Published online Aug 7, 2017. doi: 10.3748/wjg.v23.i29.5345
Peer-review started: February 2, 2017
First decision: March 17, 2017
Revised: April 25, 2017
Accepted: July 4, 2017
Article in press: July 4, 2017
Published online: August 7, 2017
Processing time: 192 Days and 19.1 Hours
To clarify the mechanisms of connexin 32 (Cx32) downregulation by potential transcriptional factors (TFs) in Helicobacter pylori (H. pylori)-associated gastric carcinogenesis.
Approximately 25 specimens at each developmental stage of gastric carcinogenesis [non-atrophic gastritis, chronic atrophic gastritis, intestinal metaplasia, dysplasia and gastric carcinoma (GC)] with H. pylori infection [H. pylori (+)] and 25 normal gastric mucosa (NGM) without H. pylori infection [H. pylori (-)] were collected. After transcriptional factor array analysis, the Cx32 and PBX1 expression levels of H. pylori-infected tissues from the developmental stages of GC and NGM with no H. pylori infection were measured by real-time polymerase chain reaction (RT-PCR) and Western blot analysis. Regarding H. pylori-infected animal models, the Cx32 and PBX1 mRNA expression levels and correlation between the gastric mucosa from 10 Mongolian gerbils with long-term H. pylori colonization and 10 controls were analyzed. PBX1 and Cx32 mRNA and protein levels were further studied under the H. pylori-infected condition as well as PBX1 overexpression and knockdown conditions in vitro.
Incremental PBX1 was first detected by TF microarray in H. pylori-related gastric carcinogenesis. The identical trend of PBX1 and Cx32 expression was confirmed in the developmental stages of H. pylori-related clinical specimens. The negative correlation of PBX1 and Cx32 was confirmed in H. pylori-infected Mongolian gerbils. Furthermore, decreased PBX1 expression was detected in the normal gastric epithelial cell line GES-1 with H. pylori infection. Enforced overexpression or RNAi-mediated knockdown of PBX1 contributed to the diminished or restored Cx32 expression in GES-1 and the gastric carcinoma cell line BGC823, respectively. Finally, dual-luciferase reporter assay in HEK293T cells showed that Cx32 promoter activity decreased by 30% after PBX1 vector co-transfection, indicating PBX1 as a transcriptional downregulator of Cx32 by directly binding to its promoters.
PBX1 is one of the determinants in the Cx32 promoter targeting site, preventing further damage of gap junction protein in H. pylori-associated gastric carcinogenesis.
Core tip: In this paper, we first report on the increasing tendency of transcriptional factor PBX1 in response to Helicobacter pylori (H. pylori) infection. This is significant because H. pylori is the predominant factor of gastric carcinoma, and the enhanced PBX1 expression in epithelial cells was first revealed as a marker of H. pylori-associated gastric carcinogenesis. Moreover, PBX1 transcriptionally downregulates the expression of connexin 32, which is vital to epithelial cell-cell communication, indicating an anti-cancer target that prevents the accumulation of PBX1 in the presence of H. pylori infection.
- Citation: Liu XM, Xu CX, Zhang LF, Huang LH, Hu TZ, Li R, Xia XJ, Xu LY, Luo L, Jiang XX, Li M. PBX1 attributes as a determinant of connexin 32 downregulation in Helicobacter pylori-related gastric carcinogenesis. World J Gastroenterol 2017; 23(29): 5345-5355
- URL: https://www.wjgnet.com/1007-9327/full/v23/i29/5345.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i29.5345
The development of gastric cancer is generally conceptualized as a gradual altering process subsequent to genetic susceptibility, carcinogens, and Helicobacter pylori (H. pylori) infection. H. pylori causes chronic gastritis and gastric ulcer, and its chronic infection greatly accelerates the process towards gastric carcinoma[1]. It has been widely suggested that inflammation with H. pylori infection triggers gastric carcinogenesis through an “inflammation-carcinoma chain” [non-atrophic gastritis (NAG) → chronic atrophic gastritis (CAG) → intestinal metaplasia (IM) → dysplasia (DYS) → gastric carcinoma (GC)][2]. CAG and IM are dynamic inflammatory processes that might be prone to severe histopathologic changes over time if left unchecked.
Intercellular communication mediated by gap junctions is known as an indispensable mechanism for maintaining tissue homeostasis. This type of communication, composed of protein subunits known as connexins (Cxs), is the only means by which small substances (< 1 kDa) flux between adjacent cells[3]. Connexins are expressed in specific and overlapping patterns, whose inhibition or dis-integrity has been virtually found in cancer cells and in cells clearly expressing oncogenes[4]. The gap junction protein connexin 32 (Cx32, an estimated molecular mass of 32 kDa), encoded by the gap junction protein β1 gene, is expressed abundantly in mammalian gastric epithelium and defines cell-specific patterns of gap junctional intracellular communication (GJIC). Cx32 in mature mucosa appears to form a critical path of biological functions by directly potentiating the cells to cooperate electrically or metabolically. The heteromeric gap junction channels such as Cx26/Cx30 might be similar to Cx32 gap junction channels. Despite the similar function of Cx proteins found in specific tissues such as the liver and cochlea[5,6], to date, no evidence has shown that other Cx proteins could compensate for the loss of gastric epithelial Cx32. The expression levels of Cx32 were found to be significantly lower in human adenocarcinomas than in the normal stomach, agreeing with our previous finding that gastric carcinoma cells do not contain detectable Cx32 protein[7]. The altering localization of Cx32 expression from cytomembrane to the cytoplasm was found in gastric cancer cells compared with normal gastric epithelium[8]. The loss of Cx32 expression and membrane localization in human gastric cancer was further found to be related to the degree of tumor cell differentiation with unrestricted growth control[9].
Although reduced or abolished Cx32 is the most common reporter in gastric carcinogenesis events, a better understanding of its expressing tendency and neoplastic transformation subjected to H. pylori chronic infection is needed[10]. The inhibited Cx32 expression was first reported to be associated with CagA-positive H. pylori infection[11]. Moreover, our previous in vivo studies confirmed significantly lower Cx32 expression in the H. pylori-infected Mongolian gerbil than in the mucosa with the same stage in the “inflammation-carcinoma” chain, paving the way to further study how H. pylori accelerates gastric carcinogenesis[12].
Cxs function with high turnover rates with half-lives of 1.5-5 h, and the kinetics of Cx32 is largely dependent on its transcriptional regulation[13]. Accumulating evidence has shown multiple binding sites of transcriptional factors (TFs) in the promoter of Cx32 (TFSEARCH, version 1.3). In the present study, we collected gastric mucosal samples from patients with an H. pylori-infected “inflammation-carcinoma chain” and analyzed the expression levels of TFs. The homeodomain transcription factor PBX1 (Pre-B-cell leukemia transcription factor 1) was considerably upregulated following H. pylori-associated carcinogenesis. Further studies delineated PBX1-related transcriptional activity, which might account for Cx32 downregulation, thereby proposing a prominent mechanism of altered Cx32 in the progression of H. pylori-related gastric carcinogenesis.
The malignant H. pylori (East-Asian type CagA+) used in this research was isolated from gastric carcinoma patients during gastroscopy and was grown on Columbia blood agar plates supplemented with antibiotics (10 mg/L vancomycin, 5 mg/L cefsulodin, 5 mg/L amphotericin, 5 mg/L trimethoprim and 10% sheep blood (Bianzhen Biotech, Nanjing, China) at 37 °C under microaerophilic conditions (5% O2, 10% CO2 and 85% N2) for 3-4 d. Next, H. pylori, which was in the early log phase with good motility and activity for subculture or intervention, was scraped and resuspended in PBS (with calcium). The concentration of H. pylori was analyzed by measuring the optical density of 1 × 108 colony-forming units at 600 nm.
The human gastric epithelial cell line GES-1 and poorly differentiated human gastric cancer cell line BGC823 were purchased from Bogu Biotech (Shanghai, China). All cells were maintained in high-glucose (4.5 g/L) Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, 15 mmol/L HEPES (pH 7.4), 2 mmol/L L-glutamine, and 1% non-essential amino acids (HyClone, Logan, UT, United States) at 37 °C in an incubator containing 5% CO2 with a certain humidified atmosphere and was passaged with 0.25% trypsin. H. pylori was added at an H. pylori/GES-1 cell ratio of 35:1.
Clinical specimens of the gastric antral mucosa were obtained from a total number of 4190 patients who underwent gastroscopy and were diagnosed at the Third Xiangya Affiliated Hospital of Central South University from August 2012 to August 2014. All included patients had signed written informed consent for the use of materials. Six specimens at the developmental stages of carcinogenesis (NAG, CAG, IM, DYS and GC) with H. pylori infection [H. pylori (+)] and five normal gastric mucosa (NGM) specimens without H. pylori infection [H. pylori (-)] were analyzed by transcriptional factor array. Additionally, 15 tissue specimens from CAG and GC with H. pylori infection and NGM without H. pylori infection were subjected to quantitative real-time polymerase chain reaction (RT-PCR) analysis, and another 15 specimens from CAG, IM, and GC tissues with and without H. pylori infection and NGM tissue without H. pylori infection were subjected to Western blot analysis. Endoscopic and pathological diagnosis was performed according to criteria depicted in our previous study[12]. Table 1 shows the clinical characteristics of the study population.
| Stage | n | M | F | Age | Duration (yr) |
| NGM no H. pylori infection | 25 | 12 | 13 | 40.00 ± 9.16 (21-55) | 0.17 (0.02-2) |
| NAG with H. pylori infection | 24 | 13 | 11 | 39.08 ± 8.85 (17-61) | 0.71 (0.01-7) |
| CAG with H. pylori infection | 25 | 14 | 11 | 50.52 ± 6.42 (39-66) | 3 (0.08-7) |
| IM with H. pylori infection | 25 | 13 | 12 | 54.04 ± 11.40 (38-76) | 4 (0.17-8) |
| DYS with H. pylori infection | 24 | 11 | 13 | 51.67 ± 10.03 (31-67) | 2.5 (0.17-27) |
| GC with H. pylori infection | 25 | 11 | 14 | 58.76 ± 9.94 (41-74) | 0.25 (0.08-2) |
Regarding animal tissues, the experimental protocol was approved by the ethics committee of the hospital. Twenty male Mongolian gerbils were divided randomly into an experimental group and a control group. After 24 h of fasting for all gerbils, the experimental group was inoculated with H. pylori (East-Asian type CagA+ from GC patients) by intragastric gavage once every two days for a week, and the control group was inoculated with saline. Forty-eight weeks after intragastric gavage, the gerbils were sacrificed, and their gastric antrum tissues were collected and maintained in liquid nitrogen immediately for further detection. All gerbils were dieted with the same food and sterile water.
Total RNA was isolated from cells, clinical specimens, and Mongolian gerbil gastric tissues according to the manufacturer’s instructions using the RNeasy Mini kit (Invitrogen, Life Technologies, Carlsbad, CA, United States). The cDNA was synthesized using random primers and M-MLV reverse transcriptase (Qiagen) with the removal of genomic DNA contamination by DNaseI treatment (Qiagen, Valencia, CA, United States). Real-time PCR reactions were performed using the MyiQ Single Color Real-time PCR Detection system (Bio-Rad, Hercules, CA, United States), the iScriptTM two-step RT-PCR kit with SYBR Green (Invitrogen) and gene primers (PBX1: forward: 5’-AAAGGAGATTGAGCGGATGGT-3’, reverse: 5’-GGGTAAGGGTTGCTGAGATGG-3’; Cx32: forward: 5’-GGATGCTCCGACAGCGTCTC-3’, reverse: 5’-GCCCTCTGCTCCTCTTACCC-3’; Cx32: forward: 5’-ATGAACTGGACAGGTTTGTAC-3’, reverse: 5’-ATGTGTTGCTGGTGCAGCCA-3’). The Ct values were normalized to those of β-actin (forward: 5’-GGCTGTGGAGACAAAAATGACCTC-3’, reverse: 5’-AGGCTTGGGCTTGAATGGAGTC-3’).
The PBX1 or Cx32 protein levels were analyzed using Western blot; however, the gastric antrum tissues from the Mongolian gerbil were not analyzed due to the lack of specific antibodies. The cells and clinical specimens were washed twice with PBS (with calcium) and then homogenized in RIPA lysis buffer (150 mmol/L NaCl, 1% NP-40, 0.5% deoxycholic acid, 0.1% SDS, 50 mmol/L Tris at pH 8.0) containing protease, phosphatase inhibitors and phenylmethylsulfonyl fluoride (all from Sigma, St Louis, MO, United States). Following sonication and centrifugation at 14000 g for 10 min at 4 °C, the supernatant (soluble proteins) was collected. The protein concentration of each sample was determined using a protein assay kit (Pierce, Rockford, IL, United States). The samples were denatured in loading buffer. Equivalent amounts of proteins were separated by SDS-PAGE and transferred onto nitrocellulose membranes (Millipore, Billerica, MA, United States). After incubation with antibodies specific for PBX1 (Santa Cruz, Dallas, TX, United States; diluted 1:200), Cx32 (Proteintech, Chicago, IL, United States; diluted 1:500), β-actin (Proteintech; diluted 1:4000), calnexin (Cell Signaling, Danvers, MA, United States; diluted 1:10000), and tubulin (Cell Signaling; diluted 1:10000) at 4 °C overnight, the membranes were incubated with a horseradish peroxidase-conjugated secondary antibody (1:5000, Jackson Immuno-Research, West Grove, PA, United States). The membranes were then visualized using the enhanced chemiluminescence Western blotting substrate (Bio-Rad).
Cx32 promoter (-2000 bp) sequences (H. sapiens) were downloaded from UCSC[14]. The candidate transcriptional factor binding sites of the Cx32 promoters were predicted with TFSEARCH (http://www.cbrc.jp/research/db/TFSEARCH.html)[15]. Nuclear extracts of gastric mucosal biopsies were subjected to the TranSignal™ Protein/DNA Array (Panomics, Redwood City, CA, United States) according to the manufacturer’s instructions. In short, biotin-labeled DNA-binding oligonucleotides (TranSignal™ Probe Mix) were incubated with 10 μg of nuclear extract at 15 °C for 30 min to form transcription factor/DNA complexes. These complexes were then separated from the free probes by 2% agarose gel electrophoresis in 0.5 × TBE at 120 V for 15 min. The probes in the complexes were released, ethanol-precipitated, and hybridized to the TranSignal™ Protein/DNA Array. The detection of signals was obtained using an enhanced chemiluminescence imaging system.
Three RNAi target sequences were selected according to stealth RNAi of the human PBX1 mRNA (Accession No. NM_001142077.1). The sequences of the synthesized oligonucleotides were as follows: siRNA-1: sense 5’-ACAAUCAACACCGUCAUUGAG-3’, antisense 5’-CUCAAUGACGGUGUUGAUUGU-3’; siRNA-2: sense 5’-AAAGGAAAAGGCUCAAACCCC-3’, antisense 5’-GGGGUUUGAGCCUUUUCCUUU-3’; siRNA-3: sense 5’-UAAGAAUCCCCAUUGAGUGAC-3’, antisense 5’-GUCACUCAAUGGGGAUUCUUA-3’. Lipofectamine reagent (Invitrogen) was used to transfect synthesized Stealth RNAi against PBX1 into BGC823 cells, and the negative control duplexes were delivered into BGC823 cells as well. BLOCK-iT Alexa Fluor Red Fluorescent (Invitrogen) was used to confirm the transfection efficiency of each duplex siRNA. Regarding PBX1 overexpression, the full-length human PBX1 3’untranslated region and coding sequence region were amplified by PCR and cloned into the pcDNA™3.1(+) plasmid (GenePharma Inc. Shanghai, China), followed by transfection into GES-1 cells according to the manufacturer’s instructions.
The pGL3 Basic vector containing the entire sequence of the Cx32 promoter was a kind gift from Yingrun Biotechnology (Changsha, China), and no mutation was previously confirmed by DNA sequencing. To validate the PBX1 binding sites in the Cx32 promoters, PBX1 cDNA was amplified by PCR using the BamH I and EcoR I digestion sites in the pDoubleEx-EGFP plasmid (sense, 5’-CGGGATCCGCCACCATGGACGAGCAGCCCAGGCTGAT-3’; antisense, 5’GGAATTCTCAGTTGGAGGTATCAGAGT-3’). The constructed CX32-pGL3 Basic and pDoubleEx-EGFP-PBX1 vectors were co-transfected into HEK293T cells. The firefly and Renilla luciferase activities in the cell lysates were triplicated and determined using a dual-luciferase assay system (Promega), and the normalized ratio of the Renilla/firefly reflected the expression activity.
Statistical analyses were performed using the Statistical Package for Social Sciences software 12 (SPSS Inc., Chicago, IL, United States). All data are expressed as mean ± SD. Multiple-group comparisons were characterized using one-way analysis of variance (ANOVA) with Dunnett’s multiple comparison tests. Two-sided t-tests and Spearmen’s correlation test were applied to determine the correlations between groups, and a value of P < 0.05 was considered statistically significant.
