Published online Jun 21, 2017. doi: 10.3748/wjg.v23.i23.4278
Peer-review started: August 28, 2016
First decision: February 27, 2017
Revised: March 12, 2017
Accepted: April 12, 2017
Article in press: April 12, 2017
Published online: June 21, 2017
Processing time: 296 Days and 20.9 Hours
To investigate the evaluation of neogalactosylalbumin (NGA) for liver function assessment based on positron emission tomography technology.
Female Kunming mice were assigned randomly to two groups: fibrosis group and normal control group. A murine hepatic fibrosis model was generated by intraperitoneal injection of 10% carbon tetrachloride (CCl4) at 0.4 mL every 48 h for 42 d. 18F-labeled NGA ([18F]FNGA) was synthesized and administered at a dosage of 3.7 MBq/mouse to both fibrosis mice and normal control mice. Distribution of [18F]FNGA amongst organs was examined, and dynamic scanning was performed. Parameters were set up to compare the uptake of tracers by fibrotic liver and healthy liver. Serologic tests for liver function were also performed.
The liver function of the fibrosis model mice was significantly impaired by the use of CCl4. In the fibrosis model mice, hepatic fibrosis was verified by naked eye assessment and pathological analysis. [18F]FNGA was found to predominantly accumulate in liver and kidneys in both control group (n = 21) and fibrosis group (n = 23). The liver uptake ability (LUA), peak time (Tp), and uptake rate (LUR) of [18F]FNGA between healthy liver (n = 8) and fibrosis liver (n = 10) were significantly different (P < 0.05, < 0.01, and < 0.05, respectively). LUA was significantly correlated with total serum protein level (TP) (P < 0.05). Tp was significantly correlated with both TP and glucose (Glu) concentration (P < 0.05 both), and LUR was significantly correlated with both total bile acid and Glu concentration (P < 0.01 and < 0.05, respectively).
[18F]FNGA mainly accumulated in liver and remained for sufficient time. Functionally-impaired liver showed a significant different uptake pattern of [18F]FNGA compared to the controls.
Core tip: Neogalactosylalbumin (NGA) is a specific ligand for asialoglycoprotein receptor that is exclusively expressed on the surface of hepatic parenchymal cells. This study showed [18F]FNGA mainly accumulated in liver and remained for sufficient time. Functionally-impaired liver showed a significant different uptake pattern of [18F]FNGA compared to controls.
- Citation: Du SD, Li SH, Jin B, Zhu ZH, Dang YH, Xing HQ, Li F, Wang XB, Lu X, Sang XT, Yang HY, Zhong SX, Mao YL. Potential application of neogalactosylalbumin in positron emission tomography evaluation of liver function. World J Gastroenterol 2017; 23(23): 4278-4284
- URL: https://www.wjgnet.com/1007-9327/full/v23/i23/4278.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i23.4278
Asialoglycoprotein receptor (ASGPR) is expressed abundantly on the surfaces of mammalian hepatic parenchymal cells but rarely on extra-hepatic cells[1-3]. It is believed that the amount of ASGPR represents the number and density of functional hepatocytes in the liver[4,5]. Studies revealed that expression of ASGPR on hepatic cell surface decreases in both cirrhosis and obstructive jaundice patients, with more significant reduction in cirrhosis patients. Sawamura et al[6] found that cell surface expression of ASGPR is very low on hepatocyte carcinoma cells. No ASGRP expression has been found in any metastatic cancer in liver. Tomiguchi et al[7] noted that among patients with chronic active hepatitis, the decrease of ASGPR level is highly correlated with liver fibrosis and necrosis. Therefore, quantitative assessment of the ASGPR expression in liver could directly reflect liver function with a desired accuracy[8].
Technetium-99-labeled neogalactosylalbumin (99mTc-NGA), a ligand with high affinity to ASGPR, was developed[9,10] and is being applied for differential diagnosis of focal nodular hyperplasia and hepatic cancer with single photon emission computed tomography (SPECT)[11]. However, it is the analogue of NGA, technetium-labeled galactosyl human serum albumin (99mTc-GSA), that became widely used in many studies due to the non-specific binding between 99mTc and NGA. 99mTc-GSA became the first approved receptor binding reagent for scintigraphy in Japan[12]. However, the sensitivity, spatial resolution, and quantitative capacity of SPECT limited its application, whereas positron emission tomography (PET) exhibited significant advantages over SPECT[13-15]. Currently, there is no study using NGA PET scan for liver function evaluation.
18F is the most popular positron-emitting isotope for PET imaging. It has a low positron energy and proper physical half-life (110 min), which makes it suitable for in vivo study. 18F-labeled deoxyglucose (FDG) has been applied widely in clinical diagnosis and evaluation of neoplasms. In this study, we successfully synthesized 18F-labeled NGA, and performed a preliminary investigation on a liver fibrosis mouse model for quantitative live function analysis.
All animal experiment protocols were approved by the Institutional Animal Care and Use Committee and carried out in accordance with guidelines of the Laboratory Animal Center of Peking Union Medical College. Female Kunming mice, 6-7 wk and 19-25 g, were obtained from Beijing Weitonglihua Experimental Animal Corporation (Beijing, China). Liver fibrosis was induced by intraperitoneal (ip) injection of 10% carbon tetrachloride oil solution (CCl4) (National Chemical Reagent Group, Beijing, China) at 0.4 mL every 48 h for 42 d[16].
18F-labeled neogalactosylalbumin ([18F]FNGA) was synthesized in Beijing Normal University Chemistry College using a protocol published earlier[17]. The radioactive purity was > 99%. The administration dose was 3.7 MBq for each mouse.
[18F]FNGA (3.7 MBq in 100 μL solution containing about 40 μg NGA) was injected through the tail vein. Blood samples were collected through orbital bleeding at 5, 30 or 60 min after injection, immediately followed by sacrifice of the animal by cervical dislocation and collection of organ samples. Radioactivity of each organ was measured using a radioactive detector (RM-905a; China) and expressed as relative radioactive dosage per gram (%ID/g), with the accumulated radioactivity in the organ divided by the total injected dose per gram of body weight.
Blood samples were collected through orbital bleeding from all animals at different periods after injection of [18F]FNGA. Testing items included total protein (TP), albumin (ALB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bile acid (TBA), lactate dehydrogenase (LD), cholinesterase (ChE), urea nitrogen (BUN), serum creatinine (Cr), blood glucose (Glu), total cholesterol (Tc), triglycerides (Tg), calcium ions (Ca), adenosine dehydrogenase (ADA), sodium ions (Na), potassium ions (K), prothrombin time (PT), and international normalized ratio (INR).
Mice were anaesthetized by chloral hydrate. Immediately after [18F]FNGA injection at 7.4 MBq/kg, a mouse was laid on a microPET scanner (Siemens, Germany) and serial static emission scanning was performed at 120 kV, 100 mA/s and with a 5 mm section cranial thickness. A whole-body PET emission scan was performed with 2-min acquisition per bed position using a 3-dimensional acquisition mode with 1 min interval in a total of 30 min. A second phase scanning was also performed immediately after the 30-min scan at 5 min per scan with 1 min interval for a total of 30 min. Region of interest (ROI) was drawn and the standardized uptake values (SUV) in each ROI was measured.
The images were semi-quantitatively analyzed by ImageJ software of the microPET for the average SUV within the ROI. Total body radioactivity (RTotal) was measured by the entire body scan, excluding the injection spot on the tail. Parameters were set up specifically as follows, to reflect the liver capacity for tracer uptake: Liver uptake ability (LUA) was calculated as the peak radioactive value (Rp) in liver ROI divided by the RTotal; Peak time (Tp) was the time when radioactivity in ROI reaches to the Rp; Clearance index (CI) was the radioactivity in the heart ROI at the time of liver Tp divided by the radioactivity of the first scanning in the heart ROI; Liver uptake rate (LUR) was defined as LUR = [(Rp-Rt1)/RTotal]/(Tp-T1), where T1 was the point time of the first scan and Rt1 was the liver radioactivity of the first scan.
Statistical analysis was performed with SPSS 13.0 software (IBM, Chicago, IL, United States). Data were expressed as mean ± SD. Shapiro-Wilk test was employed for normal distribution data. Independent t-test was used for comparison between the groups with normal distribution, and Mann-Whitney method was used for those with abnormal distribution. Pearson or Spearman examination was applied for correlation test for normal or non-normal distribution samples, respectively.
The number of blood samples was 31 from the fibrosis mice and 29 from the control mice, with the exception of 2 fibrosis mice having failed sampling. As shown in Table 1, liver function of the fibrosis model mice was significantly impaired by the use of CCl4 for 42 d. Laboratory tests for liver function demonstrated significant differences between the fibrosis model mice and the controls in TP, ALB, and Glu with P < 0.05, and in ALP and ChE with P < 0.01. Obvious pinkish fiber cords were observed, even with the naked eye, in the fibrosis model mice. Hepatic fibrosis was also verified by pathological analysis (data not shown)[16].
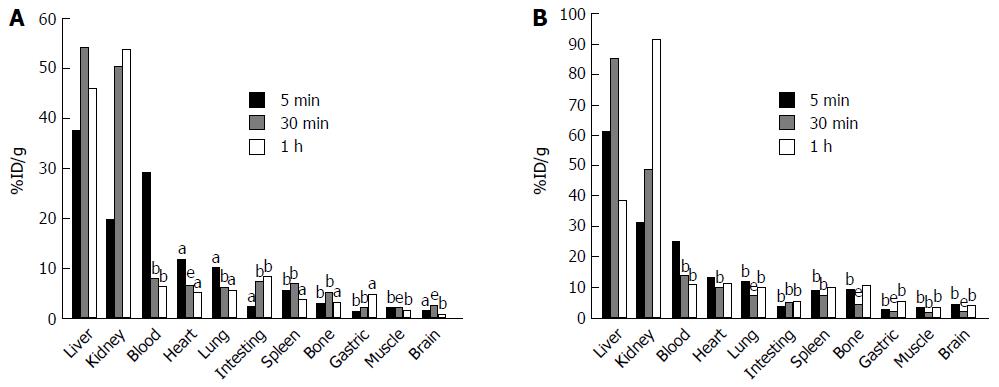
Animals were sacrificed after injection of [18F]FNGA according to the following order: 5 min (8 fibrosis and 6 control mice), 30 min (8 fibrosis and 8 control mice), and 60 min (7 fibrosis and 7 control mice). Organs were removed for radioactivity measurement. Figure 1 demonstrates the distribution of [18F]FNGA in both control mice (Figure 1A) and fibrosis model mice (Figure 1B). [18F]FNGA was found to accumulate mainly in liver and kidney over the time of observation in both control and fibrosis model mice, except for the initial high level in blood detected at 5 min after injection.
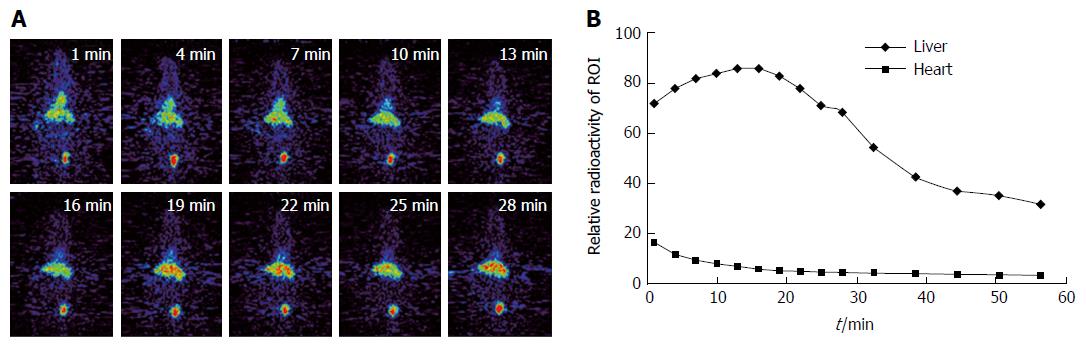
Whole body scan was performed on 10 fibrosis and 8 control mice. Clear liver images were acquired without obvious interference from other abdominal organs. Figure 2A exhibits the serial images of a control mouse over a period of 30 min. The liver outline can be clearly observed at 4 min after tracer injection. The liver radioactivity reached the peak level in the control mouse at about 19 min. However, radioactivity in the fibrosis liver reached the peak much later than that in the control group. The tracer remained in the liver at a high level throughout the scanning period. In addition, a clear heart outline was presented in the early phase. In the later phase of the scanning, high tracer accumulation was found in the bladder. Very low radioactivity was detected in the brain, lung, and limbs.
The typical dynamic curves of [18F]FNGA in liver and heart of a control mouse are shown in Figure 2B. High radioactivity was observed in both organs immediately after [18F]FNGA administration. Liver exhibited a continuous accumulation till reaching a peak level, whereas heart showed a quick clearance after [18F]FNGA injection.
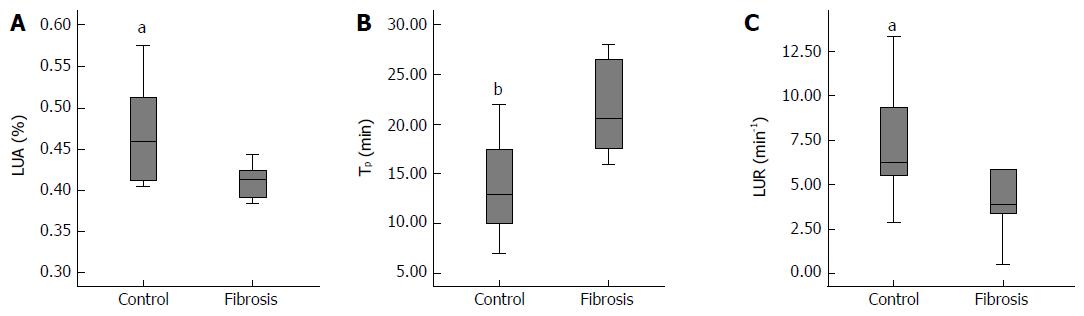
Parameters based on the PET images are shown in Figure 3. The LUA was significantly lower in fibrotic liver than that in the normal liver (P < 0.05; Figure 3A). The Tp took significantly longer in fibrotic liver to reach a peak than in the normal liver (P < 0.01; Figure 3B). The [18F]FNGA accumulating rate (LUR) of fibrotic liver was significantly slower than that of the controls (P < 0.05; Figure 3C). No significant differences were found in CI between the two groups.
Correlation was found between the PET parameters and serological tests including TP, TBA, and Glu (Table 2). LUA was significantly correlated with TP, Tp was significantly correlated with both TP and Glu, and LUR was significantly correlated with both TBA and Glu, with P < 0.05 in the above conditions.
Multiple methods have been applied in clinical assessment for liver function, including serological tests[18], Child-Pugh and model for end-stage liver disease (commonly known as MELD) scoring systems, indocyanine green clearance, and medical imaging modalities like computed tomography, magnetic resonance imaging, and SPECT[19,20]. The above-mentioned methods provide data on certain types of liver function to meet the clinical purposes for diagnosis and treatment. However, they have prominent limitations for accurate evaluation. PET has become increasingly important as a functional diagnostic tool with various applications, especially in the diagnosis and evaluation of malignant tumors. Recently, effort has been made in using PET for liver function evaluation through sophisticated calculation of 18F-FD-Galactose clearance[21]. It requires extra invasive arterial sampling, the procedure is far from practical, and the result is not visually observational.
To the best of our knowledge, this is the first study applying [18F]FNGA in the evaluation of liver function in a fibrotic liver animal model. We demonstrated the significant differences between the fibrotic liver and the normal control liver in their [18F]FNGA LUA, PT, and LUR. In addition, the tracer retained in liver for sufficient long time (> 60 min) and generated a clear image of liver, which is a critical tracer for PET scanning.
Several advantages of [18F]FNGA were observed for this application to liver function assessment. The rapid clearance from the cardiovascular system, as well as the extended retention in the liver, minimizes the influence to liver imaging and parameter calculation. The tracer was mainly excreted through kidneys, which was an obvious difference from the 99mTc-GSA, which is mainly excreted from the gallbladder-gastrointestinal (GGI) tract. Therefore, the imaging result would be less likely to be influenced by obstruction of the GGI tract, which could lead to more accurate liver function evaluation. However, concern has existed about the possible influence of renal function on scanning result. In this study, all animals were normal healthy, except for the induction of liver fibrosis. And, there is no known effect of CCl4 on renal function. Nevertheless, further study will be needed in animals with kidney defect.
The receptor index (LHL15 and HH15) in the 99mTc-GSA study was widely accepted and has been taken as reference because both NGA and GSA bind to the same receptor on hepatocytes[20]. In this study, therefore, we generated CI and LUA that played similar functional roles as HH15 and LHL15, respectively. In addition, LUR, which evaluated the liver uptake rate for [18F]FNGA, also showed significant differences between the control and fibrotic livers, with the fibrotic liver showing a much slower rate. In addition, these parameters were found to be highly correlated with traditional liver function indicators, such as TP and TBA, suggesting that these parameters are appropriate for liver function evaluation.
When liver function was impaired, blood glucose cannot be quickly converted into glycogen, thus leading to an elevated blood glucose level. In fact, some have proposed the use of an oral glucose tolerance test curve to determine the degree of liver dysfunction, tolerance for surgery, and prognosis[22]. We found that both LUR and Tp were significantly correlated with blood glucose level, suggesting that the blood glucose level indeed may indicate one of the aspects of the liver function.
This is a preliminary study investigating the use of [18F]FNGA as a PET tracer, with limitations existing in many regards. First, this study did not demonstrate the correlation of the severity of fibrosis and tracer uptake by the liver. Second, the mechanism of biological metabolism of the [18F]FNGA needs to be further investigated. Though the animal model employed in this study was well-established, the liver damage in the model could not be quantitatively defined. A progressive liver function loss would be more appropriate to evaluate the role the [18F]FNGA PET scan images in reflection of the liver function damage.
Functionally-impaired liver showed a significantly different uptake pattern of [18F]FNGA compared to control liver in PET examination. [18F]FNGA was mainly accumulated and retained in the liver, and for sufficient time for PET imaging. Excretion from the kidney could be another advantage for [18F]FNGA to avoid gastrointestinal side effect.
There are several liver function evaluation methods routinely using in the clinic, such as serological tests, Child-Pugh and model for end-stage liver disease (commonly known as MELD) scoring systems, etc. But, none of these can assess the function of any particular anatomical fraction of the liver. Asialoglycoprotein receptor (ASGPR) is expressed abundantly on the surfaces of mammalian hepatic parenchymal cells. It is believed that the amount of ASGPR represents the number and density of functional hepatocytes in the liver. A ligand with high affinity to ASGPR, neogalactosyl albumin (NGA) was developed. To date, however, there has been no study using NGA positron emission tomography (PET) scan for liver function evaluation.
This is the first study applying 18F-labeled neogalactosylalbumin ([18F]FNGA) in the evaluation of liver function in a fibrotic liver animal model. Functionally-impaired liver showed a significant different uptake pattern of [18F]FNGA compared to control liver in PET examination.
This is the first study to show [18F]FNGA mainly accumulated and retained in the liver, and for sufficient time for PET. Our data demonstrated that the parameters from the pharmacokinetics curve of NGA were significantly different between control and fibrosis mice. More importantly, the data was significantly correlated with the major traditional serological tests.
In the present manuscript, we have reported, for the first time, application of [18F]FNGA in the evaluation of liver function in a fibrotic and normal liver animal model. Under PET/computed tomography (CT) scan, it could primarily achieve the goals that present the liver function with computerized 3-D imagining techniques. Thus, this can be an intuitive tool to design operations and may predict the risk of hepatectomy. Finally, it can also be used in other medical fields for assessing liver function.
Neogalactosylalbumin, positron emission tomography, liver function, liver fibrosis, mouse model.
This is a very interesting application of the PET/CT technique to the analysis of liver pathophysiology. The data are convincing and the specificity of the labeling is adequate.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): D
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Aurello P, Boscá L, Mura VL S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Zhang FF
| 1. | Akaki S, Mitsumori A, Kanazawa S, Togami I, Takeda Y, Joja I, Hiraki Y. Technetium-99m-DTPA-galactosyl human serum albumin liver scintigraphy evaluation of regional CT/MRI attenuation/signal intensity differences. J Nucl Med. 1998;39:529-532. [PubMed] |
| 2. | Burgess JB, Baenziger JU, Brown WR. Abnormal surface distribution of the human asialoglycoprotein receptor in cirrhosis. Hepatology. 1992;15:702-706. [PubMed] |
| 3. | Morell AG, Irvine RA, Sternlieb I, Scheinberg IH, Ashwell G. Physical and chemical studies on ceruloplasmin. V. Metabolic studies on sialic acid-free ceruloplasmin in vivo. J Biol Chem. 1968;243:155-159. [PubMed] |
| 4. | Gärtner U, Stockert RJ, Morell AG, Wolkoff AW. Modulation of the transport of bilirubin and asialoorosomucoid during liver regeneration. Hepatology. 1981;1:99-106. [PubMed] |
| 5. | Meyer B, Luo HS, Bargetzi M, Renner EL, Stalder GA. Quantitation of intrinsic drug-metabolizing capacity in human liver biopsy specimens: support for the intact-hepatocyte theory. Hepatology. 1991;13:475-481. [PubMed] |
| 6. | Sawamura T, Nakada H, Hazama H, Shiozaki Y, Sameshima Y, Tashiro Y. Hyperasialoglycoproteinemia in patients with chronic liver diseases and/or liver cell carcinoma. Asialoglycoprotein receptor in cirrhosis and liver cell carcinoma. Gastroenterology. 1984;87:1217-1221. [PubMed] |
| 7. | Tomiguchi S, Kira T, Oyama Y, Nabeshima M, Nakashima R, Tsuji A, Kojima A, Takahashi M, Yoshimatsu S, Sagara K. Correlation of Tc-99m GSA hepatic studies with biopsies in patients with chronic active hepatitis. Clin Nucl Med. 1995;20:717-720. [PubMed] |
| 8. | Kaibori M, Ha-Kawa SK, Uchida Y, Ishizaki M, Saito T, Matsui K, Hirohara J, Tanaka K, Kamiyama Y. Liver regeneration in donors evaluated by Tc-99m-GSA scintigraphy after living donor liver transplantation. Dig Dis Sci. 2008;53:850-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Vera DR, Krohn KA, Scheibe PO, Stadalnik RC. Identifiability analysis of an in vivo receptor-binding radiopharmacokinetic system. IEEE Trans Biomed Eng. 1985;32:312-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Vera DR, Krohn KA, Stadalnik RC, Scheibe PO. Tc-99m galactosyl-neoglycoalbumin: in vitro characterization of receptor-mediated binding. J Nucl Med. 1984;25:779-787. [PubMed] |
| 11. | Kurtaran A, Müller C, Novacek G, Kaserer K, Mentes M, Raderer M, Pidlich J, Eibenberger K, Angelberger P, Virgolini I. Distinction between hepatic focal nodular hyperplasia and malignant liver lesions using technetium-99m-galactosyl-neoglycoalbumin. J Nucl Med. 1997;38:1912-1915. [PubMed] |
| 12. | Stadalnik RC, Vera DR. The evolution of (99m)Tc-NGA as a clinically useful receptor-binding radiopharmaceutical. Nucl Med Biol. 2001;28:499-503. [PubMed] |
| 13. | Hayakawa N, Nakamoto Y, Kurihara K, Yasoda A, Kanamoto N, Miura M, Inagaki N, Togashi K. A comparison between 11C-methionine PET/CT and MIBI SPECT/CT for localization of parathyroid adenomas/hyperplasia. Nucl Med Commun. 2015;36:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Erritzoe D, Talbot P, Frankle WG, Abi-Dargham A. Positron emission tomography and single photon emission CT molecular imaging in schizophrenia. Neuroimaging Clin N Am. 2003;13:817-832. [PubMed] |
| 15. | Parker MW, Iskandar A, Limone B, Perugini A, Kim H, Jones C, Calamari B, Coleman CI, Heller GV. Diagnostic accuracy of cardiac positron emission tomography versus single photon emission computed tomography for coronary artery disease: a bivariate meta-analysis. Circ Cardiovasc Imaging. 2012;5:700-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 184] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 16. | Li SH, Qiu L, Cheng XQ, Li J, Du SD, Mao YL. The establishment of hepatic fibrosis model in mice through intraperitoneal injection with low concentrationcarbon tetrachloride. Basic Clinical Med. 2014;34:1694-1695. |
| 17. | Yang W, Mou T, Peng C, Wu Z, Zhang X, Li F, Ma Y. Fluorine-18 labeled galactosyl-neoglycoalbumin for imaging the hepatic asialoglycoprotein receptor. Bioorg Med Chem. 2009;17:7510-7516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Limdi JK, Hyde GM. Evaluation of abnormal liver function tests. Postgrad Med J. 2003;79:307-312. [PubMed] |
| 19. | Ribero D, Curley SA, Imamura H, Madoff DC, Nagorney DM, Ng KK, Donadon M, Vilgrain V, Torzilli G, Roh M. Selection for resection of hepatocellular carcinoma and surgical strategy: indications for resection, evaluation of liver function, portal vein embolization, and resection. Ann Surg Oncol. 2008;15:986-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Taniguchi M, Okizaki A, Watanabe K, Imai K, Uchida K, Einama T, Shuke N, Miyokawa N, Furukawa H. Hepatic clearance measured with (99m)Tc-GSA single-photon emission computed tomography to estimate liver fibrosis. World J Gastroenterol. 2014;20:16714-16720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Sørensen M, Mikkelsen KS, Frisch K, Villadsen GE, Keiding S. Regional metabolic liver function measured in patients with cirrhosis by 2-[¹8F]fluoro-2-deoxy-D-galactose PET/CT. J Hepatol. 2013;58:1119-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Mao YL, Du SD. [Improvement of the methods of liver function assessment for primary hepatocellular carcinoma]. Zhonghua Waike Zazhi. 2010;48:1530-1533. [PubMed] |