity has been found to be associated with a wide array of morbidities, including cardiovascular disease, chronic kidney disease, sleeping disorder, type 2 diabetes, and several forms of cancer. Obesity is considered to be an adverse prognostic factor in malignancies, such as breast and colon cancers[18,19]. In our study, a significant difference in comorbidity of diabetes and coronary artery disease was identified between high BMI values and normal-weight patients. But survival analysis showed that BMI did not affect the survival of pancreatic cancer patients.
Previous studies conferring the role of BMI were mainly carried out in cohorts of United States adults, which presented a higher percentage of obese (BMI ≥ 30) and very obese (BMI ≥ 35) patients and the BMI categorization as opposed to our Asian criterion. For a Mayo Clinic cohort of patients covering all stages of pancreatic cancer respectively, including early-stage patients who underwent surgery, McWilliams et al[6] reported that the BMI at diagnosis had a negative impact on survival. This was particularly pronounced in the very obese patients with a BMI of 35 to 39.99 kg/m2 (HR = 1.32, 95%CI: 1.08-1.62) and > 40 kg/ m2 (HR = 1.60, 95%CI: 1.26-2.04), respectively. In a case control study designed to assess the risk of developing pancreatic cancer in overweight persons, Li et al[5] described a shorter survival period for patients who were overweight or obese during the year prior to diagnosis. Another study from Olson et al[11] demonstrated that obesity was non-significantly associated with poorer survival of PDAC patients, particularly in the resected group (HR = 1.62, 95%CI: 0.76-3.44). The experience from Chen et al[20] showed that a higher BMI increases the risk for postoperative complications after pancreatectomy in the Chinese population. However, Tsai et al[16] reported that obese patients undergoing pancreaticoduodenectomy for pancreatic cancer had improved long-term survival, independent of known clinicopathologic factors. Big controversy has lasted about the real role of BMI on pancreatic cancer for years. The results in this study need to be replicated in more studies with larger sample sizes with a greater power.
Limited information was available on the possible effect of weight loss on the survival of PDAC patients. As we know, loss of weight is usual for pancreatic cancer patients either before or after diagnosis. In our study, more than half of the patients experienced weight loss during their disease progression, especially in the obese group, which might explain why the obesity-specific co-morbidities such as diabetes were not more prevalent in the obese group compared with the normal populations. Dalal et al[21] believed that obese patients experienced higher loss in weight, skeletal muscle and visceral adipose tissue, which may contribute to poorer survival of pancreatic cancer patients. So, we carried out the survival analysis using patients’ BMIs at usual time as well as at diagnosis, but the results did not change.
The strengths of this study included a large quantity of patients and the accurate BMI categorization according the Asian criterion. To the best of our knowledge, it is the largest study in Asia to evaluate the prognostic role of BMI on the overall survival of pancreatic cancer patients. Our results showed no significant influence of BMI on the overall survival of PDAC patients and are consistent with those of many other studies[11-15]. We, however, must admit that there are several limitations for the current study. The retrospective nature of this study can be associated with selection bias as well as increased risk of differential misclassification bias. Another shortcoming was the limited generalizability because the entire study population came from a single hospital.
In conclusion, BMI has not shown any clear relationship with the survival outcome of pancreatic cancer. More studies are needed to validate this finding and evaluate the mechanism behind the observation.
Excessive adipose can impair both humoral and cellular immunity, which may be important in the development of pancreatic cancer. Although many studies have demonstrated that a high body mass index (BMI), an indirect measure of adiposity, is associated with increased risk of developing pancreatic cancer, it remains controversial whether BMI influences the prognosis of pancreatic cancer. Several studies have reported that a higher BMI is associated with decreased survival of pancreatic cancer patients, whereas other results informed that BMI was not a statistically significant prognostic factor.
The authors conducted a single-center, large-scale retrospective study to determine the associations between BMI and overall survival of Chinese pancreatic ductal adenocarcinoma (PDAC) patients.
To the best of our knowledge, this is the largest study in Asia to evaluate the prognostic role of BMI on the overall survival of pancreatic cancer patients.
In this study, BMI did not affect the survival of pancreatic cancer patients. The results provide more insight into the role of BMI on pancreatic cancer.
The present retrospective study demonstrated that BMI has no relationship with survival of PDAC in a large single center. The paper has high value for inclusion in the current literature on the topic.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cesaretti M, Luyer M S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Zhang FF
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25545] [Article Influence: 1824.6] [Reference Citation Analysis (7)] |
| 2. | Zou L, Zhong R, Shen N, Chen W, Zhu B, Ke J, Lu X, Zhang T, Lou J, Wang Z. Non-linear dose-response relationship between cigarette smoking and pancreatic cancer risk: evidence from a meta-analysis of 42 observational studies. Eur J Cancer. 2014;50:193-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Elena JW, Steplowski E, Yu K, Hartge P, Tobias GS, Brotzman MJ, Chanock SJ, Stolzenberg-Solomon RZ, Arslan AA, Bueno-de-Mesquita HB. Diabetes and risk of pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium. Cancer Causes Control. 2013;24:13-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 4. | Jaacks LM, Gordon-Larsen P, Mayer-Davis EJ, Adair LS, Popkin B. Age, period and cohort effects on adult body mass index and overweight from 1991 to 2009 in China: the China Health and Nutrition Survey. Int J Epidemiol. 2013;42:828-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Li D, Morris JS, Liu J, Hassan MM, Day RS, Bondy ML, Abbruzzese JL. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA. 2009;301:2553-2562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 347] [Cited by in RCA: 321] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 6. | McWilliams RR, Matsumoto ME, Burch PA, Kim GP, Halfdanarson TR, de Andrade M, Reid-Lombardo K, Bamlet WR. Obesity adversely affects survival in pancreatic cancer patients. Cancer. 2010;116:5054-5062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Yuan C, Bao Y, Wu C, Kraft P, Ogino S, Ng K, Qian ZR, Rubinson DA, Stampfer MJ, Giovannucci EL. Prediagnostic body mass index and pancreatic cancer survival. J Clin Oncol. 2013;31:4229-4234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 8. | Kasenda B, Bass A, Koeberle D, Pestalozzi B, Borner M, Herrmann R, Jost L, Lohri A, Hess V. Survival in overweight patients with advanced pancreatic carcinoma: a multicentre cohort study. BMC Cancer. 2014;14:728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Shi YQ, Yang J, Du P, Xu T, Zhuang XH, Shen JQ, Xu CF. Effect of Body Mass Index on Overall Survival of Pancreatic Cancer: A Meta-Analysis. Medicine (Baltimore). 2016;95:e3305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Fleming JB, Gonzalez RJ, Petzel MQ, Lin E, Morris JS, Gomez H, Lee JE, Crane CH, Pisters PW, Evans DB. Influence of obesity on cancer-related outcomes after pancreatectomy to treat pancreatic adenocarcinoma. Arch Surg. 2009;144:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Olson SH, Chou JF, Ludwig E, O’Reilly E, Allen PJ, Jarnagin WR, Bayuga S, Simon J, Gonen M, Reisacher WR. Allergies, obesity, other risk factors and survival from pancreatic cancer. Int J Cancer. 2010;127:2412-2419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Gong Z, Holly EA, Bracci PM. Obesity and survival in population-based patients with pancreatic cancer in the San Francisco Bay Area. Cancer Causes Control. 2012;23:1929-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Pelucchi C, Galeone C, Polesel J, Manzari M, Zucchetto A, Talamini R, Franceschi S, Negri E, La Vecchia C. Smoking and body mass index and survival in pancreatic cancer patients. Pancreas. 2014;43:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Dandona M, Linehan D, Hawkins W, Strasberg S, Gao F, Wang-Gillam A. Influence of obesity and other risk factors on survival outcomes in patients undergoing pancreaticoduodenectomy for pancreatic cancer. Pancreas. 2011;40:931-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Gaujoux S, Torres J, Olson S, Winston C, Gonen M, Brennan MF, Klimstra DS, D’Angelica M, DeMatteo R, Fong Y. Impact of obesity and body fat distribution on survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg Oncol. 2012;19:2908-2916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Tsai S, Choti MA, Assumpcao L, Cameron JL, Gleisner AL, Herman JM, Eckhauser F, Edil BH, Schulick RD, Wolfgang CL. Impact of obesity on perioperative outcomes and survival following pancreaticoduodenectomy for pancreatic cancer: a large single-institution study. J Gastrointest Surg. 2010;14:1143-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Esteghamati A, Mazaheri T, Vahidi Rad M, Noshad S. Complementary and alternative medicine for the treatment of obesity: a critical review. Int J Endocrinol Metab. 2015;13:e19678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Dawood S, Broglio K, Gonzalez-Angulo AM, Kau SW, Islam R, Hortobagyi GN, Cristofanilli M. Prognostic value of body mass index in locally advanced breast cancer. Clin Cancer Res. 2008;14:1718-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Siegel EM, Ulrich CM, Poole EM, Holmes RS, Jacobsen PB, Shibata D. The effects of obesity and obesity-related conditions on colorectal cancer prognosis. Cancer Control. 2010;17:52-57. [PubMed] |
| 20. | Chen YT, Deng Q, Che X, Zhang JW, Chen YH, Zhao DB, Tian YT, Zhang YW, Wang CF. Impact of body mass index on complications following pancreatectomy: Ten-year experience at National Cancer Center in China. World J Gastroenterol. 2015;21:7218-7224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Dalal S, Hui D, Bidaut L, Lem K, Del Fabbro E, Crane C, Reyes-Gibby CC, Bedi D, Bruera E. Relationships among body mass index, longitudinal body composition alterations, and survival in patients with locally advanced pancreatic cancer receiving chemoradiation: a pilot study. J Pain Symptom Manage. 2012;44:181-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |