Published online Jun 28, 2017. doi: 10.3748/wjg.v23.i24.4416
Peer-review started: January 18, 2017
First decision: March 16, 2017
Revised: March 27, 2017
Accepted: June 1, 2017
Article in press: June 1, 2017
Published online: June 28, 2017
Processing time: 220 Days and 15.7 Hours
To assess the accuracy of a new magnifying endoscopy (ME) classification for predicting depth of invasion of superficial esophageal squamous cell carcinoma (SESCC).
This study included a total of 70 lesions in 69 patients with SESCC who underwent ME with narrow-band imaging (ME-NBI) before resection from August 2010 to July 2016. Accuracy of ME-NBI for predicting depth of invasion of SESCC was analyzed by using a new ME classification proposed by the Japan Esophageal Society (JES), and interobserver agreement was assessed.
Overall accuracy of ME-NBI for estimating depth of invasion of SESCC was 78.6%. Sensitivity and specificity of type B1 for tumors limited to the epithelial layer (m1) or invading into the lamina propria (m2) were 71.4% and 100%, respectively. Sensitivity and specificity of type B2 for tumors invading into the muscularis mucosa (m3) or superficial submucosa (≤ 200 μm, sm1) were 94.4% and 73.1%, respectively, while those of type B3 for tumors invading into the deep submucosa (> 200 μm, sm2) were 75.0% and 97.8%, respectively. Interobserver agreement was excellent (κ = 0.86, 95%CI: 0.76-0.95).
The recently developed JES ME classification is useful for predicting depth of invasion of SESCC, with reliable interobserver agreement.
Core tip: Recently, the Japan Esophageal Society proposed a new magnifying endoscopic classification for superficial esophageal squamous cell carcinoma (SESCC) based on two previous classifications. This classification is simple and useful for predicting depth of invasion of SESCC, with reliable interobserver agreement.
- Citation: Kim SJ, Kim GH, Lee MW, Jeon HK, Baek DH, Lee BE, Song GA. New magnifying endoscopic classification for superficial esophageal squamous cell carcinoma. World J Gastroenterol 2017; 23(24): 4416-4421
- URL: https://www.wjgnet.com/1007-9327/full/v23/i24/4416.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i24.4416
Depth of invasion of superficial esophageal squamous cell carcinoma (SESCC) is the most important factor in selecting therapeutic options. Risk of lymph node (LN) metastasis is mainly determined by depth of tumor invasion[1-3]. Because tumors limited to the epithelial layer (m1) or invading into the lamina propria (m2) have very low risk of LN metastasis, endoscopic resection of such tumors is appropriate. However, incidence of LN metastasis increases to 26.5% for tumors invading into but not through the muscularis mucosa (m3) or invading into the superficial submucosa (≤ 200 μm, sm1), and to 30.4% to 41.9% for tumors invading into the deep submucosa (> 200 μm, sm2). Therefore, for these tumors, surgical resection with LN dissection or chemoradiation therapy is recommended[4-6]. Because endoscopic resection has many advantages over esophagectomy, such as preservation of the esophagus, maintenance of quality of life, and low post-treatment morbidity and mortality, Japanese guidelines for treatment of esophageal cancer suggest that m3 to sm1 cancers are a relative indication of endoscopic resection[7,8].
Accuracy of endoscopic diagnosis with white-light imaging (WLI) is limited because of lack of standardized diagnostic criteria for depth of invasion of SESCC. Recent advancements in magnifying endoscopy (ME) have enabled visualization of mucosal details that cannot be seen with standard endoscopy[9,10]. The combination of ME with narrow-band imaging (ME-NBI) can further enhance detailed observation of microcapillary patterns of the mucosa[11]. ME-NBI enables examination of intrapapillary capillary loops (IPCLs) of the esophageal mucosa, which works as an indicator of tissue atypia in the squamous epithelium[12,13]. To date, two different ME classifications, established by Inoue and Arima, have been reported to be useful for predicting depth of invasion of SESCC[14,15]. However, in the clinical setting, both classifications are difficult to apply because of their complicated criteria. Recently, the Japan Esophageal Society (JES) proposed a new ME classification based on these two classifications, and performed a prospective multicenter study[16]. It was demonstrated that the new ME classification is simple and useful for estimating depth of invasion of SESCC[16]. However, interobserver agreement of this new classification was not evaluated in this previous study. Therefore, we aimed to investigate the accuracy of the new ME classification for predicting depth of invasion of SESCC, and to assess the interobserver agreement of this classification.