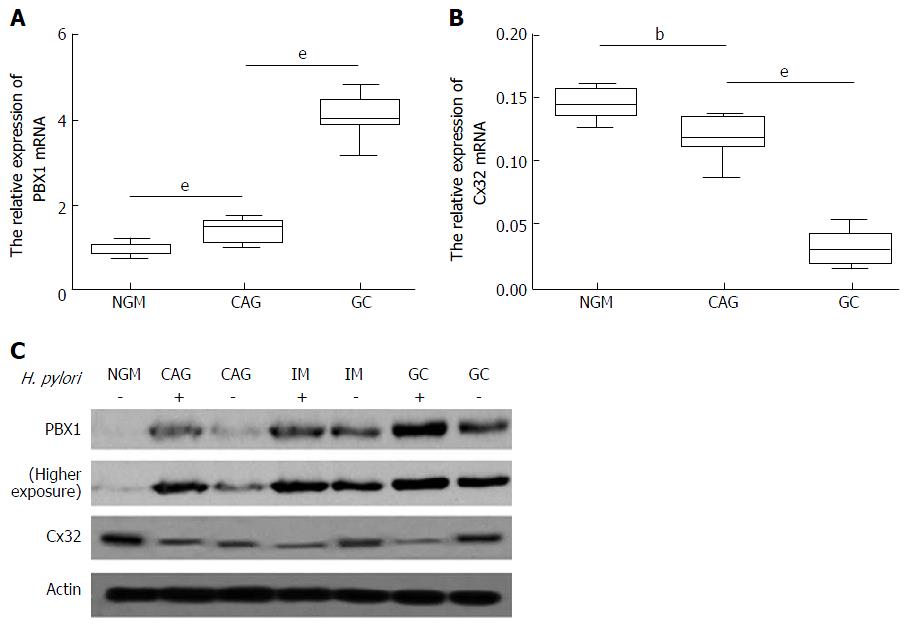
Comparison of H. pylori (-) NGM and H. pylori (+) NAG, CAG, IM, DYS and GC tissues revealed the increased expression of PBX1 as follows: (1) a consistent trend of endogenous expression along the gastric inflammation-carcinoma chain; and (2) a remarkable change with clinical significance between any two successive stages (> 2-fold or < 0.5-fold). It was previously shown by TF microarray that PBX1 is rarely found in H. pylori (-) NGM, but its accumulation is initiated in H. pylori (+) NAG and CAG, enriched in IM and DYS, and highest in GC tissues[16]. H. pylori infection accounting for stimulated PBX was, for the first time, based on the comparison between each single-stage exposure to H. pylori and NGM. Moreover, because CAG is widely accepted as the initiating phase of gastric precancerous lesions, further amounts of PBX1 shown above could be counted as the molecular event involved in gastric epithelial neoplasia. PBX1 malignancy was first classified as showing gastric epithelial specificity given the continuous high expression in IM, DYS and GC.
The pertinent roles of incremental PBX1 in the H. pylori-related gastric epithelial inflammation-carcinoma chain were further investigated. Consistent with the TF microarray results, enriched PBX1 mRNA was time-dependently subjected to H. pylori (Figure 1A, P < 0.001) and showed the opposite tendency towards Cx32 mRNA levels in the same samples (Figure 1B, P < 0.01). PBX1 and Cx32 protein levels in typical specimens from H. pylori (-) NGM, H. pylori (-/+) CAG, IM and GC demonstrated that PBX1 upregulation and Cx32 inhibition were associated with GC progression as well as the presence of H. pylori (Figure 1C). It was conceivable that the accelerated PBX1 activation and inhibited Cx32 were synchronous with gastric carcinoma progression.
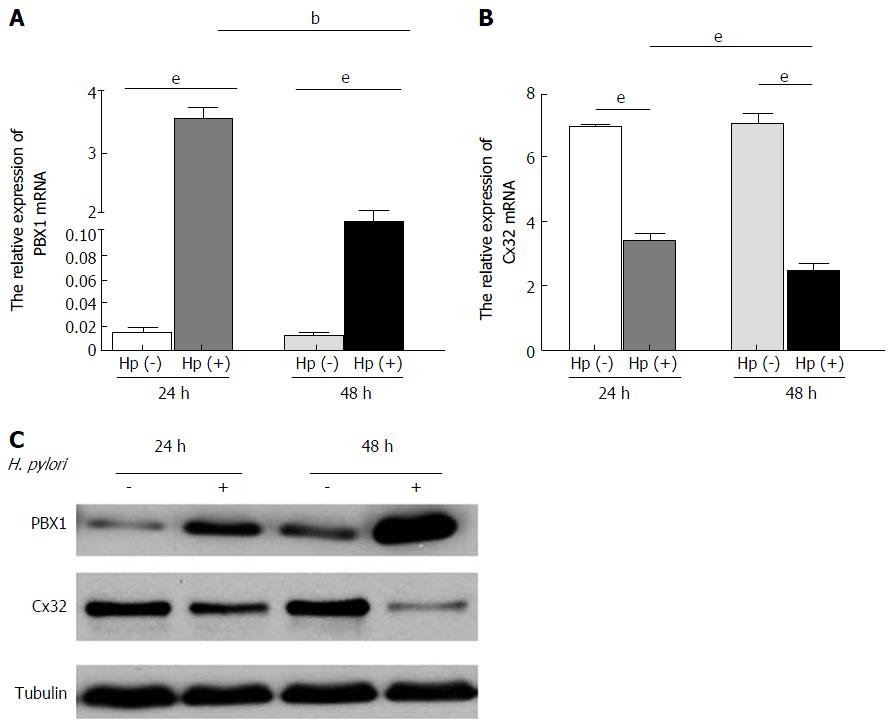
Dramatic elevation of PBX1 mRNA expression was found after 24 h co-cultures of H. pylori and GES-1 cells, and this enrichment was maintained for 48 h (Figure 2A, P < 0.01). The expected time-dependent Cx32 mRNA decrease (Figure 2B, P < 0.001) was in accordance with the malfunction of GJIC revealed by the Scrape-loading and dye transfer technique (SLDT, data not shown). The increased PBX1 protein levels, as well as decreased Cx32 protein levels, were detected with prolonged time (Figure 2C), which is consistent with a previous report that H. pylori infection is correlated with GJ inhibition[17]. These findings verified a significant stimulatory effect of short-time H. pylori on the gastric mucosa even in low-infectious titers.
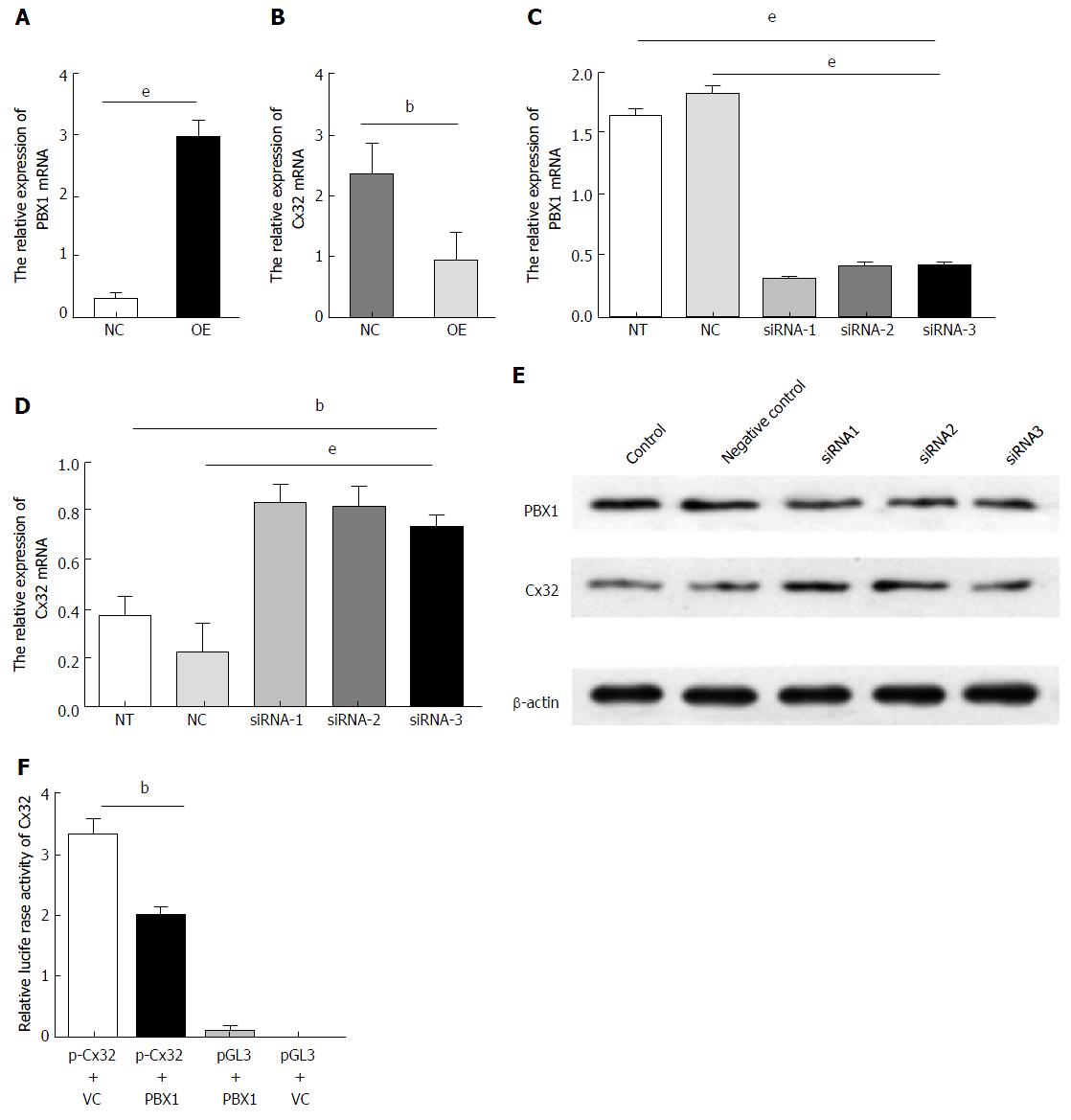
The increased PBX1 was accompanied by decreased Cx32 upon H. pylori infection as mentioned above. It was confirmed that the PBX1 overexpression in GES-1 cells (Figure 3A, P < 0.001) considerably reduced the Cx32 expression (Figure 3B, P < 0.001). Given that other PBX family proteins theoretically compensate for the endogenous PBX1 alteration if there are indeed overlapping structure and biological function among them, we additionally addressed specific functions in PBX1 attenuation studies. The relatively enriched PBX1 was previously found in the poorly differentiated gastric adenocarcinoma cell line BGC-823 (data not shown); thus, these monolayers were subjected to PBX1 silencing (siRNA-1, siRNA-2 and siRNA-3) as the downregulated PBX1 mRNA (Figure 3C, P < 0.001). Restoration of the Cx32 mRNA (Figure 3D, P < 0.01) and protein levels (Figure 3E) supported the ameliorated Cx32 expression in GC cells subsequent to PBX1 suppression. It is tempting to speculate that H. pylori-mediated Cx32 disruption and gap junctional functions could be alleviated by restraining further PBX1 activation.
Given the inverse trends of Cx32 expression upon enforced PBX1 changes, we next explored how PBX1 transcriptionally regulates the Cx32 gene using the dual-luciferase reporter assay. The PBX1-overexpressing plasmid (pDoubleEx-EGFP-PBX1, see vector map in Supplemental Figure 1) without any mutation (sequence alignment in Supplemental Figure 2) and Cx32 promoters were successfully transfected into HEK293T cells (Supplemental Figure 3). The activity of the wild-type Cx32 promoter reporter was reduced by approximately 30% shortly after pDoubleEx-EGFP-PBX1 co-transfection (Figure 3F; P < 0.001), suggesting that PBX1 reduced the Cx32 activity by binding to its promoters.
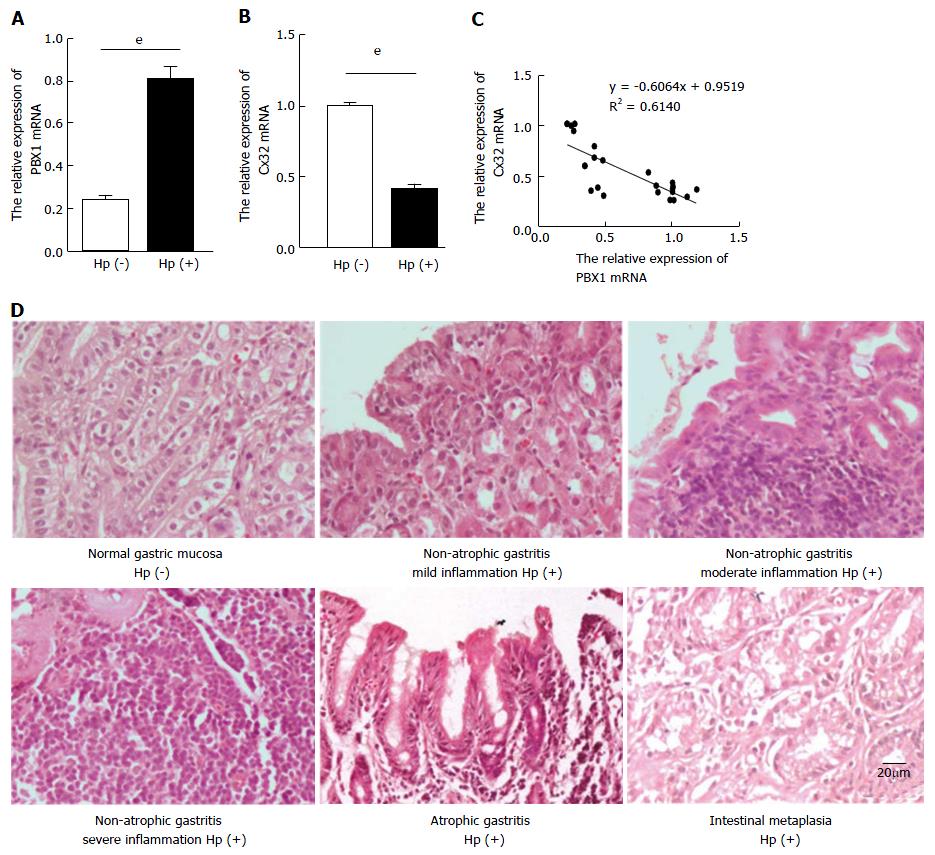
The augmented PBX1 and inhibited Cx32 mRNA levels in H. pylori (+) antral tissues were detected (Figure 4A and B) compared with the control group, showing an inverse correlation between PBX1 and Cx32 (Figure 4C). Among 21 gerbils with 48-week H. pylori infection, 17 manifested with erosion, hemorrhage, edema, hyperemia, and food retention macroscopically. As for the pathological sections, 4 were unchanged, 12 were non-atrophic gastritis (7 with mild inflammation, 4 with moderate inflammation, and 1 with severe inflammation), 3 were atrophic gastritis, and 2 were intestinal metaplasia. Alternatively, no inflammatory status were found in the control group (Figure 4D). The lack of MGs-specific antibodies hindered the further test of PBX1 and Cx32 protein expression. But overall, these in vivo data strengthened the PBX1 and Cx32 tendencies in gastric mucosa models.
Studies of gap junction proteins during the progression of gastric epithelial carcinogenesis have provided us with remarkable insights into various outcomes of host-bacterial interactions. Reduced or absent Cx32 expression has been implicated in the entire process of H. pylori-associated gastric carcinogenesis, one mechanism of which is promoter methylation[12]. Meanwhile, a growing number of transcriptional factors involved in Cx32 regulation for a potential pathway remain unidentified. One of our previous studies confirmed the augmented transcriptional factor GATA-3 in H. pylori-related GC occurrence, and GATA-3 enables the Cx32 transcriptional downregulation by directly binding to its promoters; these results are in line with the sequence prediction using a bioinformatics approach[18]. These observations raise the possibility that the emerging transcriptional factors directly regulate Cxs genes that participate in key pathways related to gastric oncogenesis.
PBX1 is a well-established transcriptional factor in the self-renewal of hematopoietic stem cells and participates in reprogramming lineage-committed blood cells into hematopoietic stem cells[19,20]. Studies in mice have suggested that PBX1 may be involved in the regulation of osteogenesis, and it is required for skeletal, neuronal, pancreatic island, spleen, and urogenital tract patterning by binding to known regulatory regions of growth-related genes[20-23]. In human cancer, PBX1 was first referred to as a translocated proto-oncogene in leukemia, and the translocated gene product (together with the TCF3/E2A gene) was shown to have oncogenic potential[24,25]. Its hematopoietic malignancy was partly addressed by the signaling networks, including those of the E2A-PBX1 target genes ZAP70, SYK, and LCK that encode kinases upstream of PLCγ2; particularly, PBX1 plays an oncogenic role in pre-B-ALL[26]. Furthermore, PBX1 is highly expressed in solid tumors, including melanoma, prostate, ovarian, and breast cancers[27-30]. For instance, PBX1 has been identified as an effector in the NOTCH3 signaling pathway in ovarian cancer and is strongly related to a high extent of prostate cancer from TF microarray analysis and is known to be positively correlated with multiple epithelial malignancies through regulation of the PI3K/Akt pathway[28,31].
The in silico analysis of the Cx32 promoter sequence data demonstrated that PBX1 may act as an upstream molecular hub by directly regulating Cx32. We report here that PBX1 is a critical transcriptional regulator required for Cx32 expression. At the tissue level, PBX1 is upregulated significantly compared with the matched stages in non-H. pylori infected tissues, and its upregulation is associated with a less favorable prognosis in patients. Moreover, the high affinity of PBX1-Cx32 transcriptional regulation was confirmed by luciferase reporter assay. These findings established a novel but determinant role of PBX1 in the event of reduced Cx32 along the development of H. pylori-related gastric carcinogenesis and provide a molecular basis for targeting PBX1 or its downstream effectors to overcome the inflammation-carcinoma progression.
The association between PBX1 expression and development of cell neoplasia suggests that the PBX1 pathway is potentially actionable for target-based therapy. In fact, PBX1 orchestrates multiple functional networks, and additional PBX1-regulated signaling pathways are yet to be discovered. A direct strategy employing a combination of PBX1 antagonists and chemotherapeutic agents may at least offer a better intervention to delay tumor advancement and recurrence. Another feasible rationale is to target downstream effectors of PBX1 such as JAK2/STAT3 (JAK2, a tyrosine kinase that directly phosphorylates and activates STAT3) that leads to transcriptional activation of various STAT3-regulated genes that work in concert to promote tumor progression[30]. Similarly, E2A-PBX1 notably expands progenitor B-cell subpopulations in pre-leukemic mice, increasing penetrance and shortening leukemia latency accompanied most notably by JAK/STAT activation, which is required for leukemia cell proliferation[32]. It was recently reported that PBX1 acts directly on the STAT3 promoter to induce STAT3 transcription. More intriguingly, STAT3 is responsible for all PBX1-mediated phenotypes[30]. Further efforts are needed to illuminate the mechanisms through which PBX1 is reactivated or its network reprogrammed in response to challenges in the tumor environment, such as experiencing inflammation or under pathogen invasion. Cx32 has a strong tumor-suppressive effect on multiple cancer cell lines via various pathways[33,34]. A previous study considers Cx32 as a tumor suppressor gene in a renal carcinoma (Caki-1 cell) since this tumor-suppressive effect partly depends on the inhibited Src-Stat3-VEGF signaling[35]. Overall, our data largely illustrate the sequential up/downstream relationship between PBX and Cx32, revealing a “convenient access” to Cx32 regulation independent of intermediate networks such as STAT3. It would be meaningful to further study potential crosstalk between these signaling pathways.
Collectively, H. pylori retarded gap junction function by reducing Cx32 expression, expediting gastric cancerous susceptibility. TF screening shows that PBX1 activation is one of novel events of H. pylori-related gastric carcinogenesis. A global view of increasing PBX1 is presented in H. pylori-associated clinical specimens, gastric epithelial cells and Mongolian gerbils. The reversed regulating trend of Cx32 by PBX1 presumably presents an approach for the restoration of Cx32 in precancerous lesion tissues. Apart from binding directly to the Cx32 promoter, the research direction of PBX1 in gastric epithelial cells is challenging but warrants further investigation. PBX1 would be a crucial mediator that unravels H. pylori-dependent microenvironment and the gastric inflammation-carcinoma chain. It could be expected that the PBX1-Cx32 targeting site sheds light on preventing further damage by H. pylori chronic infection.
Helicobacter pylori (H. pylori) infection has been widely suggested to accelerate gastric carcinogenesis through an “inflammation-carcinoma chain”. Connexin 32 (Cx32), a gap-junction protein, plays a suppressive role in gastric carcinogenesis. Our studies previously revealed the reduced Cx32 expression in H. pylori-infected gastric mucosa and its association with the carcinogenesis, but its regulating patterns, including transcriptional control, remain enigmatic.
Previous studies have demonstrated the decreasing expression of Cx32 from normal gastric mucosa to precancerous lesions and gastric carcinomas (GCs). Cx32 abnormality is a crucial molecular event for loss of gap junctional functions. The changing expression patterns of transcriptional factors (TFs) caused by H. pylori might affect Cx32 expression. It is requisite to clarify the mechanisms regarding how Cx32 is downregulated by potential TFs to unravel the transcriptional regulation mechanism of GC occurrence.
The authors draw attention to PBX1 because of its increasing tendency toward H. pylori-associated gastric carcinogenesis and its Cx32 binding sites predicted by bioinformatics tools. The multiple H. pylori-infected cell, animal and clinical specimen models were rigorously designed and performed in terms of PBX1 and Cx32 expression, whose negative correlation is in line with our previous work. It is intriguing that PBX1 downregulates Cx32 expression by directly binding to its promoters, and inhibition of PBX1 leads to Cx32 upregulation, indicating PBX1 as a promising anti-cancer target.
This study showed increased PBX1 expression during gastric carcinogenesis stages with H. pylori infection that is largely responsible for Cx32 downregulation. It is therefore expected that Cx32 inhibition could be somehow reversed by treating against potential TFs, which provides new insights for H. pylori-related GC therapy.
This is a very-well designed, performed and written experimental and clinical study for deciphering PBX1-Cx32 regulating axis under H. pylori infection and its association with gastric carcinogenesis.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: De Re V, Garcia-Olmo D, Slomiany BL, Vorobjova T S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Huang Y
| 1. | Malfertheiner P, Sipponen P, Naumann M, Moayyedi P, Mégraud F, Xiao SD, Sugano K, Nyrén O; Lejondal H. pylori-Gastric Cancer Task Force. Helicobacter pylori eradication has the potential to prevent gastric cancer: a state-of-the-art critique. Am J Gastroenterol. 2005;100:2100-2115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 2. | Marshall B. Helicobacter pylori: past, present and future. Keio J Med. 2003;52:80-85. [PubMed] |
| 3. | Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83:1359-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 895] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 4. | de Feijter AW, Matesic DF, Ruch RJ, Guan X, Chang CC, Trosko JE. Localization and function of the connexin 43 gap-junction protein in normal and various oncogene-expressing rat liver epithelial cells. Mol Carcinog. 1996;16:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Nagy JI, Ionescu AV, Lynn BD, Rash JE. Coupling of astrocyte connexins Cx26, Cx30, Cx43 to oligodendrocyte Cx29, Cx32, Cx47: Implications from normal and connexin32 knockout mice. Glia. 2003;44:205-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 165] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Degen J, Schütz M, Dicke N, Strenzke N, Jokwitz M, Moser T, Willecke K. Connexin32 can restore hearing in connexin26 deficient mice. Eur J Cell Biol. 2011;90:817-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Uchida Y, Matsuda K, Sasahara K, Kawabata H, Nishioka M. Immunohistochemistry of gap junctions in normal and diseased gastric mucosa of humans. Gastroenterology. 1995;109:1492-1496. [PubMed] |
| 8. | Radebold K, Horakova E, Gloeckner J, Ortega G, Spray DC, Vieweger H, Siebert K, Manuelidis L, Geibel JP. Gap junctional channels regulate acid secretion in the mammalian gastric gland. J Membr Biol. 2001;183:147-153. [PubMed] |
| 9. | Jee H, Nam KT, Kwon HJ, Han SU, Kim DY. Altered expression and localization of connexin32 in human and murine gastric carcinogenesis. Dig Dis Sci. 2011;56:1323-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Yokozaki H. Molecular characteristics of eight gastric cancer cell lines established in Japan. Pathol Int. 2000;50:767-777. [PubMed] |
| 11. | Mine T, Endo C, Kushima R, Kushima W, Kobayashi I, Muraoka H, Taki R, Fujita T. The effects of water extracts of CagA positive or negative Helicobacter pylori on proliferation, apoptosis and connexin formation in acetic acid-induced gastric ulcer of rats. Aliment Pharmacol Ther. 2000;14 Suppl 1:199-204. [PubMed] |
