Published online Aug 21, 2017. doi: 10.3748/wjg.v23.i31.5713
Peer-review started: March 7, 2017
First decision: April 17, 2017
Revised: June 11, 2017
Accepted: June 19, 2017
Article in press: June 19, 2017
Published online: August 21, 2017
Processing time: 165 Days and 17.1 Hours
To develop a reliable and simple method to identify important biological metabolites and relevant pathways for taurine in hepatic stellate cells (HSCs), in order to provide more data for taurine therapy.
All the biological samples were analyzed by using high-performance liquid chromatography-time electrospray ionization/quadrupole-time of flight mass spectrometry. Principal component analysis and partial least squares discriminant analysis were used to identify statistically different metabolites for taurine in HSCs, and metabolomic pathway analysis was used to do pathway analysis for taurine in HSCs. The chemical structure of the related metabolites and pathways was identified by comparing the m/z ratio and ion mode with the data obtained from free online databases.
A total of 32 significant differential endogenous metabolites were identified, which may be related to the mechanism of action of taurine in HSCs. Among the seven relevant pathways identified, sphingolipid metabolism pathway, glutathione metabolism pathway and thiamine metabolism pathway were found to be the most important metabolic pathways for taurine in HSCs.
This study showed that there were distinct changes in biological metabolites of taurine in HSCs and three differential metabolic pathways including sphingolipid pathway, glutathione pathway and thiamine metabolism pathway might be of key importance in mediating the mechanism of action of taurine in HSCs.
Core tip: At the cellular level, it is reported that the activation of hepatic stellate cells (HSCs) in the subendothelial space may result in hepatic fibrosis. Although taurine was found to increase HSC apoptosis significantly, its molecular mechanisms are still unknown. This study developed a reliable and simple method to identify important biological metabolites and relevant pathways for taurine in HSCs, in order to provide more data for taurine therapy. We found that there were distinct changes in the biological metabolites of taurine in HSCs, and identified three differential metabolic pathways that might be of key importance in mediating the mechanism of action of taurine in HSCs.
- Citation: Deng X, Liang XQ, Lu FG, Zhao XF, Fu L, Liang J. Metabolomic profiling for identification of metabolites and relevant pathways for taurine in hepatic stellate cells. World J Gastroenterol 2017; 23(31): 5713-5721
- URL: https://www.wjgnet.com/1007-9327/full/v23/i31/5713.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i31.5713
Hepatic fibrosis (HF) is a scarring process in which the liver forms scar tissue due to an abnormal deposition of extracellular matrix (ECM)[1]. Recently, more and more attention has been paid to the molecular mechanisms of hepatic fibrosis. At the cellular level, it is reported that the activation of hepatic stellate cells (HSCs) in the subendothelial space may result in hepatic fibrosis[2]. Moreover, reversal or apoptosis of activated HSCs has been proven to be beneficial for the treatment or regression of hepatic fibrosis[3].
Metabolomics methods can be used to characterize the metabolic profiles of a biological system. As metabolites with relatively low molecular weight are downstream products of biological processes, their identity and concentrations in a living biological system can provide biochemical signatures for globally tracking the physiological effects and exploring the drug effects[4-6]. A previous study[7] systematically analyzed the protective effects of traditional Chinese medicine Hongshan Capsules, and identified the potential target biomarkers through the metabonomic approach of ultra-performance liquid chromatography coupled to mass spectrometry. Therefore, metabolomics methods can be used to identify biomarkers for clinical drug therapy, especially in cancer research[8,9].
Taurine, a beta-amino acid with a simple structure that is extracted from animal tissue, has been investigated as a promising drug for the treatment of many hepatic injuries[10-13]. In previous studies, taurine was showed to significantly increase the apoptosis of HSCs and raise the levels of 19 proteins related to cellular apoptosis or oxidation in HSCs[14,15]. However, the exact metabolic pathways and molecules involved in the mechanisms of action of taurine are still unknown. Therefore, this study aimed at developing a reliable and simple method to find important biological metabolites and pathways that are related to taurine in HSCs, in order to provide more data for taurine therapy.
Human HSCs (LX-2) were obtained from Xiangya Central Experiment Laboratory of Central South University (Changsha, China) and were incubated in DMEM (Thermo Scientific Hyclone, Logan, UT, United States) containing 100 U/mL penicillin (North China Pharmaceutical, China), 100 μg/mL streptomycin (North China Pharmaceutical, China) and 10% fetal bovine serum (Biochrom AG, Berlin, Germany) at room temperature in an incubator with 50 mL/L CO2 and saturated humidity. The culture medium was replaced every two days. Trypsin (0.25%) was added for digestion when the confluence of HSCs reached 80%-90% and the supernatant was discarded after centrifugation at 1000 r/min for 5 min. Then, the cells were resuspended to a density of 5 × 105/mL.
MTT method was used to determine the optimum drug concentration of taurine for the subsequent study. Briefly, HSCs were added into a 96-well plate at a density of 5 × 104 cells per well. Natural taurine (Yuanlong Pearl Co. Ltd., Beihai, China) was dissolved in dimethyl sulphoxide (DMSO) at a concentration of 8 mol/L, and then diluted with 2% DMEM to 10, 20, 40, 60, 80 and 100 mmol/L. Six replicates were applied for each concentration of taurine and the whole reaction system was maintained for 48 h. Cells treated with DMSO alone were used as controls. Subsequently, 10 μL of MTT solution (5 mg/mL in PBS) was added and the cells were further incubated for 4 h at 37 °C. Then, the reaction was terminated by the addition of 100 μL DMSO and absorbance was measured at 495 nm using a microplate reader (Molecular Devices, United States). Based on the results, the concentration of 40 mmol/L was chosen as the optimum drug concentration of taurine for the following study.
For the following metabolomic study, HSCs were incubated in 10-cm culture dishes until approximately 70% confluence and then treated with celastrol and other control drugs for 12 h. Each dish was washed twice at 37 °C with PBS after removing the culture medium, and dried in the vacuum. Then, the cells were quenched by adding 1.5 mL HPLC-grade methanol at -80 °C and were separated from the culture dish with a cell lifter (Fisher Scientific, United States). The cell solution was subsequently transferred to a 2 mL centrifuge tube and frozen in liquid nitrogen until liquid-liquid extraction. Six replicates were applied for 40 μmol/L of natural taurine for 48 h as the test group and the same concentration of DMSO was used as the control group. To avoid cell cytotoxicity, the final concentration of DMSO must be less than 0.1% for both the taurine group and the control group. Additionally, five parallel blank culture dishes were trypsinized and counted for the normalizing the subsequent analysis.
After incubation, the culture medium was removed generally and the residual medium was removed by using 2 mL 0.9% [w/v] ice-cold isotonic saline (NaCl) to arrest cellular metabolism. Subsequently, the cells were added with 1 mL cold methanol/water (4:1) to quench cells and then collected in 2 mL centrifuge tubes. Then, the cells were ultrasonicated for 5 min (5 s ultrasonication at a 10-s interval) in an ice bath and centrifuged at 13000 g for 10 min at 4 °C. Finally, the supernatant was dried with nitrogen and stored at -80 °C before detection.
Samples were resolved with acetonitrile/water (1:1, v/v) mixture according to the cell number counted in advance and detected by high performance liquid chromatography-electrosprayionization/quadrupole-time-of-flight mass spectrometry (HPLC ESI/Q-TOF MS) (Agilent Technologies, United States). All the samples were separated on an Agilent C18 column (2.1 mm × 100 mm, 1.8 μm, Agilent Technologies, United States) with the following parameters: injection volume, 4 μL; flow rate, 0.35 mL/min; column temperature, 40 °C. The mobile phase was composed of (A) 0.1% formic acid water solution and (B) 0.1% formic acid acetonitrile solution. The gradient elution program (Table 1) was linearly increased from 5% to 20% B (0-6 min), stable at 50% B (6-8 min), linearly increased to 95% B (8-12 min) and maintained for 3 min. The quality control (QC) was prepared and injected before the injection of test samples.
| Time (min) | Flow rate (mL/min) | Solution B (%) |
| 0 | 0.35 | 5 |
| 1 | 0.35 | 5 |
| 6 | 0.35 | 20 |
| 9 | 0.35 | 50 |
| 13 | 0.35 | 95 |
| 15 | 0.35 | 95 |
Both ESI+ and ESI- ionization modes were used to find the potential specific metabolites of taurine in HSCs. The ionization mode was set at a capillary voltage of 4.0 kV (ESI+) or 3.5 kV (ESI-), cone voltage of 35V (ESI+) and 50V (ESI-), source temperature of 100 °C, cone gas flow of 50 L/h, desolvation gas flow, desolvation gas flow of 600 L/h, and desolvation gas temperature of 350 °C. Data were collected in the centroid mode with the mass range (m/z) of 50-1000, scan time of 0.03 s and inter-scan delay of 0.02 s. To lock the mass system, 100 pg/μL leucine-enkephalin (m/z 556.2771 in ESI+ mode or m/z 554.2615 in ESI- mode) at 0.05 mL/min with the frequency of 10 s was used.
After excluding the noise and background interference, raw mass spectral data of both ESI+ and ESI- modes were analyzed by using Mass Profiler (Agilent Technologies, United States) to generate data, including retention time, signal intensity and m/z ratio for peak identification. Subsequently, SIMCA-P 13.0 software (Umetric AB, CA, United States) was applied to do principal component analysis (PCA) to distinguish different scatters of the biological metabolites for taurine in HSCs, partial least squares discriminant analysis (PLS-DA) to find statistically different metabolites. Variable importance in the projection (VIP) plots (VIP > 1) of the PLS-DA was used to find the exact potential biomarkers. Finally, the structure of the related metabolites and pathways was identified by comparing the m/z ratio and ion mode with the data obtained from free online databases including Human Metabolome Database (HMDB) (http://www.hmdb.ca), Metlin metabolomics database (http://metlin.scripps.edu/) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database (http://www.genome.jp/kegg/pathway.html). MetPA network software[16] (http://metpa.metabolomics.ca/Met PA/faces/Home.jsp) was used to do pathway analysis for taurine in HSCs.