We retrospectively analyzed a database of all patients with endoscopically suspected SESCC who underwent ME-NBI for pretreatment staging at Pusan National University Hospital (Busan, South Korea) or Pusan National University Yangsan Hospital (Yangsan, South Korea) from August 2010 to July 2016. Patients who previously received chemotherapy or radiotherapy or did not undergo endoscopic or surgical resection were excluded. A total of 70 lesions in 69 patients with pathologically confirmed SESCC were included in this study. This study was conducted in accordance with the Declaration of Helsinki, and the study protocol was reviewed and approved by the Institutional Review Board of Pusan National University Hospital.
ME assessment was performed using a magnifying video endoscope (GIF-H260Z; Olympus, Tokyo, Japan) combined with NBI. A soft black hood (2-mm depth, MB-46; Olympus) was attached to the distal tip of the scope to maintain the distance from the lesion. ME-NBI was performed by two endoscopists (Kim GH and Kim SJ) who had experience with ME-NBI of the esophagus. Examinations were performed under intravenous conscious sedation (2-5 mg midazolam). After routine examination using WLI, ME-NBI assessment of the target area was performed to evaluate the microvessels.
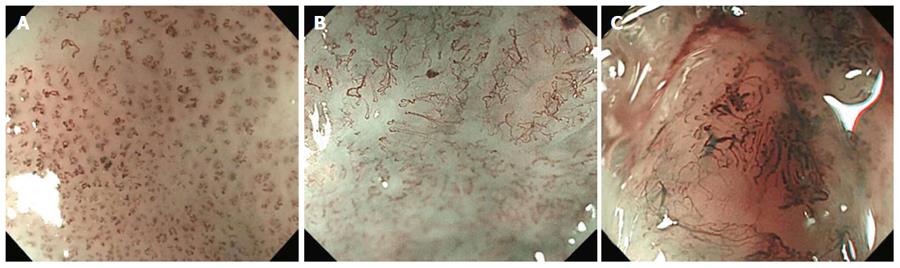
Diagnostic criteria of the new JES classification are based on changes in IPCLs observed by ME-NBI[16]. Type A tumors consist of normal or abnormal microvessels without severe irregularity that have three or fewer IPCL changes: meandering, dilation, caliber change, and various shapes; type A suggests normality, inflammation, and low-grade dysplasia. Type B tumors consist of abnormal microvessels with severe irregularity that have all four IPCL changes; type B suggests SCC and is classified into three subtypes[16]. Type B1 tumors consist of abnormal microvessels with four characteristic findings and a conserved loop-like formation; type B1 is considered to correspond to m1 or m2 SESCC (Figure 1A). Type B2 tumors consist of stretched and markedly elongated transformations without a loop-like formation; type B2 is considered to correspond to m3 or sm1 SESCC (Figure 1B). Type B3 tumors consist of highly dilated irregular vessels with a caliber that appears to be more than three times that of type B2 vessels; type B3 is considered to correspond to sm2 or deeper SESCC (Figure 1C).
Two endoscopists reviewed the recorded series of ME-NBI images independently without any pathologic information. In discordant cases, microvessel types were adjudicated according to the agreement of the two endoscopists.
We performed endoscopic resection or esophagectomy within 2 to 4 wk following ME-NBI. Resected specimens were cut serially into 2-mm slices in parallel and embedded in paraffin, and then sectioned and stained with hematoxylin and eosin. Tumor location, macroscopic shape, tumor size, tumor differentiation, and depth of invasion were reviewed according to recommendations of the World Health Organization[17]. Depth of invasion was classified as m1 to m2, m3 to sm1, or sm2 according to Japanese criteria[7].
The χ2 test or Fisher’s exact test was used to assess the accuracy of ME-NBI in relation to clinicopathologic characteristics. The Student’s t test was used for non-categorical variables. Interobserver agreement was assessed by kappa statistics using a weighted method. Kappa values were interpreted as follows: fair, 0.21 to 0.40; moderate, 0.41 to 0.60; good, 0.61 to 0.80; and excellent, > 0.81. Statistical analyses were performed using SPSS version 21.0 for Windows (SPSS, Inc., Chicago, IL, United States), with P values of < 0.05 considered statistically significant.
Clinicopathologic characteristics of patients with SESCC are summarized in Table 1. Patients comprised 62 men and 7 women with a mean age of 66 years (range, 47-81 years). Of the 70 tumors, 52 occurred in the mid-esophagus, 15 in the lower esophagus, and 3 in the upper esophagus. Macroscopically, 24 tumors were classified as elevated, 27 as flat, and 19 as depressed. Mean tumor size was 25 mm (range, 4-70 mm). Histopathologically, there were 43 mucosal cancers (m1 in 8, m2 in 20, m3 in 15) and 27 submucosal cancers (sm1 in 3, sm2 in 24). Thirty-two lesions were treated with endoscopic resection, while 38 were treated surgically. Characteristics of the lesions are summarized in Table 1.
| Characteristics | |
| Male/female | 62/7 |
| Mean age, yr (range) | 66 (47-81) |
| Location | |
| Upper esophagus | 3 (4.3) |
| Mid-esophagus | 52 (74.3) |
| Lower esophagus | 15 (21.4) |
| Macroscopic shape | |
| Elevated | 24 (34.3) |
| Flat | 27 (38.6) |
| Depressed | 19 (27.1) |
| Mean tumor size, mm (range) | 25 (4-70) |
| Differentiation | |
| Well differentiated | 34 (48.6) |
| Moderately differentiated | 31 (44.3) |
| Poorly differentiated | 5 (7.1) |
| Depth of invasion | |
| Mucosa | 43 (61.4) |
| m1 | 8 |
| m2 | 20 |
| m3 | 15 |
| Submucosa | 27 (38.6) |
| sm1 | 3 |
| sm2 | 24 |
| Resection method | |
| Endoscopic resection | 32 (45.7) |
| Surgical resection | 38 (54.3) |
Overall accuracy of ME-NBI for estimating depth of invasion of SESCC was 78.6% (55 of 70 tumors), as listed in Table 2. Type B1 tumors, corresponding to m1 to m2 cancer, showed an accuracy of 88.6% (95%CI: 78.2%-94.6%) with a sensitivity and specificity of 71.4% (95%CI: 51.1%-86.0%) and 100% (95%CI: 89.6%-100%), respectively (Table 3). Type B2 tumors, corresponding to m3 to sm1 cancer, showed an accuracy of 78.6% (95%CI: 66.8%-87.1%) with a sensitivity and specificity of 94.4% (95%CI: 70.6%-99.7%) and 54.8% (95%CI: 36.3%-72.2%), respectively. Type B3 tumors, corresponding to sm2 cancer, showed an accuracy of 90.0% (95%CI: 79.9%-95.5%) with a sensitivity and specificity of 75.0% (95%CI: 52.9%-89.4%) and 97.8% (95%CI: 87.0%-99.9%), respectively. Depth of invasion was overestimated in 8 type B2 tumors (11.4%), underestimated in 6 type B2 tumors (8.6%), and underestimated in 1 type B3 tumor (5.3%).
| ME-NBI | No. of cases | Histopathology, n | ||
| m1-m2 | m3-sm1 | sm2 | ||
| B1 | 20 | 20 | 0 | 0 |
| B2 | 31 | 8 | 17 | 6 |
| B3 | 19 | 0 | 1 | 18 |
| ME-NBI | Accuracy (95%CI) | Sensitivity (95%CI) | Specificity (95%CI) | PPV (95%CI) | NPV (95%CI) |
| B1 | 88.6 (78.2-94.6) | 71.4 (51.1-86.0) | 100 (89.6-100) | 100 (80.0-100) | 84.0 (70.3-92.4) |
| B2 | 78.6 (66.8-87.1) | 94.4 (70.6-99.7) | 73.1 (58.7-84.0) | 54.8 (36.3-72.2) | 97.4 (84.9-99.9) |
| B3 | 90.0 (79.9-95.5) | 75.0 (52.9-89.4) | 97.8 (87.0-99.9) | 94.7 (71.9-99.7) | 88.2 (75.4-95.1) |
Of 48 lesions < 3 cm in size, 20 were classified as B1 tumors, 19 as B2 tumors, and 10 as B3 tumors. Of 21 lesions ≥ 3 cm in size, 12 were classified as B2 tumors and 9 as B3 tumors. The accuracy of ME-NBI for estimating depth of invasion of SESCC was lower in lesions ≥ 3 cm than in lesions < 3 cm, but the difference was not statistically significant (61.9% vs 85.7%, P = 0.053).
Kappa value of the JES classification for estimating depth of invasion of SESCC was excellent at 0.87 (95%CI: 0.77-0.97). Kappa values of the JES classification were 0.75 (95%CI: 0.48-1.02) for m1 to m2 cancer, 0.64 (95%CI: 0.05-1.33) for m3 to sm1 cancer, and 0.80 (95%CI: 0.53-1.07) for sm2 cancer.
Pretreatment prediction of depth of invasion of SESCC is very important in deciding treatment modalities. In the present study, we used the new ME-NBI classification recently developed by the JES for predicting depth of invasion of SESCC. As a result, overall accuracy was 78.6%, and interobserver agreement was excellent.
Because the JES classification is simplified compared with two previous classifications (developed by Inoue and Arima, respectively), there is a possibility that the accuracy for predicting depth of invasion will decrease. However, in the present study, accuracy of the JES classification was 78.6%, which is similar to the results of our previous study using Inoue’s classification[18]. Recently, a prospective multicenter study using the JES classification in Japan also demonstrated a high overall accuracy rate (90.5%) for type B vessels[16]. Although the accuracy rate in that study was higher than in our present results, there were limitations including high proportion of m1 to m2 cancers (77.3%), and changes in IPCLs were analyzed by participating endoscopists without a central review. In the present study, proportions of m1 to m2, m3 to sm1, and sm2 cancers were evenly distributed (40.0%, 25.7% and 34.3%, respectively), and changes in IPCLs were adjudicated according to the agreement of two endoscopists in discordant cases.
Although the sensitivities of type B1 and type B3 tumors were suboptimal (71.4% and 75.0%, respectively), the specificities were 100% and 97.8%, respectively. Because the recommended treatment modalities for type B1 and type B3 tumors are endoscopic resection and surgical resection, respectively, type B1 or type B3 tumors can guide the physician to decide the optimal treatment. However, the specificity of type B2 tumor was 73.1%; 8 m1 to m2 cancers were overestimated to m3 to sm1 cancers, and 6 sm2 cancers were underestimated to m3 to sm1 cancers. Therefore, compared with type B1 and type B3 tumors, type B2 tumors showed an insufficiency for estimating depth of invasion. It has been reported that m3 to sm1 cancers without lymphovascular invasion have very low risk of LN metastasis[3,19], and, as such, are potential candidates for curative treatment by endoscopic resection. Thus, because it is important to detect underestimated sm2 cancers in type B2 tumors, additional use of endoscopic ultrasonography or another diagnostic modality to estimate depth of invasion may be helpful[18].
In the present study, interobserver agreement was excellent at 0.87, which is slightly high compared with that in a previous study using Inoue’s classification (κ = 0.61, 0.64 and 0.71)[14]. This result suggests that this simplified classification is reliable among observers. However, the kappa value was lowest (κ = 0.64) for type B2 tumors. Therefore, efforts to improve interobserver agreement via modification of the definition of type B2 or use of computer-aided analysis should be considered.
This study had several limitations. First, this was a retrospective study that analyzed ME-NBI images using the JES classification. Thus, there might have been potential bias when reviewing the ME-NBI images. During ME-NBI, we tried to take at least 4 images to show the characteristic features associated with depth of invasion, with hopes that this would compensate, to some degree, the limitation of this retrospective study. Second, both endoscopists involved in this study are experts. A further study including non-experts is needed to apply our results to general practice.
In conclusion, the recently developed JES classification is useful for predicting depth of invasion of SESCC, with reliable results between observers. However, its accuracy decreases for type B2 tumors. Therefore, establishment of a revised classification including modification of the definition of type B2 will be considered, and prospective multi-center studies are needed to validate our results.
In order to choose the appropriate management strategy for superficial esophageal squamous cell carcinoma (SESCC), accurate assessment of depth of tumor invasion is important. Recently, the Japan Esophageal Society (JES) proposed a new magnifying endoscopy (ME) classification for estimating depth of tumor invasion based on two previous classifications developed by Inoue and Arima.
Since the new ME classification was recently proposed by the JES, there have been few studies to evaluate its utility. Furthermore, interobserver agreement of this classification has not been evaluated.
In this study, the JES classification was useful for estimating depth of invasion of SESCC. Overall accuracy was 78.6%, and interobserver agreement was excellent at 0.87. Type B1 tumors showed an accuracy of 88.6% with a sensitivity and specificity of 71.4% and 100%, respectively. Type B2 tumors showed an accuracy of 78.6% with a sensitivity and specificity of 94.4% and 54.8%, respectively. Type B3 tumors showed an accuracy of 90.0% with a sensitivity and specificity of 75.0% and 97.8%, respectively.
The JES classification is useful for predicting depth of invasion of SESCC, with reliable results between observers. This classification was particularly useful for selecting optimal treatment for type B1 and type B3 tumors.
Diagnostic criteria of the new JES classification are based on changes in intrapapillary capillary loops (IPCLs) of the esophageal mucosa observed by ME with narrow-band imaging. Type B tumors consist of abnormal microvessels with severe irregularity that have all four IPCL changes (meandering, dilation, caliber change, and various shapes).
The work has great interest, due to the importance of the theme.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ide E, Garcia-Olmo D S- Editor: Gong ZM L- Editor: A E- Editor: Li D
| 1. | Yamashina T, Ishihara R, Nagai K, Matsuura N, Matsui F, Ito T, Fujii M, Yamamoto S, Hanaoka N, Takeuchi Y. Long-term outcome and metastatic risk after endoscopic resection of superficial esophageal squamous cell carcinoma. Am J Gastroenterol. 2013;108:544-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 220] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 2. | Endo M, Yoshino K, Kawano T, Nagai K, Inoue H. Clinicopathologic analysis of lymph node metastasis in surgically resected superficial cancer of the thoracic esophagus. Dis Esophagus. 2000;13:125-129. [PubMed] |
