Published online Aug 28, 2017. doi: 10.3748/wjg.v23.i32.5895
Peer-review started: January 6, 2017
First decision: March 16, 2017
Revised: March 30, 2017
Accepted: July 22, 2017
Article in press: July 24, 2017
Published online: August 28, 2017
Processing time: 235 Days and 14.4 Hours
To investigate the effect of (-)-epigallocatechin-3-gallate (EGCG) on polyinosinic-polycytidylic acid (poly I:C)-triggered intracellular innate immunity against hepatitis C virus (HCV) in hepatocytes.
A cell culture model of HCV infection was generated by infecting a hepatoma cell line, Huh7, with HCV JFH-1 strain (JFH-1-Huh7). Poly I:C with a high molecular weight and EGCG were used to stimulate the JFH-1-Huh7 cells. Real-time reverse transcription-polymerase chain reaction was used to detect the expression levels of intracellular mRNAs and of intracellular and extracellular HCV RNA. Enzyme-linked immunosorbent assay was used to evaluate the interferon (IFN)-λ1 protein level in the cell culture supernatant. Immunostaining was used to examine HCV core protein expression in Huh7 cells.
Our recent study showed that HCV replication could impair poly I:C-triggered intracellular innate immune responses in hepatocytes. In the current study, we showed that EGCG treatment significantly increased the poly I:C-induced expression of Toll-like receptor 3 (TLR3), retinoic acid-inducible gene I, and IFN-λ1 in JFH-1-Huh7 cells. In addition, supplementation with EGCG increased the poly I:C-mediated antiviral activity in JFH-1-Huh7 cells at the intracellular and extracellular HCV RNA and protein levels. Further investigation of the mechanisms showed that EGCG treatment significantly enhanced the poly I:C-induced expression of IFN-regulatory factor 9 and several antiviral IFN-stimulated genes, including ISG15, ISG56, myxovirus resistance A, and 2’-5’-oligoadenylate synthetase 1, which encode the key antiviral elements in the IFN signaling pathway.
Our observations provide experimental evidence that EGCG has the ability to enhance poly I:C-induced intracellular antiviral innate immunity against HCV replication in hepatocytes.
Core tip: The interactions between hepatitis C virus (HCV) and the host immune system in the liver play a key role in the immunopathogenesis of HCV-induced diseases. We showed here that (-)-epigallocatechin-3-gallate (EGCG) treatment could significantly increase the poly I:C-induced expression of TLR3, RIG-I and interferon (IFN)-λ1 in JFH-1-Huh7 cells. In addition, supplementation with EGCG enhanced poly I:C-mediated viral inhibition in JFH-1-Huh7 cells at both RNA and protein levels. Further investigation of the mechanisms showed that EGCG treatment significantly enhanced the poly I:C-induced expression of IFN-regulatory factor 9 and several IFN-stimulated genes. It would be interesting to investigate the possible use of EGCG in combination with current antiviral drugs for HCV therapy.
- Citation: Wang YZ, Li JL, Wang X, Zhang T, Ho WZ. (-)-Epigallocatechin-3-gallate enhances poly I:C-induced interferon-λ1 production and inhibits hepatitis C virus replication in hepatocytes. World J Gastroenterol 2017; 23(32): 5895-5903
- URL: https://www.wjgnet.com/1007-9327/full/v23/i32/5895.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i32.5895
Hepatitis C virus (HCV) is a hepatotropic virus, which holds a single-stranded, positive-sense RNA genome of 9.6 kb in length[1]. Currently, around 150 million people are chronically infected with HCV worldwide[2]. HCV infection causes liver diseases ranging from mild to moderate and severe, such as hepatitis, cirrhosis, and hepatocellular carcinoma[3]. The host immune responses play pivotal roles in the pathogenesis of viral infection, and the interplays of host antiviral immunity and HCV determine the outcomes of HCV infection[4]. It has been demonstrated that host immune responses are triggered by HCV, however, it is less effective in clearing the virus and leading a high rate of chronic infection in the majority of cases[5]. Acute HCV infection is accompanied by a spontaneous viral clearance in only about 20% to 30% of the subjects[2]. Recent studies showed that HCV has evolved several strategies to escape the attacks mediated by the host antiviral immunity[6]. Nonstructural (NS) 3/4A protease encoded by HCV genome has been showed to be able to impair the host antiviral innate immunity by disrupting the signaling of several pattern recognition receptors (PPRs), such as Toll-like receptor 3 (TLR3) and retinoic acid-inducible gene I (RIG-I)[7-9]. HCV NS4B blocks interferon (IFN) production by disrupting the interaction of STING with mitochondrial antiviral signaling protein (MAVS) and TBK1[10,11]. Furthermore, host genetic factors play important roles in controlling HCV infection. Studies have shown that polymorphisms located in the IFN-λ region are related with the rate of viral clearance in HCV-infected individuals without treatment, as well as the sustained viral response (SVR) rate to the IFN-α-based treatment[12-15]. To date, tremendous progress has been made in understanding the biology of HCV and its related disease. Important advances in characterizing the HCV life cycle have led to the discovery of direct-acting antivirals (DAAs), which were developed for the effective treatment of chronic HCV infection[16,17]. However, it is still challenging to dramatically decrease the incidence of HCV infection in the near future. High costs, low barrier to resistance-associated mutations, viral reinfection, failure of the DAAs in HCV-induced liver diseases, and lack of effective vaccines are obstacles to overcoming HCV infection globally[2].
(-)-Epigallocatechin-3-gallate (EGCG), the most abundant and bioactive catechin in green tea, possesses various physiological and pharmacological benefits to human health. Previous studies[18-20] revealed that EGCG exhibits abilities against viral infection and can prevent cardiovascular diseases, metabolic syndrome, neurodegenerative diseases, and cancer. It has been shown that EGCG can inhibit the replication of several viruses, such as human immunodeficiency virus[19], herpes simplex virus[21], as well as influenza virus[22]. Recent studies[23,24] have revealed that EGCG can act as an inhibitor of HCV entry and limit intercellular spread of HCV. EGCG is a potent antioxidant that has both anti-inflammatory and anti-atherogenic properties[25-27]. Our previous study[27] indicated that EGCG inhibits lipopolysaccharide-induced inflammatory cytokine expression in microvascular endothelial cells. In addition, EGCG exhibits anticancer functions through inhibition of proteasome activity and induction of endoplasmic reticulum stress[28,29]. Furthermore, it was also shown that EGCG increases the formation of lipid droplets and inhibits the secretion of very low-density lipoproteins in human hepatocytes[30].
Our recent study showed that HCV replication could impair the polyinosinic-polycytidylic acid (poly I:C)-triggered intracellular innate immune signaling pathway in hepatocytes[31]. Furthermore, we showed that EGCG possesses the ability to increase HCV dsRNA intermediate-induced expression of IFN-λ1 and IFN-stimulated genes (ISGs)[32]. In this study, we investigated the effect of EGCG on poly I:C-induced IFN pathway activation and its antiviral activities.
EGCG (purity, ≥ 95%) was purchased from SIGMA-ALDRICH, St. Louis, MO, United States (CAS#: 989-51-5; Cat# E4143). EGCG stock solution was prepared with sterile double distilled water at a concentration of 20 mmol/L.
The hepatoma cell line (Huh7), provided by Dr. Charles Rice (Rockefeller University, NY, United States), was maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 mg/mL). The generation of infectious HCV JFH-1 and infection of Huh7 cells (multiplicity of infection of 0.01) were carried out as previously described[33]. HCV JFH-1 infection of Huh7 cells was analyzed by immunostaining with mouse anti-HCV core antibody or by real-time reverse transcription-polymerase chain reaction (RT-PCR) for HCV RNA. LyoVec transfection reagent and poly I:C with a high molecular weight were purchased from InvivoGen (San Diego, CA, United States). An enzyme-linked immunosorbent assay (ELISA) kit for IFN-λ1 was purchased from eBioscience Inc. (San Diego, CA, United States). Mouse antibody against the HCV core antigen was purchased from ABR Affinity BioReagents, Thermo Scientific (Rockford, IL, United States). Hoechst 33342 was purchased from Molecular Probes (Carlsbad, CA, United States).
The JFH-1-infected Huh7 cells (72 h post-infection) were treated with EGCG (1-10 μmol/L) for 1 h prior to poly I:C (1 μg/mL) stimulation with LyoVec transfection reagent. The cells were collected for total RNA extraction after 24 h or 48 h of stimulation, and the supernatant (SN) was collected for ELISA after 48 h of stimulation. As a negative control of the transfection experiment, cells were incubated with the LyoVec transfection reagent without poly I:C.
Total RNA from the cultured cells or SN was extracted with TRI Reagent (Molecular Research Center, Cincinnati, OH, United States) and subjected to reverse transcription (RT) using the RT system (Promega, Madison, WI, United States) with random primers for 1 h at 42 °C. The reaction was terminated by incubating the reaction mixture at 99 °C for 5 min, and the mixture was kept at 4 °C. The resulting cDNA was used as a template for quantitative real-time PCR. Real-time PCR was performed with 1/10 of the cDNA with the iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA, United States). The amplified products were visualized and analyzed using the software MyiQ provided with the thermocycler (iCycler iQ real-time PCR detection system; Bio-Rad Laboratories). The oligonucleotide primers were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA, United States), and the sequences are shown in Table 1. The cDNA was amplified by PCR, and the products were measured using SYBR green I (Bio-Rad Laboratories, Inc., Hercules, CA, United States). The data were normalized to the level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and presented as the change in induction relative to that of the untreated control cells.
| Primer | Orientation | Sequences (5’-3’) |
| GAPDH | Forward | GGTGGTCTCCTCTGACTTCAACA |
| Reverse | GTTGCTGTAGCCAAATTCGTTGT | |
| IFN-λ1 | Forward | CTTCCAAGCCCACCCCAACT |
| Reverse | GGCCTCCAGGACCTTCAGC | |
| TLR3 | Forward | AGCCACCTGAAGTTGACTCAGG |
| Reverse | CAGTCAAATTCGTGCAGAAGGC | |
| RIG-I | Forward | CTTGGCATGTTACACAGCTGAC |
| Reverse | GCTTGGGATGTGGTCTACTCA | |
| IRF-9 | Forward | GCATCAGGCAGGGCACGCTGCACCCG |
| Reverse | GCCTGCATGTTTCCAGGGAATCCGG | |
| ISG15 | Forward | GGCTGGGAGCTGACGGTGAAG |
| Reverse | GCTCCGCCCGCCAGGCTCTGT | |
| ISG56 | Forward | TTCGGAGAAAGGCATTAGA |
| Reverse | TCCAGGGCTTCATTCATAT | |
| MxA | Forward | GCCGGCTGTGGATATGCTA |
| Reverse | TTTATCGAAACATCTGTGAAAGCAA | |
| OAS-1 | Forward | AGAAGGCAGCTCACGAAACC |
| Reverse | CCACCACCCAAGTTTCCTGTA | |
| HCV | Forward | RAYCACTCCCCTGTGAGGAAC |
| Reverse | TGRTGCACGGTCTACGAGACCTC |
IFN-λ1 protein expression was evaluated by ELISA. SN collected from EGCG and/or poly I:C-treated Huh7 cell cultures was directly tested for IFN-λ1 protein levels by ELISA, which was performed according to the manufacturer’s instructions.
Student’s t-test was used to evaluate the significance of difference between the groups, and multiple comparisons were performed by regression analysis and one-way analysis of variance. All data are presented as the mean ± SD. Statistical analyses were performed with SPSS 11.5 for Windows. Statistical significance was defined as P < 0.05.
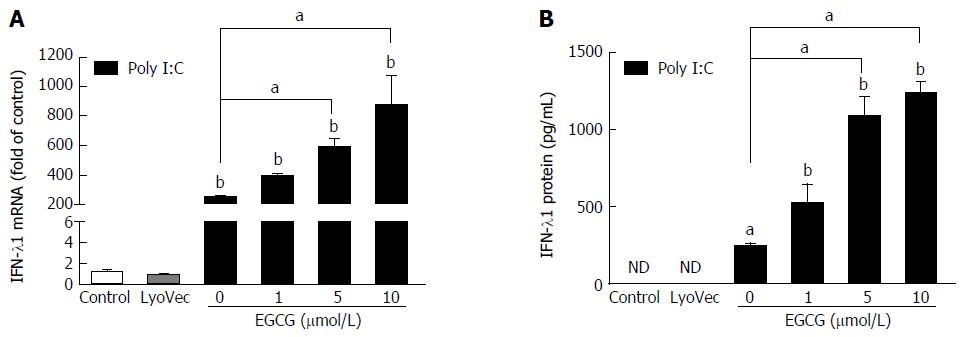
In order to test the effect of EGCG on poly I:C-induced IFN-λ1 expression, we treated JFH-1-Huh7 cells (72 h post-infection) with EGCG (1-10 μmol/L) for 1 h before poly I:C treatment. The data showed that EGCG could significantly increase poly I:C-mediated IFN-λ1 mRNA expression (Figure 1A), as well as IFN-λ1 protein production (Figure 1B), in a dose-dependent manner, whereas EGCG treatment alone had a negligible effect on IFN-λ1 expression in JFH-1-infected Huh7 cells[32].
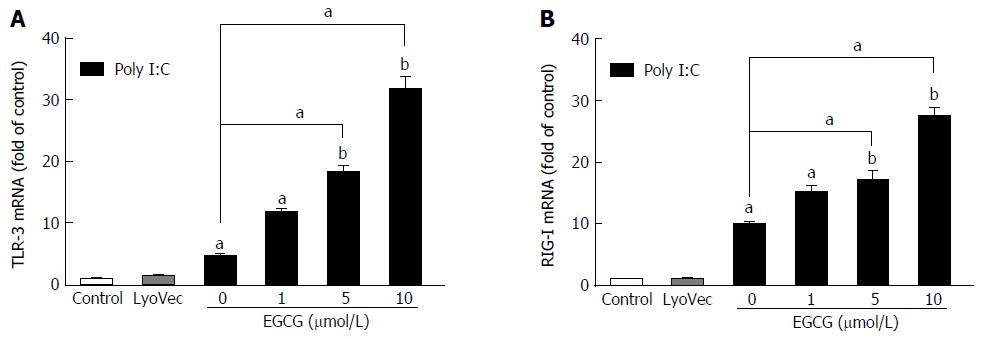
TLR3 and RIG-I are major cellular receptors that recognize pathogen-associated molecular patterns (PAMPs) during RNA virus infections. While EGCG (at a concentration lower than 10 μmol/L) treatment alone showed little effect on TLR3 and RIG-I expression[32], EGCG significantly increased the poly I:C-induced mRNA expression of TLR3 (Figure 2A) and RIG-I (Figure 2B) in JFH-1-Huh7 cells.
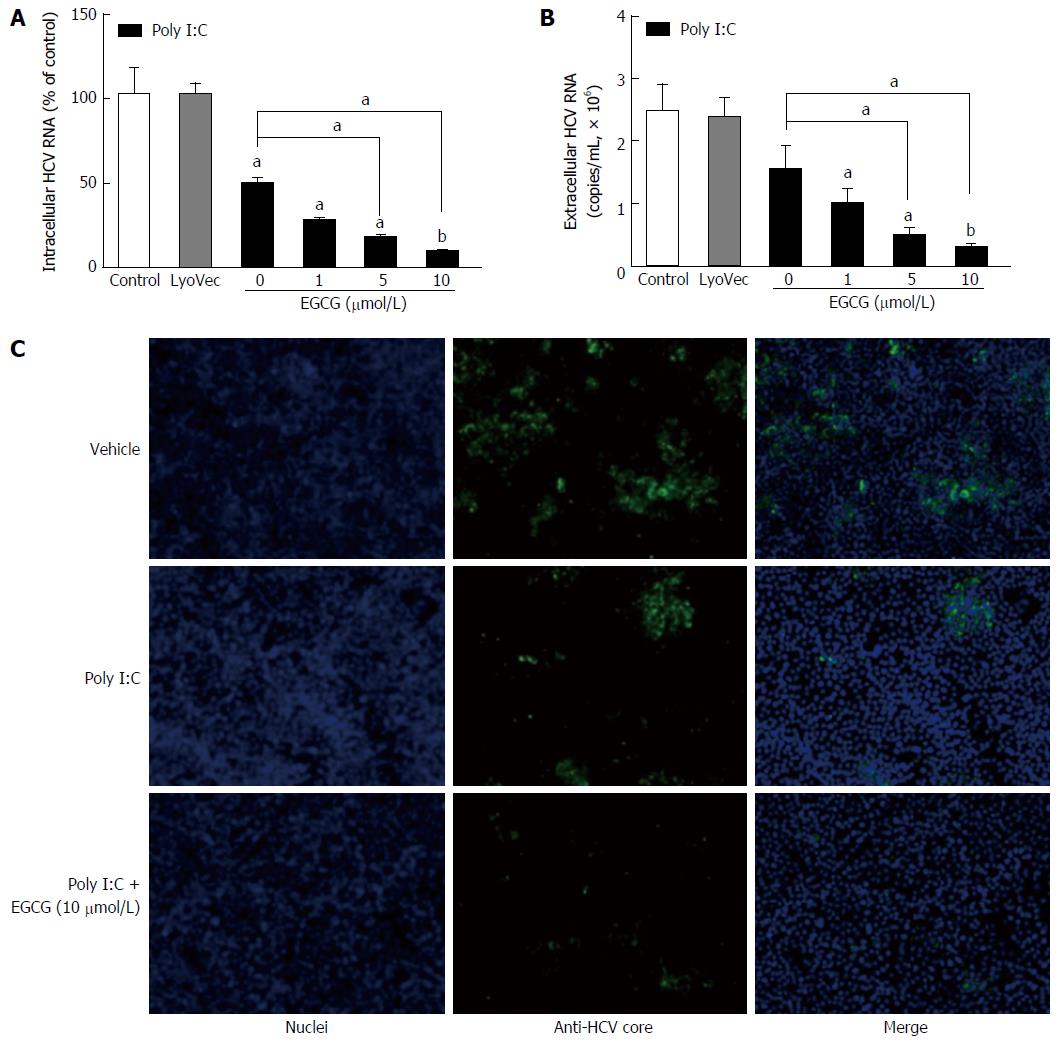
Our previous study[32] showed that when we treated JFH-1-Huh7 cells with EGCG (1-10 μmol/L) alone, EGCG could not inhibit viral replication. We found that poly I:C treatment also had limited antiviral effect on the HCV-infected Huh7 cells (Figure 3). When the JFH-1-Huh7 cells were treated with EGCG 1 h before poly I:C stimulation, EGCG enhanced the poly I:C-mediated HCV inhibition in Huh7 cells in a dose-dependent manner (Figure 3). The inhibition of HCV by EGCG and poly I:C was confirmed by examining the intracellular (Figure 3A) and extracellular levels (Figure 3B) of HCV RNA and the level of HCV core protein (Figure 3C).
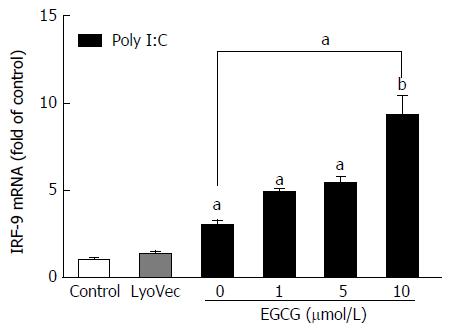
As IRF-9 plays a critical role in the induction of antiviral ISGs, we further investigated the impact of EGCG on poly I:C-induced IRF-9 mRNA expression. While EGCG alone had little impact on IRF-9 mRNA expression (data not shown), EGCG pretreatment could significantly enhance poly I:C-induced IRF-9 mRNA expression in JFH-1-Huh7 cells (Figure 4).
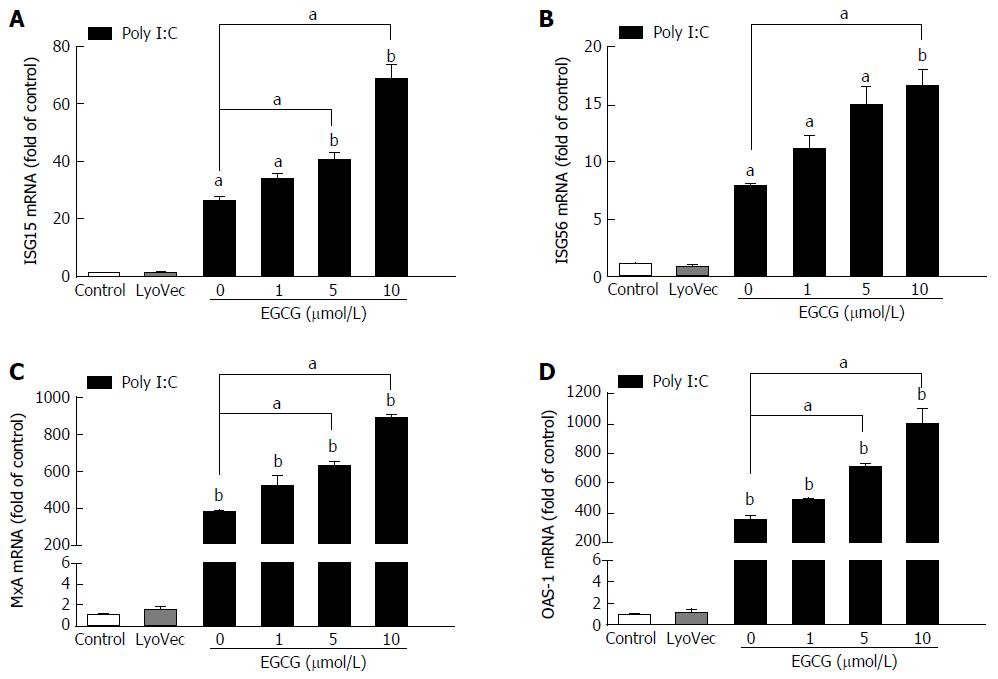
EGCG dose-dependently enhanced the poly I:C-induced expression of ISG15 (Figure 5A), ISG56 (Figure 5B), MxA (Figure 5C), and OAS-1 (Figure 5D) in JFH-1-infected cells. However, EGCG treatment alone showed a negligible impact on the expression of those ISGs (data not shown).
In the current study, we showed that EGCG could enhance the poly I:C-induced innate immune responses in hepatocytes, which contributed to poly I:C-mediated HCV inhibition. Although poly I:C could induce the expression of IFN-λ1 and several antiviral ISGs in JFH-1-infected Huh7 cells, the expression level was restricted by existing virus[31]. Our data indicated that EGCG significantly enhanced the poly I:C-induced expression of IFN-λ1, TLR3, RIG-I, ISG15, ISG56, MxA, and OAS-1 in JFH-1-infected Huh7 cells. More importantly, pretreatment with EGCG enhanced poly I:C-mediated viral inhibition in JFH-1-infected cells. It has been demonstrated that IFN-λ could inhibit HCV replication, and recombinant IFN-λ1 was used as an antiviral drug in HCV treatment trials[34,35]. The basic level of IFN-λ1 is very low in human hepatocytes, but it could be triggered by viral infections through activation of PPRs, such as TLR3[31,36]. Our previous study[31] indicated that poly I:C treatment induces IFN-λ1 expression; however, HCV replication could impair the poly I:C-triggered TLR3 signaling pathway and decrease IFN-λ1 induction in Huh7 cells. In addition, HCV induced IFN-λ1 expression in primary human hepatocytes could resist against HCV[36]. Thus, the anti-HCV effect mediated by EGCG and poly I:C combination treatment in JFH-1-infected cells was likely through the enhancement of intracellular IFN-λ1 expression.
The interplays of HCV and the host antiviral immunity play important roles in the pathogenesis of HCV-related diseases. The innate immunity is the front line of host defenses against pathogen infections. TLRs and RIG-I-like receptors (RLRs) are major cellular receptors that recognize PAMPs during viral infections[37]. Among these TLR members, TLR3 is triggered by dsRNAs from the genome of some RNA viruses or intermediates formed during viral genome replication, such as HCV dsRNA intermediates[38,39]. In addition to TLR3, RIG-I has been demonstrated to recognize HCV genome, inducing immune antiviral responses through the type I IFN signaling activation[40]. Our data showed that EGCG enhanced poly I:C-induced TLR3 and RIG-I expression in HCV-infected hepatocytes, which may contribute to the activation of IFN signaling to inhibit viral replication.
In order to maintain a persistent infection, HCV evolves several strategies to escape host antiviral immune responses[6]. It has been shown that a protease of HCV, NS3/4A, could disrupt both TLR3 and RIG-I activation through cleaving the cellular adaptor molecules, TRIF and MAVS, respectively[7-9]. HCV NS4B blocks IFN production by disrupting the interaction of STING with MAVS and TBK1[10,11]. Studies also revealed that HCV impairs IRF-7 translocation, and inhibits both IFN-α and IFN-λ1 expression in hepatocytes[31,41,42]. Therefore, how to rescue the host antiviral immunity impaired by existing viruses is very important for the HCV eradication. In the current study, we found that EGCG enhanced poly I:C-induced IFN-λ1, TLR3, RIG-I and ISGs expression in JFH-1-Huh7 cells, showing that EGCG could improve intracellular antiviral immune responses in viral-infected cells.
In recent years, the development of effective DAAs has greatly increased the opportunity to cure HCV and achieve a milestone of HCV therapy. Following the approval of high effective DDAs in 2013, all-oral, IFN-free regimens were available for chronic HCV management[43]. Clinical applications of DAAs including inhibitors of NS3/4A, NS5A, and NS5B nucleotide or non-nucleotide inhibitors can achieve a high SVR rate of 90% to 100%[2,44]. However, challenges still exist in the future management of HCV infection. DAAs are unavailable in poor areas and most of developing countries due to the high prices. It is also important to investigate the clinical effects of DAAs on advanced or decompensated liver diseases caused by HCV, as well as the clinical use of DAAs in HCV-infected children and pregnant women[45]. In addition, the majority of current DAAs have a low barrier to resistance, which leads to a high risk of selection of drug-resistant viral strains[46]. In the absence of an effective vaccine against HCV, reinfection is probable in cured patients under continuous HCV exposure[16].
In summary, EGCG enhances both poly I:C- and HCV dsRNA intermediate-induced innate immune responses in hepatocytes[32]. It would be interesting to investigate the possible use of EGCG in combination with current antiviral drugs for HCV therapy in the future.
Chronic hepatitis C virus (HCV) infection is a major cause of chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. New direct-acting antivirals (DAAs) therapies are able to achieve a sustainable virological response of up to 90% against the most prevalent HCV genotypes. However, continued efforts are still needed due to the high cost, low barrier to viral resistance, and the inability to treat HCV-related diseases of the DAAs as well as the lack of an effective HCV vaccine.
The authors previously reported that HCV replication can impair the poly I:C-triggered innate immune response in hepatocytes. They also showed that epigallocatechin-3-gallate (EGCG) enhances HCV dsRNA intermediate-induced expression of IFN-λ1 and ISGs in hepatocytes. Effects of EGCG on the poly I:C-mediated expression of antiviral factors and inhibition of HCV were investigated in the present study. They found that EGCG increased poly I:C-induced IFN-λ1 and ISG expression and contributed to poly I:C-mediated HCV inhibition.
The present study showed for the first time that EGCG can increase poly I:C-induced IFN-λ1 and ISG expression and contribute to poly I:C-mediated HCV inhibition.
The authors demonstrated the effects of EGCG on poly I:C-triggered innate immune responses in hepatocytes. However, our observations only provided in vitro evidence due to the lack of in vivo data. It would be interesting to investigate the possible use of EGCG in combination with current antiviral drugs for HCV therapy in the future.
EGCG is the most abundant and bioactive catechin in green tea and has been considered to have a number of physiological and pharmacological health benefits. Polyinosinic-polycytidylic acid (poly I:C) is a synthetic analog of dsRNA, a molecular pattern associated with viral infection. The average size of HMW poly I:C ranges from 1.5 kb to 8 kb.
This paper reports a well detailed investigation of the effect of EGCG on poly I:C-triggered intracellular innate immunity against HCV in hepatocytes. The authors demonstrated that EGCG had the ability to enhance poly I:C-induced innate immune responses in hepatocytes, which contributed to poly I:C-mediated HCV inhibition. Thus, it would be interesting to investigate the possible use of EGCG in combination with current antiviral drugs for HCV therapy in the future.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chiellini G S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Zhang FF
| 1. | Manns MP, Buti M, Gane E, Pawlotsky JM, Razavi H, Terrault N, Younossi Z. Hepatitis C virus infection. Nat Rev Dis Primers. 2017;3:17006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 342] [Article Influence: 42.8] [Reference Citation Analysis (1)] |
| 2. | Douam F, Ding Q, Ploss A. Recent advances in understanding hepatitis C. F1000Res. 2016;5:pii: F1000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Alter MJ. Prevention of spread of hepatitis C. Hepatology. 2002;36:S93-S98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3:47-52. [PubMed] |
| 5. | Battaglia AM, Hagmeyer KO. Combination therapy with interferon and ribavirin in the treatment of chronic hepatitis C infection. Ann Pharmacother. 2000;34:487-494. [PubMed] |
| 6. | Gale M Jr, Foy EM. Evasion of intracellular host defence by hepatitis C virus. Nature. 2005;436:939-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 455] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 7. | Li K, Foy E, Ferreon JC, Nakamura M, Ferreon AC, Ikeda M, Ray SC, Gale M Jr, Lemon SM. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc Natl Acad Sci USA. 2005;102:2992-2997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 847] [Cited by in RCA: 817] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 8. | Foy E, Li K, Sumpter R Jr, Loo YM, Johnson CL, Wang C, Fish PM, Yoneyama M, Fujita T, Lemon SM, Gale M Jr. Control of antiviral defenses through hepatitis C virus disruption of retinoic acid-inducible gene-I signaling. Proc Natl Acad Sci USA. 2005;102:2986-2991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 430] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 9. | Yang K, Puel A, Zhang S, Eidenschenk C, Ku CL, Casrouge A, Picard C, von Bernuth H, Senechal B, Plancoulaine S. Human TLR-7-, -8-, and -9-mediated induction of IFN-alpha/beta and -lambda Is IRAK-4 dependent and redundant for protective immunity to viruses. Immunity. 2005;23:465-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 214] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 10. | Ding Q, Cao X, Lu J, Huang B, Liu YJ, Kato N, Shu HB, Zhong J. Hepatitis C virus NS4B blocks the interaction of STING and TBK1 to evade host innate immunity. J Hepatol. 2013;59:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 11. | Nitta S, Sakamoto N, Nakagawa M, Kakinuma S, Mishima K, Kusano-Kitazume A, Kiyohashi K, Murakawa M, Nishimura-Sakurai Y, Azuma S. Hepatitis C virus NS4B protein targets STING and abrogates RIG-I-mediated type I interferon-dependent innate immunity. Hepatology. 2013;57:46-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 12. | Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O’Huigin C, Kidd J, Kidd K, Khakoo SI, Alexander G. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798-801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1706] [Cited by in RCA: 1687] [Article Influence: 105.4] [Reference Citation Analysis (0)] |
| 13. | Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399-401. [PubMed] |
| 14. | Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, Nakagawa M, Korenaga M, Hino K, Hige S. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1779] [Cited by in RCA: 1775] [Article Influence: 110.9] [Reference Citation Analysis (0)] |
| 15. | Suppiah V, Moldovan M, Ahlenstiel G, Berg T, Weltman M, Abate ML, Bassendine M, Spengler U, Dore GJ, Powell E. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41:1100-1104. [PubMed] |
| 16. | Kumar S, Jacobson IM. Antiviral therapy with nucleotide polymerase inhibitors for chronic hepatitis C. J Hepatol. 2014;61:S91-S97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Gentile I, Coppola N, Buonomo AR, Zappulo E, Borgia G. Investigational nucleoside and nucleotide polymerase inhibitors and their use in treating hepatitis C virus. Expert Opin Investig Drugs. 2014;23:1211-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Lambert JD. Does tea prevent cancer? Evidence from laboratory and human intervention studies. Am J Clin Nutr. 2013;98:1667S-1675S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Nance CL, Siwak EB, Shearer WT. Preclinical development of the green tea catechin, epigallocatechin gallate, as an HIV-1 therapy. J Allergy Clin Immunol. 2009;123:459-465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Liu JB, Zhou L, Wang YZ, Wang X, Zhou Y, Ho WZ, Li JL. Neuroprotective Activity of (-)-Epigallocatechin Gallate against Lipopolysaccharide-Mediated Cytotoxicity. J Immunol Res. 2016;2016:4962351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Isaacs CE, Wen GY, Xu W, Jia JH, Rohan L, Corbo C, Di Maggio V, Jenkins EC Jr, Hillier S. Epigallocatechin gallate inactivates clinical isolates of herpes simplex virus. Antimicrob Agents Chemother. 2008;52:962-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 22. | Song JM, Lee KH, Seong BL. Antiviral effect of catechins in green tea on influenza virus. Antiviral Res. 2005;68:66-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 448] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 23. | Calland N, Albecka A, Belouzard S, Wychowski C, Duverlie G, Descamps V, Hober D, Dubuisson J, Rouillé Y, Séron K. (-)-Epigallocatechin-3-gallate is a new inhibitor of hepatitis C virus entry. Hepatology. 2012;55:720-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 200] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 24. | Ciesek S, von Hahn T, Colpitts CC, Schang LM, Friesland M, Steinmann J, Manns MP, Ott M, Wedemeyer H, Meuleman P. The green tea polyphenol, epigallocatechin-3-gallate, inhibits hepatitis C virus entry. Hepatology. 2011;54:1947-1955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 216] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 25. | Wu CC, Hsu MC, Hsieh CW, Lin JB, Lai PH, Wung BS. Upregulation of heme oxygenase-1 by Epigallocatechin-3-gallate via the phosphatidylinositol 3-kinase/Akt and ERK pathways. Life Sci. 2006;78:2889-2897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 192] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 26. | Rezai-Zadeh K, Arendash GW, Hou H, Fernandez F, Jensen M, Runfeldt M, Shytle RD, Tan J. Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 2008;1214:177-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 322] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 27. | Li J, Ye L, Wang X, Liu J, Wang Y, Zhou Y, Ho W. (-)-Epigallocatechin gallate inhibits endotoxin-induced expression of inflammatory cytokines in human cerebral microvascular endothelial cells. J Neuroinflammation. 2012;9:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Kuhn D, Lam WH, Kazi A, Daniel KG, Song S, Chow LM, Chan TH, Dou QP. Synthetic peracetate tea polyphenols as potent proteasome inhibitors and apoptosis inducers in human cancer cells. Front Biosci. 2005;10:1010-1023. [PubMed] |
| 29. | Rizzi F, Naponelli V, Silva A, Modernelli A, Ramazzina I, Bonacini M, Tardito S, Gatti R, Uggeri J, Bettuzzi S. Polyphenon E(R), a standardized green tea extract, induces endoplasmic reticulum stress, leading to death of immortalized PNT1a cells by anoikis and tumorigenic PC3 by necroptosis. Carcinogenesis. 2014;35:828-839. [PubMed] |
| 30. | Li L, Stillemark-Billton P, Beck C, Boström P, Andersson L, Rutberg M, Ericsson J, Magnusson B, Marchesan D, Ljungberg A. Epigallocatechin gallate increases the formation of cytosolic lipid droplets and decreases the secretion of apoB-100 VLDL. J Lipid Res. 2006;47:67-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Wang Y, Li J, Wang X, Ye L, Zhou Y, Thomas RM, Ho W. Hepatitis C virus impairs TLR3 signaling and inhibits IFN-λ 1 expression in human hepatoma cell line. Innate Immun. 2014;20:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Wang Y, Li J, Wang X, Peña JC, Li K, Zhang T, Ho W. (-)-Epigallocatechin-3-Gallate Enhances Hepatitis C Virus Double-Stranded RNA Intermediates-Triggered Innate Immune Responses in Hepatocytes. Sci Rep. 2016;6:21595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Zhong J, Gastaminza P, Cheng G, Kapadia S, Kato T, Burton DR, Wieland SF, Uprichard SL, Wakita T, Chisari FV. Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci USA. 2005;102:9294-9299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1475] [Cited by in RCA: 1466] [Article Influence: 73.3] [Reference Citation Analysis (0)] |
| 34. | Pagliaccetti NE, Robek MD. Interferon-λ in HCV Infection and Therapy. Viruses. 2010;2:1589-1602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Muir AJ, Shiffman ML, Zaman A, Yoffe B, de la Torre A, Flamm S, Gordon SC, Marotta P, Vierling JM, Lopez-Talavera JC. Phase 1b study of pegylated interferon lambda 1 with or without ribavirin in patients with chronic genotype 1 hepatitis C virus infection. Hepatology. 2010;52:822-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 186] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 36. | Marukian S, Andrus L, Sheahan TP, Jones CT, Charles ED, Ploss A, Rice CM, Dustin LB. Hepatitis C virus induces interferon-λ and interferon-stimulated genes in primary liver cultures. Hepatology. 2011;54:1913-1923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 37. | Kawai T, Akira S. Toll-like receptor and RIG-I-like receptor signaling. Ann N Y Acad Sci. 2008;1143:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 754] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 38. | Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1-14. [PubMed] |
| 39. | Li K, Li NL, Wei D, Pfeffer SR, Fan M, Pfeffer LM. Activation of chemokine and inflammatory cytokine response in hepatitis C virus-infected hepatocytes depends on Toll-like receptor 3 sensing of hepatitis C virus double-stranded RNA intermediates. Hepatology. 2012;55:666-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 40. | Saito T, Owen DM, Jiang F, Marcotrigiano J, Gale M Jr. Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature. 2008;454:523-527. [PubMed] |
| 41. | Zhang T, Lin RT, Li Y, Douglas SD, Maxcey C, Ho C, Lai JP, Wang YJ, Wan Q, Ho WZ. Hepatitis C virus inhibits intracellular interferon alpha expression in human hepatic cell lines. Hepatology. 2005;42:819-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Raychoudhuri A, Shrivastava S, Steele R, Dash S, Kanda T, Ray R, Ray RB. Hepatitis C virus infection impairs IRF-7 translocation and Alpha interferon synthesis in immortalized human hepatocytes. J Virol. 2010;84:10991-10998. [PubMed] |
| 43. | Nyalakonda H, Utay NS. A new era of therapy for hepatitis C virus infection. Curr Opin Infect Dis. 2015;28:471-478. [PubMed] |
| 44. | Lanini S, Easterbrook PJ, Zumla A, Ippolito G. Hepatitis C: global epidemiology and strategies for control. Clin Microbiol Infect. 2016;22:833-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 45. | Liang TJ, Ghany MG. Therapy of hepatitis C--back to the future. N Engl J Med. 2014;370:2043-2047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 46. | Sarrazin C, Zeuzem S. Resistance to direct antiviral agents in patients with hepatitis C virus infection. Gastroenterology. 2010;138:447-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 412] [Article Influence: 27.5] [Reference Citation Analysis (0)] |