In the current study, all the biological metabolites of taurine in HSCs were detected by HPLC-ESI/Q-TOFMS, which might provide useful information for unveiling the underlying molecular mechanisms of taurine in HSCs. The total ion current spectra of the samples between the control and taurine-treated HSCs differed greatly, suggesting that there might be some metabolic changes for taurine in HSCs.
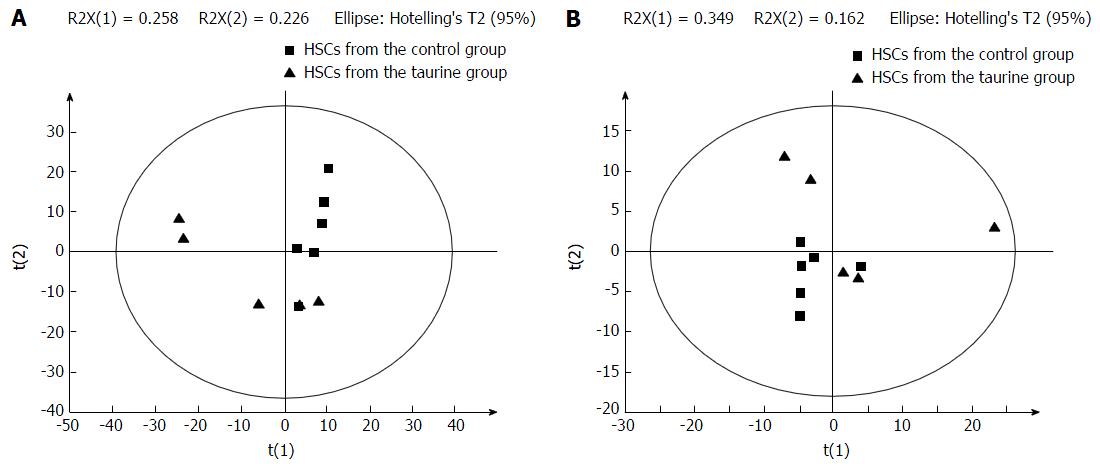
PCA statistically divided all the detected metabolites into smaller clusters as principal components (PCs) to find the potential biomarkers. In this study, all the samples were classified into two small clusters between the control and taurine-treated cells in the PCA score plots (Figure 1), demonstrating that there were significant metabolic differences between the control and taurine-treated HSCs.
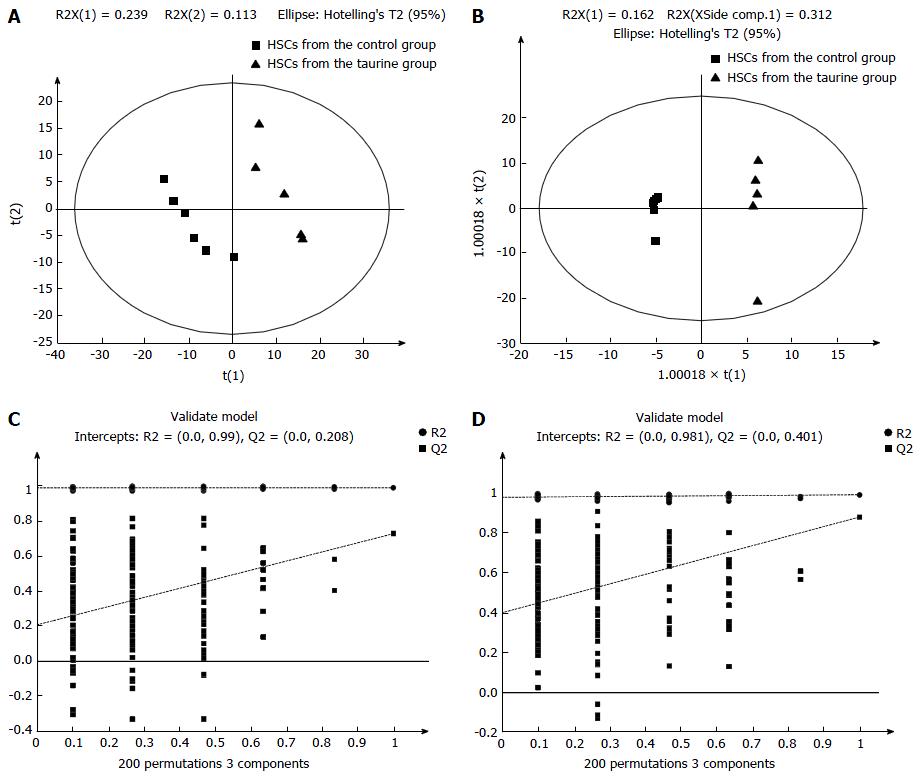
Then, the potential important metabolites were further analyzed by using PLS-DA. Consistent with the results of PCA, PLS-DA plots showed two clear groups between the control and taurine-treated cells (Figure 2). Moreover, a total of 27 metabolites in ESI+ mode and five metabolites in ESI- mode were found to be the significant metabolites between the control and taurine-treated cells (VIP > 1) (P < 0.05) (Table 2).
| Number | Mode | VIP | RT (min) | m/z ratio | Compound name | Fold change (B/A) | P value |
| 1 | ESI+ | 1.312 | 11.16 | 495.3338 | PC (16:0) | -0.758↓ | 0.037 |
| 2 | ESI+ | 1.973 | 14.02 | 148.0160 | 2-oxo-4-methylthiobutanoic acid | -3.094↓ | 0.001 |
| 3 | ESI+ | 1.326 | 7.51 | 215.1890 | Amino-dodecanoic acid | 1.249↑ | 0.043 |
| 4 | ESI+ | 1.316 | 3.89 | 231.1475 | Butyryl-L-carnitine | -1.711↓ | 0.041 |
| 5 | ESI+ | 1.644 | 8.87 | 273.2679 | C16 Sphinganine | -0.837↓ | 0.034 |
| 6 | ESI+ | 1.329 | 0.74 | 161.1047 | Carnitine | 2.324↑ | 0.049 |
| 7 | ESI+ | 1.570 | 13.27 | 375.3125 | Docosatetraenoyl Ethanolamide | 2.200↑ | 0.017 |
| 8 | ESI+ | 1.706 | 11.46 | 399.3362 | Palmitoylcarnitine | -1.419 | 0.005 |
| 9 | ESI+ | 1.484 | 11.01 | 199.1943 | Dodecanamide | 1.521↑ | 0.022 |
| 10 | ESI+ | 1.590 | 1.09 | 307.0848 | Glutathione | -1.372↓ | 0.019 |
| 11 | ESI+ | 1.268 | 6.03 | 259.1792 | Hexanoylcarnitine | -1.216↓ | 0.046 |
| 12 | ESI+ | 1.565 | 4.59 | 282.1684 | Hydroxydesipramine | 2.210↑↓ | 0.013 |
| 13 | ESI+ | 1.601 | 11.01 | 569.3486 | LysoPC(22:5) | 17.551↑ | 0.048 |
| 14 | ESI+ | 1.483 | 1.01 | 175.0482 | N-acetylaspartate | -1.160↓ | 0.018 |
| 15 | ESI+ | 1.495 | 12.63 | 369.3254 | N-palmitoyl isoleucine | 1.375↑ | 0.017 |
| 16 | ESI+ | 1.557 | 12.15 | 369.3254 | N-palmitoyl isoleucine | 2.683↑ | 0.039 |
| 17 | ESI+ | 1.245 | 10.48 | 371.3045 | N-stearoyl serine | -1.027↓ | 0.048 |
| 18 | ESI+ | 1.312 | 13.05 | 281.2728 | Oleamide | -0.852↓ | 0.036 |
| 19 | ESI+ | 1.469 | 10.13 | 212.1417 | Oxo-dodecenoic acid | 1.750↑ | 0.019 |
| 20 | ESI+ | 1.595 | 11.24 | 479.3025 | PC (15:1)/PE (18:1) | -1.141↓ | 0.018 |
| 21 | ESI+ | 1.284 | 11.30 | 521.3494 | PC (18:1) | -0.727↓ | 0.048 |
| 22 | ESI+ | 2.021 | 8.93 | 317.2939 | Phytosphingosine | -1.274↓ | 0.001 |
| 23 | ESI+ | 1.290 | 0.65 | 202.2163 | Spermine | -0.874↓ | 0.039 |
| 24 | ESI+ | 1.443 | 9.90 | 301.2992 | Sphinganine | -0.807↓ | 0.040 |
| 25 | ESI+ | 1.394 | 11.60 | 299.2835 | Sphingosine | -0.949↓ | 0.026 |
| 26 | ESI+ | 1.430 | 12.34 | 427.3675 | Stearoylcarnitine | -1.006↓ | 0.026 |
| 27 | ESI+ | 1.818 | 0.80 | 117.0791 | Valine | -0.681↓ | 0.037 |
| 28 | ESI- | 2.017 | 1.13 | 192.0270 | Citric acid | -0.870↓ | 0.001 |
| 29 | ESI- | 1.678 | 11.11 | 453.2859 | Glycerophospho-N-Palmitoyl Ethanolamine | -1.125↓ | 0.011 |
| 30 | ESI- | 2.076 | 11.46 | 479.3016 | PC (15:1) | -1.697↓ | 0.000 |
| 31 | ESI- | 1.648 | 0.69 | 264.1045 | Thiamine | 0.394↑ | 0.040 |
| 32 | ESI- | 1.448 | 9.25 | 250.1204 | Ubiquinone-1 | 1.538↑ | 0.031 |
The definite identity of the significant metabolites in the biological samples and their contributions to the biological processes are important for the current metabolomics study. Therefore, the structure of the related metabolites and pathways was identified by comparing the m/z ratio and ion mode with the data obtained from on-line free databases including HMDB (http://www.hmdb.ca), Metlin metabolomics database (http://metlin.scripps.edu/) and KEGG pathway database (http://www.genome.jp/kegg/pathway.html).
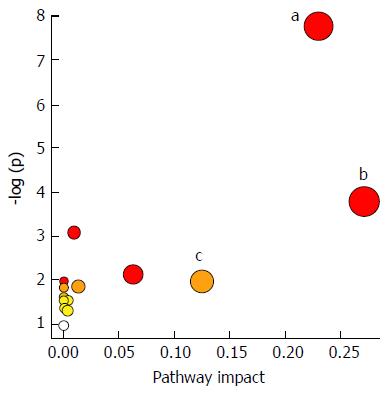
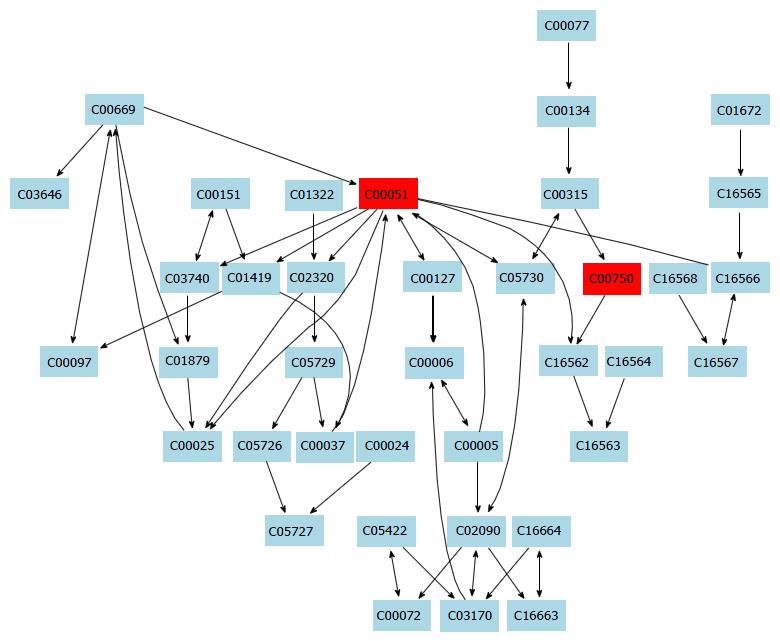
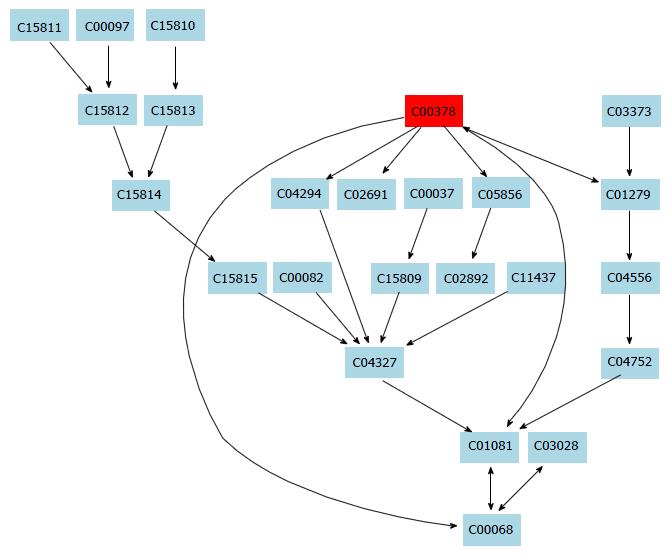
Additionally, 16 potential metabolic pathways were analyzed by MetPA network software and three significant metabolic pathways (sphingolipid metabolism, glutathione metabolism and thiamine metabolism) were found to be the most important metabolic pathways for taurine in HSCs (Table 3 and Figure 3).
| Pathway name | Compounds1 | Expected2 | Hits | Raw P3 | FDR P4 | Impact |
| Glutathione metabolism | 38 | 0.23681 | 2 | 0.022 | 0.896 | 0.272 |
| Sphingolipid metabolism | 25 | 0.1558 | 3 | 0.000 | 0.033 | 0.231 |
| Thiamine metabolism | 24 | 0.14956 | 1 | 0.140 | 1 | 0.125 |
| Citrate cycle (TCA cycle) | 20 | 0.12464 | 1 | 0.118 | 1 | 0.063 |
| Valine, leucine and isoleucine biosynthesis | 27 | 0.16826 | 1 | 0.156 | 1 | 0.013 |
| Cysteine and methionine metabolism | 56 | 0.34898 | 2 | 0.046 | 1 | 0.009 |
| Glyoxylate and dicarboxylate metabolism | 50 | 0.31159 | 1 | 0.271 | 1 | 0.003 |
| Glycerophospholipid metabolism | 39 | 0.24304 | 1 | 0.218 | 1 | 0.003 |
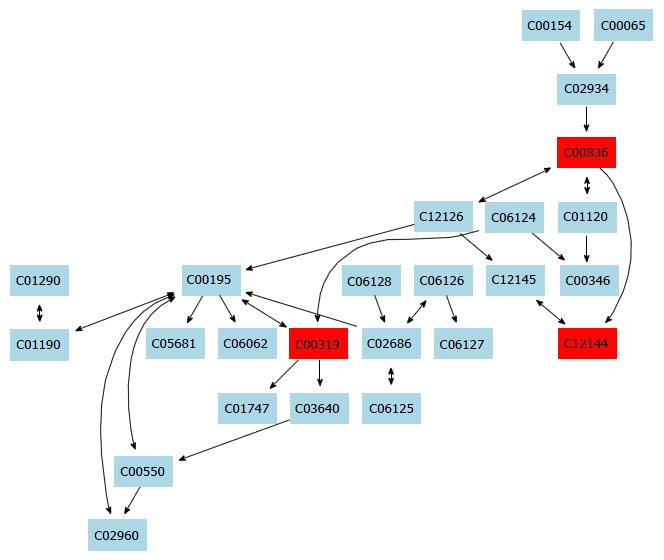
In this study, a simple chromatography method coupled with pathway analysis by using high-performance liquid chromatography-time electrospray ionization/quadrupole-time of flight mass spectrometry (HPLC-ESI/Q-TOF-MS) was successfully performed for analyzing the mechanism of taurine in HSCs. A total of 32 significant differential endogenous metabolites were identified. Among the seven relevant pathways for taurine, sphingolipid metabolism pathway (Figure 4), glutathione metabolism pathway (Figure 5) and thiamine metabolism pathway were found to be the most important metabolic pathways for taurine in HSCs.
The sphingolipid metabolism pathway contributes greatly to structural functions and cellular signaling. For example, membrane sphingolipids can regulate cell proliferation, differentiation and death[17-19]. Increasing evidence shows that sphingolipids are important in stress and ligand-induced hepatocellular death, which can cause several liver diseases like steatohepatitis, ischaemia-reperfusion hepatic damage or hepatic cancer[20]. Additionally, increased levels of sphingolipids in specific cell subcompartments can cause liver dysfunctions and some inherited diseases. Tumour necrosis factor (TNF) is an important death receptor, and abnormal expression of TNF can cause many liver diseases[21]. Thus, TNF becomes an important target for liver disease. In this study, taurine was found to be able to regulate sphingolipid metabolism. Therefore, we hypothesize that taurine might be able to intervene the activation of HSCs and regulate TNF expression. However, the exact mechanism of the action of taurine in the HSCs needs further investigation.
Besides, lysophosphatidylcholine (LysoPC) can affect HSCs through several mechanisms. First, LysoPC can inhibit proliferation of endothelial cells and increase their apoptosis, thereby affecting the molecular structure of the endothelium and the proliferation of vascular smooth muscle cells[22,23]. Second, LysoPC can downregulate endothelium-derived NO and activate the LOX-1 receptor to desensitize eNOS, thus causing endothelial dysfunction[24]. Furthermore, activation of HSCs can lead to increased expression of genes relevant to lipid accumulation and increased levels of intracellular lipids. In this study, LysoPC was found as the significantly different metabolite between the control and taurine-treated groups. LysoPC is an important plasma lipid cell signaling molecule for LDL oxidization and can be generated by phospholipase A2 hydrolysis or phosphatidylcholine (PC) oxidation[25]. Thus, we hypothesize that the interventional effect of taurine on HSCs is related with lipid metabolism. This was consistent with the finding of a previous study which showed that taurine can obviously decrease LDL-C level[26].
Moreover, amino-dodecanoic acid, 2-oxo-4-methylthiobutanoic acid, oxo-dodecenoic acid, valine, citric acid, thiamine, and N-acetylaspartate were also identified in the current study. It is therefore proposed that taurine may intervene HSCs through the valine, leucine and isoleucine biosynthesis pathway, cysteine and methionine metabolism pathway, citrate cycle (TCA cycle) pathway, alanine, aspartate and glutamate metabolism pathway, lysine degradation pathway, glyoxylate and dicarboxylate metabolism pathway, arginine and proline metabolism pathway and aminoacyl-tRNA biosynthesis pathway.
This study has several limitations. Validation of this current chromatography method using targeted metabolomics and application of this current chromatography method on other cell lines or in vivo models need to be studied in the future. In addition, dose-dependence of taurine should be clarified in the future.
To conclude, this study has found that there were distinct changes in the biological metabolites of taurine in HSCs by using HPLC-ESI/Q-TOF-MS. A total of 32 significant differential endogenous metabolites and 3 differential metabolic pathways including sphingolipid pathway, glutathione pathway and thiamine metabolism pathway (Figure 6) were identified, which might be of key importance in mediating the mechanism of action of taurine in HSCs.
It is well-known that quiescent hepatic stellate cells (HSC) may become matrix-secreting myofibroblasts upon activation and are the primary sources of extracellular matrix (ECM) during liver fibrogenesis. Thus, a novel therapeutic method for hepatic fibrosis can rely on the inhibition of activated HSCs. Taurine, a beta-amino acid with a simple structure that is extracted from animal tissue, has been investigated as a promising drug for the treatment of many hepatic injuries. In previous studies, taurine was found to significantly increase the apoptosis of HSCs and raise the concentrations of 19 proteins related to cellular apoptosis or oxidation in HSCs. However, the exact metabolic pathways and molecular mechanisms for taurine are still unknown.
Metabolomics methods can be used to characterize the metabolic profiles of a biological system. Since metabolites with relatively low molecular weight are downstream products of biological processes, their identity and concentrations in a living biological system can provide biochemical signatures for globally tracking the physiological effects and exploring the drug effects. Therefore, metabolomics methods can be used to identify biomarkers for clinical drug therapy, especially in cancer research.
In this study, a simple chromatography method coupled with pathway analysis by using high-performance liquid chromatography-time electrospray ionization/quadrupole-time of flight mass spectrometry was successfully performed for analyzing the mechanism of action of taurine in HSCs. A total of 32 significantly differential endogenous metabolites were identified, which may mediate the mechanism of action of taurine in HSCs. Among the seven relevant pathways identified for taurine, sphingolipid metabolism pathway, glutathione metabolism pathway and thiamine metabolism pathway were found to be the most important metabolic pathways for taurine in HSCs.
Taurine, a beta-amino acid with a simple structure that is extracted from animal tissue, has been investigated as a promising drug for the treatment of many hepatic injuries. In previous studies, taurine was found to significantly increase the apoptosis of HSCs and raise the concentrations of 19 proteins related to cellular apoptosis or oxidation in HSCs. However, the exact metabolic pathways and molecular mechanisms for taurine are still unknown. Therefore, this study aimed at developing a reliable and simple method to identify important biological metabolites and relevant pathways for taurine in HSCs, in order to provide more data for taurine therapy.
Hepatic fibrosis is a pathological condition characterized by excessive deposition of ECM proteins, which may led to the development of liver cirrhosis or even hepatocellular carcinoma in the absence of effective treatment.
This is an interesting paper looking to identify biological pathways and metabolites for taurine in hepatocytes.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Tsoulfas G S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Li D
| 1. | Roderburg C, Luedde M, Vargas Cardenas D, Vucur M, Mollnow T, Zimmermann HW, Koch A, Hellerbrand C, Weiskirchen R, Frey N. miR-133a mediates TGF-β-dependent derepression of collagen synthesis in hepatic stellate cells during liver fibrosis. J Hepatol. 2013;58:736-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (1)] |
| 2. | Zhang CY, Yuan WG, He P, Lei JH, Wang CX. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World J Gastroenterol. 2016;22:10512-10522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 358] [Cited by in RCA: 452] [Article Influence: 50.2] [Reference Citation Analysis (4)] |
| 3. | Jung YK, Yim HJ. Reversal of liver cirrhosis: current evidence and expectations. Korean J Intern Med. 2017;32:213-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 4. | Li JP, Guo JM, Shang EX, Zhu ZH, Liu Y, Zhao BC, Zhao J, Tang ZS, Duan JA. Quantitative determination of five metabolites of aspirin by UHPLC-MS/MS coupled with enzymatic reaction and its application to evaluate the effects of aspirin dosage on the metabolic profile. J Pharm Biomed Anal. 2017;138:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Ilievski V, Kinchen JM, Prabhu R, Rim F, Leoni L, Unterman TG, Watanabe K. Experimental Periodontitis Results in Prediabetes and Metabolic Alterations in Brain, Liver and Heart: Global Untargeted Metabolomic Analyses. J Oral Biol (Northborough). 2016;3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Tang Z, Liu L, Li Y, Dong J, Li M, Huang J, Lin S, Cai Z. Urinary Metabolomics Reveals Alterations of Aromatic Amino Acid Metabolism of Alzheimer’s Disease in the Transgenic CRND8 Mice. Curr Alzheimer Res. 2016;13:764-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Liu Y, Lin ZB, Tan GG, Chu ZY, Lou ZY, Zhang JP, Hong ZY, Chai YF. Metabonomic studies on potential plasma biomarkers in rats exposed to ionizing radiation and the protective effects of hong shan, capsule. Metabolomics. 2013;9:1082-1095. [DOI] [Full Text] |
| 8. | Maertens A, Bouhifd M, Zhao L, Odwin-DaCosta S, Kleensang A, Yager JD, Hartung T. Metabolomic network analysis of estrogen-stimulated MCF-7 cells: a comparison of overrepresentation analysis, quantitative enrichment analysis and pathway analysis versus metabolite network analysis. Arch Toxicol. 2017;91:217-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Ganti S, Weiss RH. Urine metabolomics for kidney cancer detection and biomarker discovery. Urol Oncol. 2011;29:551-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Zhao XF, Liang XQ, Zhu CY, Liang J, Fu L, Deng X. Effect of natural taurine combine with traditional Chinese medicine on hepatic fibrosis in rats. Internal Medicine. 2016;819-822. |
| 11. | Zhao XF, Liang J, Zhu CY, Liang XQ, Wen B, Deng X. Effects of natural taurine combined with Chinese medicine on hepatic stellate cells. Zhongguo Yixue Gongcheng. 2016;24:5-9. |
| 12. | Deng X, Liang J, Li YZ, Huang B, Zhang XL. Protective effect of natural taurine on mitochondria of hepatic fibrosis in rats. Xi’an Jiaotong Daxue Xuebao. 2007;28:648-703. |
| 13. | Miyazaki T, Matsuzaki Y. Taurine and liver diseases: a focus on the heterogeneous protective properties of taurine. Amino Acids. 2014;46:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Liang J, Deng X, Wu JY, Yang GY, Huang RB. The effect of natural taurine on hepatic stellate cell of rat. Guangxi Yixue. 2006;1:35-37. |
| 15. | Deng X, Liang J, Lin ZX, Wu FS, Zhang YP, Zhang ZW. Natural taurine promotes apoptosis of human hepatic stellate cells in proteomics analysis. World J Gastroenterol. 2010;16:1916-1923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Xia J, Wishart DS. MetPA: a web-based metabolomics tool for pathway analysis and visualization. Bioinformatics. 2010;26:2342-2344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 558] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 17. | Green CL, Mitchell SE, Derous D, Wang Y, Chen L, Han JJ, Promislow DEL, Lusseau D, Douglas A, Speakman JR. The effects of graded levels of calorie restriction: IX. Global metabolomic screen reveals modulation of carnitines, sphingolipids and bile acids in the liver of C57BL/6 mice. Aging Cell. 2017;16:529-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Bilal F, Pérès M, Le Faouder P, Dupuy A, Bertrand-Michel J, Andrieu-Abadie N, Levade T, Badran B, Daher A, Ségui B. Liquid Chromatography-High Resolution Mass Spectrometry Method to Study Sphingolipid Metabolism Changes in Response to CD95L. Methods Mol Biol. 2017;1557:213-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Ozbayraktar FB, Ulgen KO. Molecular facets of sphingolipids: mediators of diseases. Biotechnol J. 2009;4:1028-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Wang SY, Zhang JL, Zhang D, Bao XQ, Sun H. [Recent advances in study of sphingolipids on liver diseases]. Yao Xue Xue Bao. 2015;50:1551-1558. [PubMed] |
| 21. | Kiraz Y, Adan A, Kartal Yandim M, Baran Y. Major apoptotic mechanisms and genes involved in apoptosis. Tumour Biol. 2016;37:8471-8486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 417] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 22. | Takahashi M, Okazaki H, Ogata Y, Takeuchi K, Ikeda U, Shimada K. Lysophosphatidylcholine induces apoptosis in human endothelial cells through a p38-mitogen-activated protein kinase-dependent mechanism. Atherosclerosis. 2002;161:387-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Hirsova P, Ibrahim SH, Krishnan A, Verma VK, Bronk SF, Werneburg NW, Charlton MR, Shah VH, Malhi H, Gores GJ. Lipid-Induced Signaling Causes Release of Inflammatory Extracellular Vesicles From Hepatocytes. Gastroenterology. 2016;150:956-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 392] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 24. | Campos-Mota GP, Navia-Pelaez JM, Araujo-Souza JC, Stergiopulos N, Capettini LSA. Role of ERK1/2 activation and nNOS uncoupling on endothelial dysfunction induced by lysophosphatidylcholine. Atherosclerosis. 2017;258:108-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Kume N, Gimbrone MA Jr. Lysophosphatidylcholine transcriptionally induces growth factor gene expression in cultured human endothelial cells. J Clin Invest. 1994;93:907-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 259] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 26. | Dan H, Wu J, Peng M, Hu X, Song C, Zhou Z, Yu S, Fang N. Hypolipidemic effects of Alismatis rhizome on lipid profile in mice fed high-fat diet. Saudi Med J. 2011;32:701-707. [PubMed] |