| 3. | Moon JY, Kim GH, Kim JH, Kim HH, Ryu KD, Park SO, Lee BE, Song GA. Clinicopathologic factors predicting lymph node metastasis in superficial esophageal squamous cell carcinoma. Scand J Gastroenterol. 2014;49:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Takubo K, Aida J, Sawabe M, Kurosumi M, Arima M, Fujishiro M, Arai T. Early squamous cell carcinoma of the oesophagus: the Japanese viewpoint. Histopathology. 2007;51:733-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Araki K, Ohno S, Egashira A, Saeki H, Kawaguchi H, Sugimachi K. Pathologic features of superficial esophageal squamous cell carcinoma with lymph node and distal metastasis. Cancer. 2002;94:570-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Kodama M, Kakegawa T. Treatment of superficial cancer of the esophagus: a summary of responses to a questionnaire on superficial cancer of the esophagus in Japan. Surgery. 1998;123:432-439. [PubMed] |
| 7. | Kuwano H, Nishimura Y, Oyama T, Kato H, Kitagawa Y, Kusano M, Shimada H, Takiuchi H, Toh Y, Doki Y. Guidelines for Diagnosis and Treatment of Carcinoma of the Esophagus April 2012 edited by the Japan Esophageal Society. Esophagus. 2015;12:1-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 345] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 8. | Merkow RP, Bilimoria KY, Keswani RN, Chung J, Sherman KL, Knab LM, Posner MC, Bentrem DJ. Treatment trends, risk of lymph node metastasis, and outcomes for localized esophageal cancer. J Natl Cancer Inst. 2014;106:pii: dju133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 9. | Gono K, Obi T, Yamaguchi M, Ohyama N, Machida H, Sano Y, Yoshida S, Hamamoto Y, Endo T. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004;9:568-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 611] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 10. | Kumagai Y, Inoue H, Nagai K, Kawano T, Iwai T. Magnifying endoscopy, stereoscopic microscopy, and the microvascular architecture of superficial esophageal carcinoma. Endoscopy. 2002;34:369-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 164] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Inoue H, Honda T, Yoshida T. Ultra-high magnification endoscopy of the normal esopageal mucosa. Dig Endosc. 1996;134-138. |
| 12. | Inoue H, Kumagai Y, Yoshida T, Kawano T, Endo M, Iwai T. High-magnification endoscopic diagnosis of the superficial esophageal cancer. Dig Endosc. 2000;12:S32-S35. |
| 13. | Inoue H, Kaga M, Ikeda H, Sato C, Sato H, Minami H, Santi EG, Hayee B, Eleftheriadis N. Magnification endoscopy in esophageal squamous cell carcinoma: a review of the intrapapillary capillary loop classification. Ann Gastroenterol. 2015;28:41-48. [PubMed] |
| 14. | Sato H, Inoue H, Ikeda H, Sato C, Onimaru M, Hayee B, Phlanusi C, Santi EG, Kobayashi Y, Kudo SE. Utility of intrapapillary capillary loops seen on magnifying narrow-band imaging in estimating invasive depth of esophageal squamous cell carcinoma. Endoscopy. 2015;47:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Arima M, Tada T, Arima H. Evaluation of microvascular patterns of superficial esophageal cancers by magnifying endoscopy. Esophagus. 2005;191-197. |
| 16. | Oyama T, Inoue H, Arima M, Momma K, Omori T, Ishihara R, Hirasawa D, Takeuchi M, Tomori A, Goda K. Prediction of the invasion depth of superficial squamous cell carcinoma based on microvessel morphology: magnifying endoscopic classification of the Japan Esophageal Society. Esophagus. 2016;1-8. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 223] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 17. | Travis WD, Brambilla E, Konrad Muller-Hermelink HK. World Health Organization classification of tumors. Lyon: IARC; 2000; . |
| 18. | Lee MW, Kim GH, I H, Park DY, Baek DH, Lee BE, Song GA. Predicting the invasion depth of esophageal squamous cell carcinoma: comparison of endoscopic ultrasonography and magnifying endoscopy. Scand J Gastroenterol. 2014;49:853-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Cho JW, Choi SC, Jang JY, Shin SK, Choi KD, Lee JH, Kim SG, Sung JK, Jeon SW, Choi IJ. Lymph Node Metastases in Esophageal Carcinoma: An Endoscopist’s View. Clin Endosc. 2014;47:523-529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |