Published online Aug 21, 2017. doi: 10.3748/wjg.v23.i31.5755
Peer-review started: April 10, 2017
First decision: June 1, 2017
Revised: June 15, 2017
Accepted: June 18, 2017
Article in press: June 19, 2017
Published online: August 21, 2017
Processing time: 107 Days and 18.5 Hours
To evaluate factors that influence the diagnostic accuracy of endoscopic ultrasound (EUS)-guided tissue acquisition for lymph node enlargement in the absence of an on-site pathologist.
A retrospective analysis of patients who underwent EUS-guided tissue acquisition for the pathological diagnosis of lymph node enlargement between April 2012 and June 2015 is reported. Tissue acquisition was performed with both cytology and biopsy needles of different calibers. The variables evaluated were lymph node location and size, number of passes and type of needle used. Final diagnosis was based on surgical histopathology or, in non-operated cases, on EUS-guided tissue acquisition and imaging assessment with a minimum clinical follow-up of 6 mo.
During the study period, 168 lymph nodes with a median size of 20.3 mm (range 12.5-27) were sampled from 152 patients. Ninety lymph nodes (53.6%) were located at mediastinum, and 105 (62.5%) were acquired with biopsy needles. The final diagnosis was benign/reactive origin in 87 cases (51.8%), malignant in 65 cases (38.7%), and lymphoma in 16 cases (9.5%). The sensitivity, specificity, positive predictive value and negative predictive value for the detection of malignancy were 74.1%, 100%, 100% and 80.6%, respectively. The overall accuracy was 87.5% (95%CI: 81.7-91.7). No variables were independently associated with a correct final diagnosis according to the multivariate analysis.
EUS-guided tissue acquisition is a highly accurate technique for assessing lymph node enlargement. None of the variables evaluated were associated with diagnostic accuracy.
Core tip: This study shows that the accuracy of endoscopic ultrasound-guided tissue acquisition in enlarged lymph nodes is not affected by the type of needle used, the number of needle passes, or the location or characteristics of the enlarged lymph nodes. Histological specimens are essential for establishing the diagnosis of lymphoproliferative disease. Employing complementary imaging techniques, such as contrast enhancement and elastography, might help improve the diagnostic yield.
- Citation: Chin YK, Iglesias-Garcia J, de la Iglesia D, Lariño-Noia J, Abdulkader-Nallib I, Lázare H, Rebolledo Olmedo S, Dominguez-Muñoz JE. Accuracy of endoscopic ultrasound-guided tissue acquisition in the evaluation of lymph nodes enlargement in the absence of on-site pathologist. World J Gastroenterol 2017; 23(31): 5755-5763
- URL: https://www.wjgnet.com/1007-9327/full/v23/i31/5755.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i31.5755
The advent of advanced diagnostic imaging modalities, such as computer-aided tomography (CT) scanning and magnetic resonance imaging (MRI), has led to increased detection rates of enlarged mediastinal and intra-abdominal lymph nodes. When no primary malignant lesion is evident, the differential diagnosis of these enlarged lymph nodes can be difficult. Open thoracic surgery, laparotomy or other surgical procedures such as mediastinoscopy or laparoscopy are often required in this setting. However, these procedures are invasive and not cost-effective[1].
Endoscopic ultrasound (EUS)-guided tissue acquisition with either fine-needle aspiration (FNA) or fine-needle biopsy (FNB) is an essential tool used to facilitate the diagnosis of periluminal lymphadenopathy adjacent to the gastrointestinal tract, particularly around the esophagus, stomach, and duodenum[2,3]. This is pivotal in patient care because malignant nodal disease will alter prognosis and overall disease management, requiring neoadjuvant therapy or a shift from futile curative treatment to palliative treatment[4]. In contrast, the diagnosis of non-malignant conditions, such as tuberculosis (TB) or sarcoidosis, not only guides the appropriate treatment but also reduces patient anxiety[5-8].
In the context of EUS-guided FNA and/or FNB, the presence of an on-site pathological evaluation during the procedure is very useful. Several studies have demonstrated the positive impact of such evaluations on the diagnostic yield of EUS-guided tissue acquisition[9-12], although not all centers are able to perform on-site evaluations due to costs and/or logistic issues. However, other factors have been identified to influence the accuracy of EUS-guided tissue acquisition, such the characteristics of the target lesion, the number of needle passes and the needle size[13]. In this retrospective analysis, we aimed to evaluate the factors that might influence the diagnostic accuracy of EUS-guided tissue acquisition of lymph node enlargement in the absence of an on-site cytopathological evaluation.
We conducted a retrospective analysis of a prospectively maintained endoscopy database with a specific EUS registry of a single tertiary referral hospital. The study was approved by the local ethics committee and was conducted in accordance with the Declaration of Helsinki and its amendments as well as Good Clinical Practice guidelines.
Consecutive patients who were referred for EUS-guided tissue acquisition of enlarged lymph nodes between April 2012 and June 2015 and who required a cytopathological evaluation were included in the analysis. We excluded patients whose information regarding the procedure was not complete or only partially available and those who were lost to follow-up and for whom sufficient information to establish the final diagnosis of the lymph nodes was unavailable.
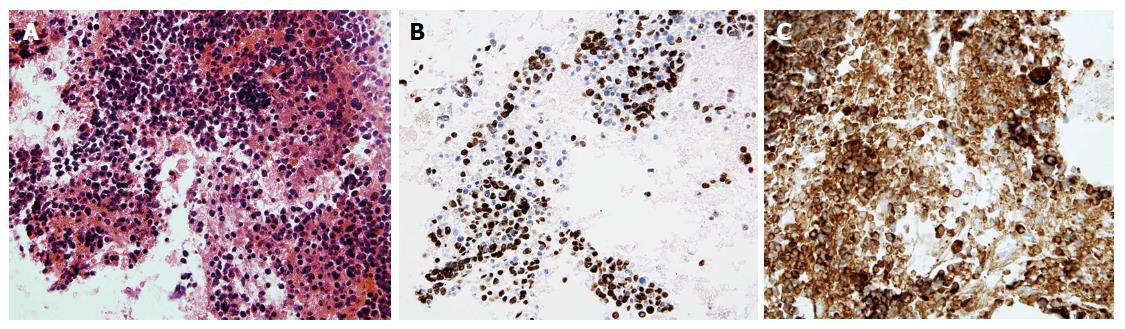
All EUS-guided tissue acquisition procedures were performed by two experienced operators (Iglesias-Garcia J and Lariño-Noia J), each of whom had performed more than 1000 EUS-guided tissue acquisitions. All patients received conscious sedation. The procedures were performed using Pentax curvilinear array echoendoscopes (EG-3870UTK and EG-3270UK) and a HITACHI ultrasound device. The needles used included 19-G, 22-G and 25-G cytology and histology needles (Echotip Ultra and Echotip ProcoreTM; Cook-Medical, Winston-Salem, NC, United States, and ExpectTM Slimline, Boston Scientific) and 20-G ProcoreTM histology needles (Cook-Medical, Winston-Salem). The selection of the needle was at the endosonographer’s discretion. All procedures were performed by first localizing the lymph node using an electronic curvilinear array echoendoscope and confirming the absence of intervening vessels via color flow and/or fine flow Doppler. A stylet was routinely used when puncturing the lymph node. Prior to puncturing the lymph node, the stylet was withdrawn 1 cm when using cytology needles; no adjustment of the stylet was required when using ProcoreTM needles. Once the needle was within the lesion (Figure 1), the stylet was advanced to the tip of the needle to expel any mucosal tissue from the gut wall and then removed. A 10-mL syringe was attached to the hub of the needle, and negative suction was then applied. Five to 10 to-and-fro movements were made within the lymph node in a fanning approach. Finally, the needle was withdrawn into the sheath, and the entire system was then withdrawn from the biopsy channel. The specimen was expelled into a tube containing a cytological solution (ThinPrep®; Cytyc Co., Marlborough, MA, United States) (Figure 2). Further needle passes were performed at the discretion of the endosonographer after gross visual assessment of the initial specimen. The puncture procedure was repeated until whitish material became macroscopically visible but was limited to a maximum of five passes if no material was obtained. All procedures were performed in the absence of an on-site pathologist. After the samples were processed, they were embedded in paraffin. Tissue sections of 3 to 4 μm were stained with hematoxylin-eosin for morphological evaluation and/or different immunohistochemical analysis (Figure 3). If the pathologists were unable to obtain a core for histological evaluation, they processed the same material as a cell block for cytological evaluation.
The procedure was performed on an outpatient basis, unless the patient had been hospitalized for other medical conditions. The outpatients were observed for immediate adverse events in the recovery room for 2 h before being discharged from the unit. Outpatients were also ambulatorily monitored for a minimum of 48 h for the detection of further complications. All adverse events were documented.
The final diagnosis was made according to one of the following reference methods: (1) definite benign or malignant histological diagnosis based on surgical resection of specimens from operated patients; (2) cytology or histology findings with definite proof of malignancy in patients with unresectable lesions according to EUS, multidetector CT scan and/or PET scan findings and compatible clinical follow-up; or (3) cytology or histology findings without proof of malignancy and compatible imaging evaluation, including EUS multidetector CT scan and/or PET scan and a minimum clinical follow-up time of 12 mo.
In patients with a high probability of an inflammatory disorder (such as sarcoidosis), the detection of specific types of granulomas was considered diagnostic of benign disease, but in patients suspected of disseminated malignancy, the detection of granulomas was considered a true negative for malignancy. Inconclusive or benign cytology was considered a false negative if further diagnostic workup or clinical follow-up showed signs positive for malignancy. Negative cytology was considered a true negative when histology did not show any abnormality or when imaging studies during follow-up showed spontaneous resolution or lack of progression of the lymph nodes under evaluation.
The statistical analysis was performed with SATA version 13. Categorical variables are presented as numbers (percentages). Continuous variables are presented as the means ± SDs. The number of needle passes is presented as the median (range). A multivariate logistic regression analysis was performed to identify the variables (lymph node location and size, needle type and number of needle passes) that affect the diagnostic yield. Performance characteristics of the EUS-guided tissue acquisition, including the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy, were calculated. These values were determined by comparing the EUS-guided tissue acquisition results with the final diagnosis of the lesions based on the abovementioned criteria. Accuracy was defined as the ratio of the sum of true positive and true negative values divided by the number of lesions. P < 0.05 was considered statistically significant.
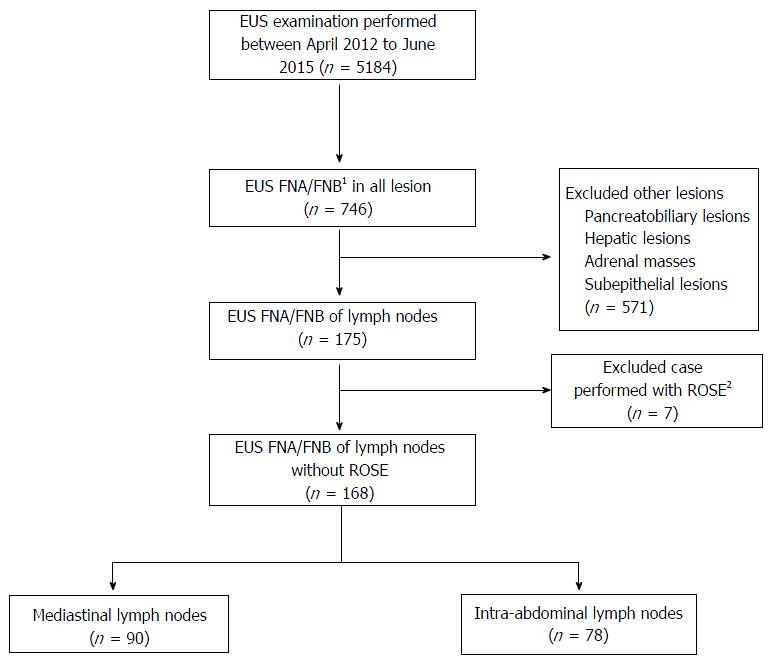
A total of 5184 EUS examinations were performed during the study period. Of these, 152 patients fulfilled the inclusion and exclusion criteria. A total of 168 EUS-guided tissue acquisitions of lymph nodes were performed (Figure 4), and 117 (69.6%) and 35 (30.4%) of these were males and females, respectively. Eight patients had two lymph nodes sampled from different sites. The mean age of the patients was 63.8 ± 15 years. Ninety (53.6%) cases presented enlarged mediastinal lymph nodes, and in 78 cases (46.4%), the enlarged lymph nodes were located in the intra-abdominal region. The mean size of the enlarged lymph nodes was 20.3 ± 9.9 mm.
EUS biopsy needles were used in 105 (62.5%) of the procedures, whereas cytology needles were used in only 63 cases (37.5%). The distribution of the needles used is shown in Table 1. A total of 214 needle passes were performed, with a median number of 1 (range of 1 to 4). Of these, 115 were performed on mediastinal lymph nodes, and 99 were performed on intra-abdominal lymph nodes (Table 1). There were no adverse events reported (0%).
| Characteristics | Total (n = 168) | Abdominal (n = 78) | Mediastinal (n = 90) | P value |
| Size (mm), mean ± SD | 20.3 ± 9.9 | 19.3 ± 9.0 | 21.4 ± 18.4 | 0.280 |
| Histology needle | 105 (61.3) | 48 (61.5) | 57 (61.1) | 0.955 |
| 19-G | 17 (10.1) | 5 (6.4) | 12 (13.3) | |
| 20-G | 7 (4.2) | 3 (3.9) | 4 (4.4) | |
| 22-G | 48 (28.6) | 23 (29.5) | 25 (27.8) | |
| 25-G | 33 (19.6) | 17 (21.8) | 16 (17.8) | |
| Cytology needle | 63 (38.7) | 30 (38.5) | 33 (38.9) | 0.955 |
| 19-G | 4 (2.4) | - | 4 (5.6) | |
| 22-G | 30 (17.9) | 14 (18.0) | 16 (17.8) | |
| 25-G | 29 (17.3) | 16 (20.5) | 13 (14.4) | |
| No. of passes (median, range) | 214 | 99 (11, 4) | 115 (1, 1-3) |
The final diagnosis was established based on surgical specimen evaluations for three cases (1.8%) and on the results of EUS-guided tissue acquisition and follow-up for the remaining 165 cases (98.2%). According to the defined gold standard, 87 (51.8%) cases were benign, and 81 (48.2%) were malignant. According to the EUS-guided tissue acquisition pathological results, 108 lymph nodes were considered benign (inflammatory, granulomatous), and 60 were considered malignant (carcinoma, neuroendocrine tumor (NET), melanoma and lymphoma) (Table 2). The sensitivity, specificity, PPV and NPV for the detection of malignancy were 74.1%, 100%, 100% and 80.6%, respectively. The overall accuracy was 87.5% (95%CI: 81.7-91.7) (Table 3).
| EUS FNA/FNB diagnoses | Final diagnoses | |
| Metastasis | ||
| Carcinoma | 51 (30.3) | 62 (36.9) |
| Neuroendocrine | 2 (1.2) | 2 (1.2) |
| Melanoma | 1 (0.6) | 1 (0.6) |
| Benign/reactive | ||
| Granulomatous | 8 (4.8) | 21 (12.5) |
| Unspecific reactive | 100 (59.5) | 66 (39.3) |
| Lymphoma | 6 (3.6) | 16 (9.5) |
| Overall (n = 168) | Estimate (%) | 95%CI |
| Sensitivity | 74.1% | 63.6-82.4 |
| Specificity | 100% | 95.8-100 |
| Positive predictive value | 100% | 94.0-100 |
| Negative predictive value | 80.6% | 72.1-86.9 |
| Accuracy | 87.5% | 81.7-91.7 |
The analysis of factors that might influence the diagnostic outcome of EUS-guided tissue acquisition techniques revealed that none of these factors demonstrated a significant effect. There was no correlation between the number of needle passes and the diagnostic accuracy. Only one case required four passes to procure sufficient material for pathological assessment. There was also no significant association between lymph node size and diagnostic accuracy. The presence of larger lymph nodes was not associated with better accuracy. The subgroup analysis of the needles used for the procedure also revealed no significant correlation with diagnostic accuracy (Table 4).
| Variable | OR (95%CI) | P value | OR (95%CI) | P value |
| Mediastinal location | 1.05 (0.42-2.64) | 0.907 | 0.93 (0.30-2.92) | 0.900 |
| Histology needle | 1.02 (0.40-2.64) | 0.952 | 1.01 (0.31-3.31) | 0.993 |
| No. of passes | 0.58 (0.27-1.24) | 0.161 | 1.54 (0.41-5.80) | 0.522 |
| Size | 0.98 (0.93-1.03) | 0.383 | 0.98 (0.93-1.03) | 0.411 |
The results of this study demonstrate that EUS-guided tissue acquisition has a high rate of clinical success and diagnostic accuracy in sampling tissue from enlarged mediastinal and intra-abdominal lymph nodes. EUS has the ability to identify and sample even small lymph nodes just a few millimeters in size. We did not identify any specific factor related to the procedure that affected the final diagnostic accuracy of the procedure (lymph node size and location, number of passes and/or needle type and size). In this large series of cases, no adverse events were reported, highlighting the safety of this technique.
When enlarged lymph nodes are detected based on different imaging techniques, such as MRI, CT scan or even EUS, there is often a need for these lesions to be further characterized to facilitate patient management. Malignant morphological predictors on EUS for lymph nodes include a rounded shape, size greater than 10 mm, hypoechoic echotexture and well-defined margins. If a lymph node exhibits all four features, the accuracy of malignant diagnosis ranges from 80% to 100%[14,15]. However, only 25% of malignant lymph nodes present all four features[16], and benign lymph nodes can also fulfill these criteria. Hence, tissue sampling from enlarged lymph nodes is important for obtaining a pathological diagnosis, thus optimizing and determining patient treatment. Previous studies have shown that the use of EUS-guided tissue acquisition greatly increases the diagnostic yield and accuracy with a good safety profile[2,3,14,17,18]. In fact, no adverse events were observed in our study. This low rate of complications could be related to the high spatial resolution of EUS and the short needle tract used to access the target lesions. EUS allows the presence of interposed vessels to be identified, allowing them to be avoided during the process of tissue acquisition. Since the first report of EUS-FNA in 1992[19], there have been significant advances in both the techniques and equipment used for tissue sampling.
Another important finding of our study was that few needle passes (1-2) were needed to obtain sufficient material for the pathological assessment in the absence of on-site pathological evaluation. This observation could be related to needle selection because the majority of the samples included in our study were acquired with biopsy needles (61.3%), although the choice of needle was not significant. A plausible explanation is the ability of biopsy needles to procure a larger amount of specimen with preserved cellular architecture, which is crucial for certain diagnoses, such as lymphoproliferative diseases and some inflammatory conditions[20]. Our findings are in agreement with previous studies that achieved adequate specimens in one to two needle passes[21,22]. In contrast to our study, LeBlanc et al[23] suggested a maximum of five needle passes for achieving sufficient sample because further needle passes did not increase the diagnostic sensitivity. Despite the preferential use of biopsy needles, the diagnostic accuracy for lymphoproliferative disease in our study cohort was suboptimal. This result could have been related to the procurement of a non-diagnostic part of the lymph node, e.g., the necrotic portion. Evidence suggests that employing complementary tools, such as contrast-enhanced EUS or EUS elastography, might help in guiding to the area for tissue procurement, thus improving the diagnostic yield[24,25]. However, further studies are required to validate the benefits of these advanced imaging techniques associated with EUS. In our series, suction was used as a standard in all cases. Whether the application of suction during EUS-guided tissue acquisition could have contributed to the overall results was not evaluated in the current study. However, previous studies published in the literature have shown that the application of suction does not affect the diagnostic accuracy but is associated with bloody contamination of the specimens[26-29].
In our study, intrinsic (size and location of the enlarged lymph nodes) and extrinsic factors (type or size of the needle and number of needle passes) had no bearing on the diagnostic yield of EUS-guided tissue acquisition. A possible explanation for these findings could be that the procedure is highly dependent on the operator. The endosonographers (Iglesias-Garcia J and Lariño-Noia J) who performed the procedures in this study were very experienced operators, and their technique could have contributed to the diagnostic yield. In addition, a high level of expertise in the interpretation of the acquired specimens is a key element in the diagnostic success of EUS-guided tissue acquisition. In our center, a dedicated cytopathologist examined all specimens from the EUS-guided tissue acquisition, which were dispatched to the ThinPrep laboratory (Cytyc Co, Marlborough, MA, United States). Liquid-based cytology has the advantage of a monolayer cell dispersion, avoiding the contamination of samples by mucus and blood and ensuring consistent cell preparation without artifacts. Whether the methods used for processing the specimens could have influenced the diagnostic yield remains to be determined.
There were 21 (19.4%) false-negative results. Sampling error might explain the relatively low sensitivity (74.1%). This was especially true for the presence of multiple enlarged lymph nodes; even given the efforts that were taken to target the most “malignant appearing” lymph node based on EUS features, the diagnostic yield was still suboptimal. This finding is in accordance with previous studies published in the literature. There are no reliable endosonographic features that indicate the malignant potential of enlarged lymph nodes[16,30]; hence, EUS-guided tissue acquisition will remain an important tool for discerning the nature of enlarged lymph nodes. According to recent reports, techniques such as EUS elastography, which helps distinguish between benign and malignant lesions, have gained much attention over the last decade[31,32]. Malignant lesions tend to be solid due to pathologic processes that decrease tissue elasticity and hence increase tissue stiffness, resulting in a blue pattern on qualitative EUS elastography[25]. Targeting the needle to the “solid area” during the tissue acquisition process could potentially improve the diagnostic yield of malignant lesions. Another factor that might have influenced these results, as previously mentioned, is the low number of needle passes performed, which was based on gross visual inspection by the endosonographers. Although we did not evaluate this specific variable in the present study, gross visual inspection might have hindered the procurement of better results. Performing another session of EUS-guided tissue acquisition may improve the diagnostic yield in situations where there is high clinical suspicion[33].
This study has certain limitations. It was a single-center study, and the results might not reflect practices or technologies used at other institutions. The lack of an on-site pathologist evaluation may influence the final results. Previous studies have established that on-site pathological evaluation improves the diagnostic yield by 10%-15%[9,34]. However, in many occasions and in many centers, on-site pathological evaluation is not possible due to manpower and cost limitations. The type of needle used was based on the endosonographer’s preference and was not randomized; however, there was a tendency toward the use of smaller and more flexible needles for lesions that pose technical difficulties or a high risk of bleeding. We did not include data corresponding to the gold standard analysis, which is considered the analysis of the surgical specimen. In fact, this was available in only three cases. However, this reflects the real clinical practice in the evaluation of enlarged lymph nodes. It is logical that lymph node enlargement in almost any disease is indicative of an advanced condition that does not merit surgical management. In addition, the presence of benign lymph nodes precludes the need for surgery. However, to overcome this limitation, we included a robust clinical follow-up protocol over a minimum of 12 mo, coupled with advanced imaging studies (MRI, CT scan and PET). The duration of follow-up was deemed sufficient because any malignancy with significant nodal involvement would be at least stage II disease[35-37]. The primary malignancy would be clearly evident or the patient could have succumbed to the disease within the follow-up period; hence, a 12-mo follow-up period was deemed appropriate in our study cohort.
In conclusion, EUS-guided tissue acquisition is a highly accurate technique for the evaluation of enlarged lymph nodes. No factor was found to affect the operating characteristics or accuracy of this technique. Increasing the number of needle passes could be an option to improve the diagnostic yield but might be associated with a theoretically increased risk of adverse events. Whether the results of the present study can be replicated in other centers remains questionable because the level of expertise of the endosonographer and cytopathologist could be crucial for the high diagnostic yield of this technique. Future studies should include multicenter approaches with different operators to draw firmer conclusions.
Lymph node enlargement is increasingly detected on various imaging modalities. In the absence of primary malignancy, it is difficult to establish a diagnosis for lymph node enlargement. Previously, a surgical approach was the main diagnostic tool in such circumstances despite its disadvantages. Recently, endoscopic ultrasound-fine-needle aspiration/biopsy (EUS-FNA/B) has become a pivotal diagnostic tool to guide appropriate treatment. In this study, we evaluated factors that might influence the diagnostic accuracy of EUS-guided tissue acquisition of lymph node enlargement in the absence of on-site cytopathological evaluation.
EUS-FNA/B is important for determining the nature of lymph node enlargement in the absence of an on-site pathologist. The results of this study contribute to the clarification that there are no factors associated with the diagnostic accuracy of this technique.
The results of this study showed that no factor appeared to affect the operating characteristics and accuracy of EUS-FNA/B. This finding raises the question of the possible role of complementary techniques, such as EUS-elastography and contrast-enhanced EUS, to help improve the accuracy of this technique. This issue requires further study.
This study suggests that EUS-guided tissue acquisition is a highly accurate technique for the evaluation of enlarged lymph nodes. No factors were identified to be associated with the operating characteristics or accuracy of this technique.
The author of this paper evaluated the accuracy of EUS-guided tissue acquisition of lymph node enlargement in the absence of an on-site pathologist. None of the variables evaluated were associated with the diagnostic accuracy of this technique. Further studies to assess the role of EUS elastography and contrast-enhanced EUS in this context might be valuable.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: McHenry L, Schmidt J S- Editor: Wang JL L- Editor: A E- Editor: Li D
| 1. | Yasuda I, Tsurumi H, Omar S, Iwashita T, Kojima Y, Yamada T, Sawada M, Takami T, Moriwaki H, Soehendra N. Endoscopic ultrasound-guided fine-needle aspiration biopsy for lymphadenopathy of unknown origin. Endoscopy. 2006;38:919-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | Bardales RH, Stelow EB, Mallery S, Lai R, Stanley MW. Review of endoscopic ultrasound-guided fine-needle aspiration cytology. Diagn Cytopathol. 2006;34:140-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Mohammad Alizadeh AH, Shahrokh S, Hadizadeh M, Padashi M, Zali MR. Diagnostic potency of EUS-guided FNA for the evaluation of pancreatic mass lesions. Endosc Ultrasound. 2016;5:30-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Gheonea DI, Săftoiu A, Popescu C, Ciurea T, Iordache S, Filip M, Maloş A. EUS and cytological EUS-FNA prognostic factors in patients with unresectable pancreatic cancer receiving chemotherapy. Hepatogastroenterology. 2010;57:155-161. [PubMed] |
| 5. | Puri R, Vilmann P, Sud R, Kumar M, Taneja S, Verma K, Kaushik N. Endoscopic ultrasound-guided fine-needle aspiration cytology in the evaluation of suspected tuberculosis in patients with isolated mediastinal lymphadenopathy. Endoscopy. 2010;42:462-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Berzosa M, Tsukayama DT, Davies SF, Debol SM, Cen YY, Li R, Mallery S. Endoscopic ultrasound-guided fine-needle aspiration for the diagnosis of extra-pulmonary tuberculosis. Int J Tuberc Lung Dis. 2010;14:578-584. [PubMed] |
| 7. | Sharma M, Rafiq A, Kirnake V. Dysphagia due to tubercular mediastinal lymphadenitis diagnosed by endoscopic ultrasound fine-needle aspiration. Endosc Ultrasound. 2015;4:348-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Rana SS, Chaudhary V, Sharma V, Sharma R, Gupta N, Sampath S, Mittal BR, Gupta R, Dutta U, Bhasin DK. Unusual cause of obstructive jaundice revealed by endoscopic ultrasound guided fine-needle aspiration of mediastinal lymph node. Endosc Ultrasound. 2015;4:73-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Klapman JB, Logrono R, Dye CE, Waxman I. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol. 2003;98:1289-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 362] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 10. | Cleveland P, Gill KR, Coe SG, Woodward TA, Raimondo M, Jamil L, Gross SA, Heckman MG, Crook JE, Wallace MB. An evaluation of risk factors for inadequate cytology in EUS-guided FNA of pancreatic tumors and lymph nodes. Gastrointest Endosc. 2010;71:1194-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Alsohaibani F, Girgis S, Sandha GS. Does onsite cytotechnology evaluation improve the accuracy of endoscopic ultrasound-guided fine-needle aspiration biopsy? Can J Gastroenterol. 2009;23:26-30. [PubMed] |
| 12. | Iglesias-Garcia J, Lariño-Noia J, Abdulkader I, Domínguez-Muñoz JE. Rapid on-site evaluation of endoscopic-ultrasound-guided fine-needle aspiration diagnosis of pancreatic masses. World J Gastroenterol. 2014;20:9451-9457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 13. | Savides TJ. Tricks for improving EUS-FNA accuracy and maximizing cellular yield. Gastrointest Endosc. 2009;69:S130-S133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Catalano MF, Sivak MV Jr, Rice T, Gragg LA, Van Dam J. Endosonographic features predictive of lymph node metastasis. Gastrointest Endosc. 1994;40:442-446. [PubMed] |
| 15. | Wiersema MJ, Hassig WM, Hawes RH, Wonn MJ. Mediastinal lymph node detection with endosonography. Gastrointest Endosc. 1993;39:788-793. [PubMed] |
| 16. | Bhutani MS, Hawes RH, Hoffman BJ. A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest Endosc. 1997;45:474-479. [PubMed] |
| 17. | Williams DB, Sahai AV, Aabakken L, Penman ID, van Velse A, Webb J, Wilson M, Hoffman BJ, Hawes RH. Endoscopic ultrasound guided fine needle aspiration biopsy: a large single centre experience. Gut. 1999;44:720-726. [PubMed] |
| 18. | Fisher L, Segarajasingam DS, Stewart C, Deboer WB, Yusoff IF. Endoscopic ultrasound guided fine needle aspiration of solid pancreatic lesions: Performance and outcomes. J Gastroenterol Hepatol. 2009;24:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Wiersema MJ, Hawes RH, Tao LC, Wiersema LM, Kopecky KK, Rex DK, Kumar S, Lehman GA. Endoscopic ultrasonography as an adjunct to fine needle aspiration cytology of the upper and lower gastrointestinal tract. Gastrointest Endosc. 1992;38:35-39. [PubMed] |
| 20. | Guo J, Sun B, Wang S, Ge N, Wang G, Wu W, Liu X, Sun S. Diagnosis of lymphoma by endoscopic ultrasound-assisted transendoscopic direct retroperitoneal lymph node biopsy: A case report (with video). Endosc Ultrasound. 2015;4:69-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000;51:184-190. [PubMed] |
| 22. | Berzosa M, Villa N, El-Serag HB, Sejpal DV, Patel KK. Comparison of endoscopic ultrasound guided 22-gauge core needle with standard 25-gauge fine-needle aspiration for diagnosing solid pancreatic lesions. Endosc Ultrasound. 2015;4:28-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | LeBlanc JK, Ciaccia D, Al-Assi MT, McGrath K, Imperiale T, Tao LC, Vallery S, DeWitt J, Sherman S, Collins E. Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest Endosc. 2004;59:475-481. [PubMed] |
| 24. | Dietrich CF, Sharma M, Hocke M. Contrast-enhanced endoscopic ultrasound. Endosc Ultrasound. 2012;1:130-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Iglesias-Garcia J, Lindkvist B, Lariño-Noia J, Domínguez-Muñoz JE. Endoscopic ultrasound elastography. Endosc Ultrasound. 2012;1:8-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Wallace MB, Kennedy T, Durkalski V, Eloubeidi MA, Etamad R, Matsuda K, Lewin D, Van Velse A, Hennesey W, Hawes RH. Randomized controlled trial of EUS-guided fine needle aspiration techniques for the detection of malignant lymphadenopathy. Gastrointest Endosc. 2001;54:441-447. [PubMed] |
| 27. | Puri R, Vilmann P, Săftoiu A, Skov BG, Linnemann D, Hassan H, Garcia ES, Gorunescu F. Randomized controlled trial of endoscopic ultrasound-guided fine-needle sampling with or without suction for better cytological diagnosis. Scand J Gastroenterol. 2009;44:499-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 28. | Nakai Y, Isayama H, Chang KJ, Yamamoto N, Hamada T, Uchino R, Mizuno S, Miyabayashi K, Yamamoto K, Kawakubo K. Slow pull versus suction in endoscopic ultrasound-guided fine-needle aspiration of pancreatic solid masses. Dig Dis Sci. 2014;59:1578-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 29. | Villa NA, Berzosa M, Wallace MB, Raijman I. Endoscopic ultrasound-guided fine needle aspiration: The wet suction technique. Endosc Ultrasound. 2016;5:17-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Song HJ, Kim JO, Eun SH, Cho YD, Jung IS, Cheon YK, Moon JH, Lee JS, Lee MS, Shim CS. Endoscopic Ultrasonograpic Findings of Benign Mediastinal and Abdominal Lymphadenopathy Confirmed by EUS-guided Fine Needle Aspiration. Gut Liver. 2007;1:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Săftoiu A, Vilmann P, Ciurea T, Popescu GL, Iordache A, Hassan H, Gorunescu F, Iordache S. Dynamic analysis of EUS used for the differentiation of benign and malignant lymph nodes. Gastrointest Endosc. 2007;66:291-300. [PubMed] |
| 32. | Giovannini M, Thomas B, Erwan B, Christian P, Fabrice C, Benjamin E, Geneviève M, Paolo A, Pierre D, Robert Y. Endoscopic ultrasound elastography for evaluation of lymph nodes and pancreatic masses: a multicenter study. World J Gastroenterol. 2009;15:1587-1593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 204] [Cited by in RCA: 199] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 33. | Téllez-Ávila FI, Martínez-Lozano JA, Rosales-Salinas A, Bernal-Méndez AR, Guerrero-Velásquez C, Ramírez-Luna MÁ, Valdovinos-Andraca F. Repeat endoscopic ultrasound fine needle aspiration after a first negative procedure is useful in pancreatic lesions. Endosc Ultrasound. 2016;5:258-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Jhala NC, Jhala D, Eltoum I, Vickers SM, Wilcox CM, Chhieng DC, Eloubeidi MA. Endoscopic ultrasound-guided fine-needle aspiration biopsy: a powerful tool to obtain samples from small lesions. Cancer. 2004;102:239-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; European Society for Medical Oncology (ESMO); European Society of Surgical Oncology (ESSO); European Society of Radiotherapy and Oncology (ESTRO). Gastric cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi57-vi63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 219] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 36. | Stahl M, Mariette C, Haustermans K, Cervantes A, Arnold D; ESMO Guidelines Working Group. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi51-vi56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 37. | Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goere D, Seufferlein T, Haustremans K, Van Laethem JL, Conroy T. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26:v56-v68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 905] [Cited by in RCA: 930] [Article Influence: 93.0] [Reference Citation Analysis (0)] |