| 12. | Wang Y, Huang LH, Xu CX, Xiao J, Zhou L, Cao D, Liu XM, Qi Y. Connexin 32 and 43 promoter methylation in Helicobacter pylori-associated gastric tumorigenesis. World J Gastroenterol. 2014;20:11770-11779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Leithe E, Rivedal E. Ubiquitination of gap junction proteins. J Membr Biol. 2007;217:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Goldman M, Craft B, Swatloski T, Ellrott K, Cline M, Diekhans M, Ma S, Wilks C, Stuart J, Haussler D. The UCSC Cancer Genomics Browser: update 2013. Nucleic Acids Res. 2013;41:D949-D954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 15. | Wingender E. The TRANSFAC project as an example of framework technology that supports the analysis of genomic regulation. Brief Bioinform. 2008;9:326-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 309] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 16. | Hu TZ, Huang LH, Xu CX, Liu XM, Wang Y, Xiao J, Zhou L, Luo L, Jiang XX. Expressional profiles of transcription factors in the progression of Helicobacter pylori-associated gastric carcinoma based on protein/DNA array analysis. Med Oncol. 2015;32:265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Xu C, Chen Y, Chen X, Wang F. Effects of different types of Helicobacter pylori on the gap junction intercellular communication in GES-1 cells. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2011;36:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Liu X, Cao K, Xu C, Hu T, Zhou L, Cao D, Xiao J, Luo L, Guo Y, Qi Y. GATA-3 augmentation down-regulates Connexin43 in Helicobacter pylori associated gastric carcinogenesis. Cancer Biol Ther. 2015;16:987-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Riddell J, Gazit R, Garrison BS, Guo G, Saadatpour A, Mandal PK, Ebina W, Volchkov P, Yuan GC, Orkin SH. Reprogramming committed murine blood cells to induced hematopoietic stem cells with defined factors. Cell. 2014;157:549-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 260] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 20. | Ficara F, Murphy MJ, Lin M, Cleary ML. Pbx1 regulates self-renewal of long-term hematopoietic stem cells by maintaining their quiescence. Cell Stem Cell. 2008;2:484-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 185] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 21. | Grebbin BM, Hau AC, Groß A, Anders-Maurer M, Schramm J, Koss M, Wille C, Mittelbronn M, Selleri L, Schulte D. Pbx1 is required for adult subventricular zone neurogenesis. Development. 2016;143:2281-2291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Selleri L, Depew MJ, Jacobs Y, Chanda SK, Tsang KY, Cheah KS, Rubenstein JL, O’Gorman S, Cleary ML. Requirement for Pbx1 in skeletal patterning and programming chondrocyte proliferation and differentiation. Development. 2001;128:3543-3557. [PubMed] |
| 23. | Brendolan A, Ferretti E, Salsi V, Moses K, Quaggin S, Blasi F, Cleary ML, Selleri L. A Pbx1-dependent genetic and transcriptional network regulates spleen ontogeny. Development. 2005;132:3113-3126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Nourse J, Mellentin JD, Galili N, Wilkinson J, Stanbridge E, Smith SD, Cleary ML. Chromosomal translocation t(1;19) results in synthesis of a homeobox fusion mRNA that codes for a potential chimeric transcription factor. Cell. 1990;60:535-545. [PubMed] |
| 25. | Kamps MP, Look AT, Baltimore D. The human t(1;19) translocation in pre-B ALL produces multiple nuclear E2A-Pbx1 fusion proteins with differing transforming potentials. Genes Dev. 1991;5:358-368. [PubMed] |
| 26. | Duque-Afonso J, Lin CH, Han K, Wei MC, Feng J, Kurzer JH, Schneidawind C, Wong SH, Bassik MC, Cleary ML. E2A-PBX1 Remodels Oncogenic Signaling Networks in B-cell Precursor Acute Lymphoid Leukemia. Cancer Res. 2016;76:6937-6949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Shiraishi K, Yamasaki K, Nanba D, Inoue H, Hanakawa Y, Shirakata Y, Hashimoto K, Higashiyama S. Pre-B-cell leukemia transcription factor 1 is a major target of promyelocytic leukemia zinc-finger-mediated melanoma cell growth suppression. Oncogene. 2007;26:339-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Yeh HY, Cheng SW, Lin YC, Yeh CY, Lin SF, Soo VW. Identifying significant genetic regulatory networks in the prostate cancer from microarray data based on transcription factor analysis and conditional independency. BMC Med Genomics. 2009;2:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Magnani L, Stoeck A, Zhang X, Lánczky A, Mirabella AC, Wang TL, Gyorffy B, Lupien M. Genome-wide reprogramming of the chromatin landscape underlies endocrine therapy resistance in breast cancer. Proc Natl Acad Sci USA. 2013;110:E1490-E1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 30. | Jung JG, Shih IM, Park JT, Gerry E, Kim TH, Ayhan A, Handschuh K, Davidson B, Fader AN, Selleri L. Ovarian Cancer Chemoresistance Relies on the Stem Cell Reprogramming Factor PBX1. Cancer Res. 2016;76:6351-6361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 31. | Wang J, Wakeman TP, Lathia JD, Hjelmeland AB, Wang XF, White RR, Rich JN, Sullenger BA. Notch promotes radioresistance of glioma stem cells. Stem Cells. 2010;28:17-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 427] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 32. | Duque-Afonso J, Feng J, Scherer F, Lin CH, Wong SH, Wang Z, Iwasaki M, Cleary ML. Comparative genomics reveals multistep pathogenesis of E2A-PBX1 acute lymphoblastic leukemia. J Clin Invest. 2015;125:3667-3680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Katoch P, Mitra S, Ray A, Kelsey L, Roberts BJ, Wahl JK 3rd, Johnson KR, Mehta PP. The carboxyl tail of connexin32 regulates gap junction assembly in human prostate and pancreatic cancer cells. J Biol Chem. 2015;290:4647-4662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Kawasaki Y, Omori Y, Li Q, Nishikawa Y, Yoshioka T, Yoshida M, Ishikawa K, Enomoto K. Cytoplasmic accumulation of connexin32 expands cancer stem cell population in human HuH7 hepatoma cells by enhancing its self-renewal. Int J Cancer. 2011;128:51-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Fujimoto E, Sato H, Shirai S, Nagashima Y, Fukumoto K, Hagiwara H, Negishi E, Ueno K, Omori Y, Yamasaki H. Connexin32 as a tumor suppressor gene in a metastatic renal cell carcinoma cell line. Oncogene. 2005;24:3684-3690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |