Published online Jun 21, 2017. doi: 10.3748/wjg.v23.i23.4262
Peer-review started: December 20, 2016
First decision: March 16, 2017
Revised: March 29, 2017
Accepted: May 19, 2017
Article in press: May 19, 2017
Published online: June 21, 2017
Processing time: 184 Days and 21.2 Hours
To evaluate the usefulness of total colonoscopy (TCS) for patients undergoing gastric endoscopic submucosal dissection (ESD) and to assess risk factors for colorectal neoplasms.
Of the 263 patients who underwent ESD at our department between May 2010 and December 2013, 172 patients undergoing TCS during a one-year period before and after ESD were targeted. After excluding patients with a history of surgery or endoscopic therapy for colorectal neoplasms, 158 patients were analyzed. Of the 868 asymptomatic patients who underwent TCS during the same period because of positive fecal immunochemical test (FIT) results, 158 patients with no history of either surgery or endoscopic therapy for colorectal neoplasms who were matched for age and sex served as the control group for comparison.
TCS revealed adenoma less than 10 mm in 53 patients (33.6%), advanced adenoma in 17 (10.8%), early colorectal cancer in 5 (3.2%), and advanced colorectal cancer in 4 (2.5%). When the presence or absence of adenoma less than 10 mm, advanced adenoma, and colorectal cancer and the number of adenomas were compared between patients undergoing ESD and FIT-positive patients, there were no statistically significant differences in any of the parameters assessed. The patients undergoing ESD appeared to have the same risk of colorectal neoplasms as the FIT-positive patients. Colorectal neoplasms were clearly more common in men than in women (P = 0.031). Advanced adenoma and cancer were significantly more frequent in patients with at least two of the following conditions: hypertension, dyslipidemia, and diabetes mellitus (P = 0.019).
In patients undergoing gastric ESD, TCS appears to be important for detecting synchronous double neoplasms. Advanced adenoma and cancer were more common in patients with at least two of the following conditions: hypertension, dyslipidemia, and diabetes mellitus. Caution is therefore especially warranted in patients with these risk factors.
Core tip: This is a retrospective study to evaluate the usefulness of total colonoscopy (TCS) for patients undergoing gastric endoscopic submucosal dissection (ESD). The frequency of detecting colorectal lesions, especially advanced adenoma and carcinoma, was higher in patients with early gastric cancer or gastric adenoma. This observation suggests that such patients are at a risk equivalent to that of fecal immunochemical test positive patients, suggesting that screening TCS should be performed as extensively as possible in patients undergoing ESD.
- Citation: Tsuchida C, Yoshitake N, Kino H, Kaneko Y, Nakano M, Tsuchida K, Tominaga K, Sasai T, Masuyama H, Yamagishi H, Imai Y, Hiraishi H. Clinical importance of colonoscopy in patients with gastric neoplasm undergoing endoscopic submucosal dissection. World J Gastroenterol 2017; 23(23): 4262-4269
- URL: https://www.wjgnet.com/1007-9327/full/v23/i23/4262.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i23.4262
Gastric endoscopic submucosal dissection (ESD) has been established as a common curative resection procedure for gastric neoplasms including early gastric cancer and gastric adenoma[1,2]. ESD showed a superior efficacy with respect to endoscopic mucosal resection in a recent meta-analysis[3]. As adoption of this procedure becomes ever more widespread, the indications for endoscopic therapy are being expanded[4]. The curative resection rate with gastric ESD is approximately 85%[5,6], and the 5-year cancer-specific survival rate is reported to be approximately 99% in patients undergoing curative resection[7,8]. As these rates indicate, the gastric cancer mortality rate is very low after endoscopic resection of gastric neoplasms. To further improve outcomes, screening for neoplasms in other organs is assumed to be important.
Colorectal neoplasms are among the most common malignancies in Europe and North America. The incidence of these malignancies has markedly increased in the past 20 to 30 years in Asia, including Japan[9,10].
Colorectal neoplasms are the most commonly observed tumors outside of the stomach in patients with gastric cancer. Synchronous or metachronous colorectal cancer is reportedly detected in approximately 1% of patients with gastric cancer[11,12]. Therefore, screening colonoscopy before surgical interventions for the stomach is now well established. However, the usefulness of screening colonoscopy in patients undergoing gastric ESD for gastric adenoma or early gastric cancer has yet to be clarified, and there are many facilities that do not perform colonoscopy for such patients.
In the present study, we determined the prevalence of synchronous colorectal neoplasms in patients undergoing gastric ESD and compared this prevalence with the prevalence in patients with positive fecal occult blood test results to assess the usefulness of screening colonoscopy for patients undergoing gastric ESD. The risk factors for colorectal neoplasms in patients undergoing gastric ESD were also assessed.
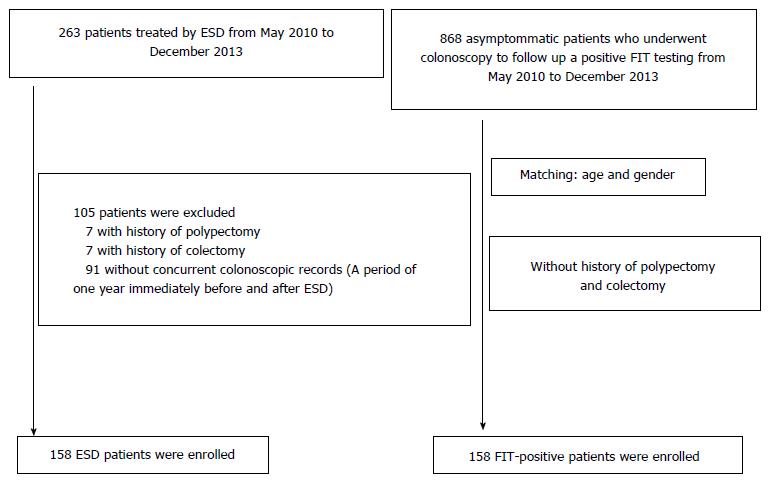
Of the 263 patients who underwent ESD for gastric adenoma or early gastric cancer at the Department of Gastroenterology, Dokkyo Medical University, between May 2010 and December 2013, 172 patients receiving total colonoscopy (TCS) during a one-year period before and after ESD were targeted. None of these patients had familial adenomatous polyposis or inflammatory bowel disease. Seven patients who had undergone endoscopic resection of the large bowel in the past and 7 who had undergone colectomy were excluded. In total, 158 patients were included (ESD group) in this study. There were 130 men and 28 women. Of the 868 asymptomatic patients who underwent TCS during the same period because of positive fecal immunochemical test (FIT) results, we excluded those who had undergone endoscopic resection of the large bowel or colectomy in the past. Therefore, 158 randomly selected subjects who were matched for age and sex served as the control group for comparison (FIT group) (Figure 1).
The study protocol was approved by the ethics committee of Dokkyo Medical University. Written informed consent was obtained from all participants before the procedures.
The number, size, and histology of colorectal neoplasms were examined. The number was assessed by dividing the patients into those with 1 to 3 neoplasms and those with 4 or more neoplasms. Sizes were determined employing biopsy forceps. Regarding histology, neoplasms measuring 6 mm or more were resected and pathologically assessed, in principle, whereas those measuring 5 mm or less were assessed by macroscopic evaluation including magnifying endoscopy. The pathological assessment was performed according to the Japanese classification of cancer of the colon and rectum[13], while magnifying endoscopy was performed according to the pit pattern and narrow band imaging classifications[14,15]. Non-neoplastic polyps, such as inflammatory and hyperplastic polyps, were excluded. Neoplasms were classified into low grade adenoma measuring less than 10 mm, advanced adenoma (adenoma measuring 10 mm or more, adenoma containing villous components, and adenoma with high-grade dysplasia), early cancer (cancer with infiltration limited to the mucosa or submucosa), advanced cancer (cancer with infiltration reaching the proper muscular layer), and neuroendocrine tumors.
For each patient, electronic and paper medical records were reviewed in terms of history of treatment and surgery, endoscopic findings, images, and pathological findings. To identify risk factors for colorectal neoplasms, benign vs malignant neoplasms, neoplasm diameter, neoplasm site, sex, age, body mass index (BMI), history of cancer, underlying diseases (hypertension, diabetes mellitus, and dyslipidemia), and lifestyle history (smoking and drinking history) were assessed. Whether gastric lesions were benign or malignant was determined by differentiating between adenoma and cancer according to the histopathological results. The sites of gastric neoplasms were classified into 3 regions. The upper (U) region was defined as the cardia, fundus, and proximal third of the body of the stomach. The middle (M) region was defined as the distal two-thirds of the body and the angle of the stomach. The lower (L) region was defined as the vestibule and anterior part of the pylorus. BMI was calculated by dividing the weight by the square of the height. Levels of cholesterol, triglyceride, and fasting blood glucose were measured with autoanalyzers. Hypertension was defined as a systolic blood pressure of 140 mmHg or more and/or a diastolic blood pressure of 90 mmHg or more. Diabetes mellitus was defined as a fasting blood glucose level of 126 mg/dL or more, a 2-h level of 200 mg/dL or more on 75-g glucose tolerance test, or a casual blood glucose level of 200 mg/dL or more. Dyslipidemia was defined as a low-density lipoprotein (LDL) cholesterol level of more than 140, a high-density lipoprotein (HDL) cholesterol level of less than 45, and a triglyceride level of more than 150. For smoking history, patients currently or previously smoking 10 cigarettes or more per day for 5 years were regarded as having a smoking history, and all others were considered as having no smoking history. For alcohol consumption history, patients who were current or former non-drinkers or social drinkers only were regarded as having no drinking history, and all others were considered as having a drinking history.
Univariate analyses were performed for each parameter. Student’s t-test was used for age, neoplasm diameter, and BMI. For the other parameters, the χ2 test was performed, except for those with an expected value of 5 or less, for which the Fisher exact test was used. Multivariate analyses for independent risk factors for overall colorectal neoplasms or for advanced adenoma and carcinoma were performed by logistic regression. Odds ratios (OR) and 95%CI were calculated for each variable. A statistically significant difference was defined as a P value of less than 0.05.
In the ESD group undergoing TCS, adenoma less than 10 mm was detected in 53 patients (33.6%), advanced adenoma in 17 (10.8%), early colorectal cancer in 5 (3.2%), advanced colorectal cancer in 4 (2.5%), and a rectal neuroendocrine tumor (NET) in 1 (0.6%) (Table 1).
| Colorectal neoplasm | ESD group (n = 158) |
| Non-lesion | 78 (49.4) |
| Adenoma (< 1 cm) | 53 (33.5) |
| Advanced adenoma | 17 (10.8) |
| Large tubular adenoma (≥ 1 cm) | |
| Tubulovillous/villous adenoma | |
| High grade dysplasia | |
| Early cancer (depth of mucosa/submucosa) | 5 (3.2) |
| Advanced cancer | 4 (2.5) |
| Neuroendocrine tumor | 1 (0.6) |
Regarding clinicopathological characteristics, no difference was observed between the ESD and FIT groups (Table 2). These two groups were compared for the presence or absence of adenoma less than 10 mm, advanced adenoma, colorectal cancer or NET, and the number of adenomas. Adenoma less than 10 mm was observed in 33.5% of patients in the ESD group and in 41.8% of those in the FIT group. Patients with 1 to 3 adenomas accounted for 37.3% of the ESD group and 44.9% of the FIT group, whereas those with 4 adenomas or more accounted for 7.0% and 12.0%, respectively. Advanced adenoma was observed in 10.8% of patients in the ESD group and 15.2% of those in the FIT group, while colorectal cancer or NET was observed in 6.3% of patients in both groups. Because there were no statistically significant differences among the types of neoplasms, the ESD group was assumed to have a colorectal neoplasm risk equal to that of the FIT group (Table 3).
| ESD group (n = 158) | FIT group (n = 158) | P value | |
| Age (year, mean ± SD) | 69.5 ± 8.9 | 69.5 ± 8.9 | 0.999 |
| Gender, male/female | 130 (82.3)/28 (17.7) | 130 (82.3)/28 (17.7) | 0.999 |
| BMI (kg/m2) | 23.0 ± 3.1 | 22.8 ± 3.4 | 0.589 |
| Smoking | 100 (63.0) | 86 (54.4) | 0.137 |
| Alcohol | 74 (46.8) | 66 (41.8) | 0.428 |
| Diabetes mellitus | 20 (12.7) | 27 (17.1) | 0.343 |
| Dyslipidemia | 25 (15.8) | 40 (25.3) | 0.051 |
| Hypertension | 73 (46.2) | 88 (55.7) | 0.115 |
| History of other organs’ cancer | 24 (15.2) | 17 (10.8) | 0.315 |
| Colorectal neoplasm | ESD group (n = 158) | FIT group (n = 158) | P value |
| Adenoma (< 1 cm) | 53 (33.5) | 66 (41.8) | 0.164 |
| Advanced adenoma | 17 (10.8) | 24 (15.2) | 0.315 |
| Large tubular adenoma (≥ 1 cm) | |||
| Tubulovillous/villous adenoma | |||
| High grade dysplasia | |||
| Number of adenomas | 0.507 | ||
| 1 to 3 | 59 (37.3) | 71 (44.9) | |
| ≥ 4 | 11 (7.0) | 19 (12.0) | |
| Cancer or NET | 10 (6.3) | 10 (6.3) | 0.999 |
When benign vs malignant neoplasms, neoplasm diameter, neoplasm site, sex, age, BMI, history of cancer, underlying disorders (hypertension, diabetes mellitus, and dyslipidemia), and tobacco and alcohol consumption were assessed to identify risk factors for colorectal neoplasms in the ESD group, it was evident that colorectal neoplasms were more common in men than in women (P = 0.031) (Table 4). Regarding the risk factors for advanced adenoma and carcinoma in the ESD group, the risk was significantly higher in patients with at least two of the following conditions: hypertension, dyslipidemia, and diabetes mellitus (life related disease, P = 0.019) (Table 5).
| CRN group (n = 80) | Non-CRN group (n = 78) | P value | |
| Gender, male/female | 71 (88.8)/9 (11.3) | 59 (75.6)/19 (24.4) | 0.031 |
| Age (yr, mean ± SD) | 70.8 ± 8.3 | 68.9 ± 10.4 | 0.215 |
| Histopathology (adenoma/carcinoma) | 69 (86.3)/11 (13.8) | 70 (89.7)/8 (10.3) | 0.667 |
| Tumor size (mm, mean ± SD) | 19.3 ± 12.3 | 19.0 ± 17.2 | 0.926 |
| Tumor location | |||
| U | 17 (21.3) | 16 (20.5) | 0.999 |
| M | 33 (41.3) | 28 (35.9) | 0.598 |
| L | 30 (37.5) | 34 (43.6) | 0.538 |
| BMI | 23.3 ± 3.3 | 22.7 ± 2.8 | 0.219 |
| Lifestyle related disease1 | 16 (20.0) | 13 (16.7) | 0.737 |
| Smoking | 55 (68.8) | 45 (57.7) | 0.202 |
| Alcohol | 41 (51.3) | 33 (42.3) | 0.334 |
| History of other organs’ cancer | 16 (20.0) | 9 (11.5) | 0.215 |
| AAC group (n = 26) | Non-AAC group (n = 132) | P value | |
| Gender, male/female | 23 (88.5)/3 (11.5) | 107 (81.1)/25 (18.9) | 0.574 |
| Age (yr, mean ± SD) | 70.4 ± 8.5 | 69.7 ± 9.6 | 0.732 |
| Histopathology (adenoma/carcinoma) | 6 (23.1)/20 (76.9) | 13 (9.85/119 (90.2) | 0.117 |
| Tumor size (mm, mean ± SD) | 21.9 ± 12.5 | 18.6 ± 12.7 | 0.305 |
| Tumor location | |||
| U | 4 (15.4) | 29 (22.0) | 0.601 |
| M | 13 (50.0) | 48 (36.4) | 0.278 |
| L | 9 (34.6) | 55 (41.7) | 0.652 |
| BMI | 23.9 ± 3.8 | 22.8 ± 2.9 | 0.074 |
| Lifestyle related disease1 | 9 (34.6) | 20 (15.2) | 0.019 |
| Smoking | 20 (76.9) | 80 (60.6) | 0.175 |
| Alcohol | 15 (57.7) | 59 (44.7) | 0.318 |
| History of other organs’ cancer | 6 (23.1) | 19 (14.4) | 0.415 |
We also performed multivariate analyses correcting for gender and life related disease, which have significant differences in univariate analyses, age and BMI, which are well known as risk factors of colorectal carcinoma. These analyses showed that male sex was the independent risk factor for colorectal neoplasm (OR = 2.65, 95%CI: 1.11-6.36, P = 0.029) and that life related disease was the independent risk factor for advanced adenoma and carcinoma in the ESD group (OR = 3.01, 95%CI: 1.16-7.86, P = 0.024) (Table 6).
| Overall colorectal neoplasm | AAC | |||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age (≥ 65 yr) | 1.42 (0.68-3.00) | 0.350 | 0.70 (0.27-1.82) | 0.460 |
| Gender (male) | 2.65 (1.11-6.36) | 0.029 | 1.85 (0.49-6.97) | 0.362 |
| BMI (≥ 25) | 1.21 (0.57-2.56) | 0.613 | 1.80 (0.71-4.58) | 0.217 |
| Lifestyle related disease1 | 1.24 (0.54-2.85) | 0.608 | 3.01 (1.16-7.86) | 0.024 |
In the present study, colorectal neoplastic lesions were observed in 50.6% of patients in the ESD group undergoing TCS, including advanced adenoma in 10.8% and colorectal cancer in 5.7%. It has been reported that in patients who underwent operations for gastric malignancies including advanced gastric cancer, preoperative TCS detects colorectal cancer in approximately 5% and colorectal adenoma in approximately 40%[16,17]. These rates are essentially consistent with the results of our present study targeting only early gastric cancer and gastric adenoma. Recently, a small number of studies have assessed the risk of colorectal neoplasms in patients undergoing gastric ESD. Kim et al[18], who examined 416 patients (123 with gastric adenoma and 293 with gastric cancer) undergoing gastric ESD, reported that colorectal lesions were observed in 50.2% of their patients including 9.4% with advanced adenoma and 1.4% with colorectal cancer. Lee et al[19], who examined 107 patients (54 with gastric adenoma and 53 with gastric cancer) undergoing gastric ESD, reported that colorectal lesions were observed in 56.1% of their patients, including 26.2% with high-risk colorectal neoplasms (advanced adenoma and adenocarcinoma). Moreover, Joo et al[20], in a study of 186 patients (81 with gastric adenoma and 105 with gastric cancer) undergoing gastric ESD, found that colorectal lesions were observed in 40.9%, including 15.6% with advanced adenoma and 4.3% with colorectal cancer. These results are consistent with ours, suggesting that the prevalence of colorectal neoplastic lesions including colorectal cancer is high even in patients undergoing gastric ESD for gastric lesions other than advanced cancer.
While the risk of colorectal neoplasms was assessed by comparison to healthy volunteers in past studies, we herein assessed the risk by comparison to FIT-positive patients who were matched for age and sex. The fecal occult blood test is a noninvasive method that is widely used for colorectal cancer screening. While this test could be performed employing chemical or immunological methods, the latter method is commonly used in Japan. The ESD and FIT groups, which did not differ in clinicopathological characteristics, showed no significant differences in detection rates of adenoma (33.5% vs 41.8%), advanced adenoma (10.8% vs 15.2%), and cancer (5.7% vs 6.3%), or in the number of adenomas (1-3 adenomas, 37.3% vs 44.9%; ≥ 4 adenomas, 7.0% vs 12.0%). We can reasonably assume that the ESD group has a risk of developing colorectal neoplasms equivalent to that of the FIT group. According to past reports, the detection rates of colorectal neoplasms in FIT-positive patients are approximately 30%, 10% and 1% for adenoma, advanced adenoma, and cancer[21,22], respectively, i.e., slightly lower than the rates in the our FIT group. Because the FIT group was matched to the ESD group for age and sex in the present study, the mean age of the FIT group was approximately 70 years, and approximately 80% were men. On the other hand, in past reports, the mean age of FIT-positive patients was lower, at approximately 60 years, and the proportion of men was also smaller, at approximately 50%. A possible reason for the differences in the detection rates might be that our study included patients with a higher risk of colorectal cancer.
There are several possible explanations for the association between gastric and colorectal neoplasms. In patients with colorectal neoplasms, the rate of infection with Helicobacter pylori (H. pylori) is reportedly high[23]. It has been indicated that H. pylori infection may promote the development of colorectal cancer through increased secretion of gastrin[24]. Moreover, there might be effects of vacuolating cytotoxin A and cytotoxin-associated gene-A protein, which are frequently detected in patients with colorectal cancer[25]. There is a study in which the risk of colorectal neoplasms was assessed according to types of gastric neoplasms, which were classified into adenoma, intestinal-type cancer, and diffuse-type cancer. Although no difference was detected in the morbidity of colorectal lesions between adenoma and intestinal-type cancer, morbidity was found to be significantly lower in patients with diffuse-type cancer[18]. There is strong evidence for the association of H. pylori infection with gastric adenoma and intestinal-type cancer, and this result appears to support the association between colorectal neoplasms and H. pylori infection. Abnormalities in genes, such as P53, APC (adenomatous polyposis coli), DCC (deleted in colorectal carcinoma), and K-ras, are also known to be associated with development of gastrointestinal cancer of both the stomach and the large bowel[26-28]. In addition, microsatellite instability was reported to frequently be observed in synchronous multiple gastrointestinal cancers[29]. Although these genetic abnormalities could reasonably be speculated to affect the association between gastric and colorectal neoplasms, reports of studies on these abnormalities remain limited. This issue merits further investigation.
In the present analysis of risk factors for colorectal neoplasms, sex was the only significant risk factor in the ESD group. Colorectal neoplasms were significantly more common in men than in women. Schoenfeld et al[30], who examined more than 50000 participants in a colorectal cancer screening program using colonoscopy, reported that colorectal neoplasia was detected at a 73% higher frequency in men than in women. Moreover, male gender is also considered an important predictor of colorectal adenomatous polyps[31]. Because there are several reports similar to that of Schoenfeld et al[30], we are confident that our current results are valid[32,33]. Although the analysis of patients with advanced adenoma and carcinoma revealed no clearly significant difference according to sex, the prevalence of these conditions tended to be higher in men. It was assumed that the small number of patients might have accounted for the lack of a significant difference. Although no significant differences were detected when the hypertension, dyslipidemia, and diabetes mellitus parameters were examined separately, advanced adenoma and carcinoma were significantly more common in patients with at least two of these conditions. Jinjuvadia et al[34] conducted a systematic review and meta-analysis, showing the risk of colorectal neoplasia to be increased by 34% in patients with metabolic syndrome. Moreover, Park et al[35], who conducted a study of 492 patients with gastric neoplasm, reported that synchronous colorectal neoplasm was 1.96 times more frequent in patients with metabolic syndrome. These reports appear to provide support for the results of our present study. Our results suggest that abdominal circumference, which is a diagnostic criterion for metabolic syndrome, might not be a risk factor for advanced adenoma and carcinoma. In our view, the effects of abdominal circumference should be clarified by future studies. Meanwhile, our study revealed no significant difference in age, which is known to be a major risk factor for colorectal adenoma and cancer. A preceding report describing a systematic review and meta-analysis showed that the risk of colorectal neoplasms increases in individuals aged 65 years or older who are otherwise at average risk[36]. However, because the present study included patients with a mean age of 69.5 years who underwent ESD, the reason for age not being identified as a risk factor in the present study was assumed to be the inclusion of elderly patients.
The limitations of the present study include, the single-center retrospective study design, which might involve selection bias. To reduce this bias as much as possible, age-matched and sex-matched FIT-positive patients were randomly selected. Moreover, patients with a history of colorectal treatment interventions, such as polypectomy and colectomy, were excluded. Other limitations include the fact that patients with small, untreated, previously detected colorectal adenomas were included in the present study, and that factors that have been reported to affect the development of colorectal neoplasm (e.g., diet, exercise, nonsteroidal anti-inflammatory drugs, and other chemopreventive agents) were not assessed.
The frequency of detecting colorectal lesions, especially advanced adenoma and carcinoma, was higher in patients with early gastric cancer or gastric adenoma. This observation indicates that such patients are at a risk equivalent to that of FIT-positive patients, suggesting that screening colonoscopy should be performed as extensively as feasible in patients undergoing ESD. Most notably, greater caution appears to be warranted in patients with at least two of the following risk factors: hypertension, dyslipidemia, and diabetes mellitus. The frequencies of advanced adenoma and cancer are significantly increased in these patients. In the future, multicenter prospective studies need to be conducted to assess the results of our present investigation.
Gastric cancer mortality rate is very low after endoscopic resection of gastric neoplasms. To further improve outcomes, screening for neoplasms in other organs is assumed to be important. Colorectal neoplasms are the most commonly observed tumors outside of the stomach in patients with gastric cancer. Screening colonoscopy before surgical interventions for the stomach is now well established.
There are few studies that assessed the risk of colorectal neoplasms in patients undergoing gastric endoscopic submucosal dissection (ESD). Therefore, the usefulness of screening colonoscopy in patients undergoing gastric ESD for gastric adenoma or early gastric cancer has yet to be clarified.
The frequency of detecting colorectal lesions, especially advanced adenoma and carcinoma, was higher in patients with early gastric cancer or gastric adenoma. This observation indicates that such patients are at a risk equivalent to that of fecal immunochemical test positive patients, suggesting that screening colonoscopy should be performed as extensively as possible in patients undergoing ESD.
This study will contribute to the reduction of colorectal cancer deaths in patients undergoing gastric ESD.
ESD has been established as a common curative resection procedure for gastric neoplasms, including early gastric cancer and gastric adenoma. As the adoption of this procedure becomes ever more widespread, indications for endoscopic therapy are being expanded.
This is well designed and performed study with important clinical and epidemiological information.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Facciorusso A, Seow-Choen F, Vujasinovic M S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
| 1. | Oda I, Gotoda T, Hamanaka H, Eguchi T, Saito Y, Matsuda T, Bhandari P, Emura F, Saito D, Ono H. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Dig Endosc. 2005;17:54-58. [RCA] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 333] [Article Influence: 16.7] [Reference Citation Analysis (2)] |
| 2. | Isomoto H, Shikuwa S, Yamaguchi N, Fukuda E, Ikeda K, Nishiyama H, Ohnita K, Mizuta Y, Shiozawa J, Kohno S. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 520] [Article Influence: 32.5] [Reference Citation Analysis (1)] |
| 3. | Facciorusso A, Antonino M, Di Maso M, Muscatiello N. Endoscopic submucosal dissection vs endoscopic mucosal resection for early gastric cancer: A meta-analysis. World J Gastrointest Endosc. 2014;6:555-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 110] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (2)] |
| 4. | Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219-225. [PubMed] |
| 5. | Sugimoto T, Okamoto M, Mitsuno Y, Kondo S, Ogura K, Ohmae T, Mizuno H, Yoshida S, Isomura Y, Yamaji Y. Endoscopic submucosal dissection is an effective and safe therapy for early gastric neoplasms: a multicenter feasible study. J Clin Gastroenterol. 2012;46:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Emura F, Mejía J, Donneys A, Ricaurte O, Sabbagh L, Giraldo-Cadavid L, Oda I, Saito Y, Osorio C. Therapeutic outcomes of endoscopic submucosal dissection of differentiated early gastric cancer in a Western endoscopy setting (with video). Gastrointest Endosc. 2015;82:804-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Tanabe S, Ishido K, Higuchi K, Sasaki T, Katada C, Azuma M, Naruke A, Kim M, Koizumi W. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a retrospective comparison with conventional endoscopic resection in a single center. Gastric Cancer. 2014;17:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 8. | Oda I, Oyama T, Abe S, Ohnita K, Kosaka T, Hirasawa K, Ishido K, Nakagawa M, Takahashi S. Preliminary results of multicenter questionnaire study on long-term outcomes of curative endoscopic submucosal dissection for early gastric cancer. Dig Endosc. 2014;26:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Sung JJ, Lau JY, Goh KL, Leung WK; Asia Pacific Working Group on Colorectal Cancer. Increasing incidence of colorectal cancer in Asia: implications for screening. Lancet Oncol. 2005;6:871-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 598] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 10. | Tamura K, Ishiguro S, Munakata A, Yoshida Y, Nakaji S, Sugawara K. Annual changes in colorectal carcinoma incidence in Japan. Analysis of survey data on incidence in Aomori Prefecture. Cancer. 1996;78:1187-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Eom BW, Lee HJ, Yoo MW, Cho JJ, Kim WH, Yang HK, Lee KU. Synchronous and metachronous cancers in patients with gastric cancer. J Surg Oncol. 2008;98:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Lee JH, Bae JS, Ryu KW, Lee JS, Park SR, Kim CG, Kook MC, Choi IJ, Kim YW, Park JG. Gastric cancer patients at high-risk of having synchronous cancer. World J Gastroenterol. 2006;12:2588-2592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Japanese Research Society for Cancer of the Colon and Rectum. General Rules for Clinical and Pathological Studies on Cancer of the Colon, Rectum and Anus: Histopathological Classification, 6th ed. Tokyo: Kanehara Syuppan, 1998: 60-90. . |
| 14. | Kudo S, Rubio CA, Teixeira CR, Kashida H, Kogure E. Pit pattern in colorectal neoplasia: endoscopic magnifying view. Endoscopy. 2001;33:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 309] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 15. | Hewett DG, Kaltenbach T, Sano Y, Tanaka S, Saunders BP, Ponchon T, Soetikno R, Rex DK. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology. 2012;143:599-607.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 417] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 16. | Saito S, Hosoya Y, Togashi K, Kurashina K, Haruta H, Hyodo M, Koinuma K, Horie H, Yasuda Y, Nagai H. Prevalence of synchronous colorectal neoplasms detected by colonoscopy in patients with gastric cancer. Surg Today. 2008;38:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Suzuki A, Koide N, Takeuchi D, Okumura M, Ishizone S, Suga T, Miyagawa S. Prevalence of synchronous colorectal neoplasms in surgically treated gastric cancer patients and significance of screening colonoscopy. Dig Endosc. 2014;26:396-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Kim SY, Jung SW, Hyun JJ, Koo JS, Choung RS, Yim HJ, Lee SW, Choi JH. Is colonoscopic screening necessary for patients with gastric adenoma or cancer? Dig Dis Sci. 2013;58:3263-3269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Lee KJ, Kim JH, Kim SI, Jang JH, Lee HH, Hong SN, Lee SY, Sung IK, Park HS, Shim CS. Clinical significance of colonoscopic examination in patients with early stage of gastric neoplasm undergoing endoscopic submucosal dissection. Scand J Gastroenterol. 2011;46:1349-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Joo MK, Park JJ, Lee WW, Lee BJ, Hwang JK, Kim SH, Jung W, Kim JH, Yeon JE, Kim JS. Differences in the prevalence of colorectal polyps in patients undergoing endoscopic removal of gastric adenoma or early gastric cancer and in healthy individuals. Endoscopy. 2010;42:114-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Hundt S, Haug U, Brenner H. Comparative evaluation of immunochemical fecal occult blood tests for colorectal adenoma detection. Ann Intern Med. 2009;150:162-169. [PubMed] |
| 22. | de Wijkerslooth TR, Stoop EM, Bossuyt PM, Meijer GA, van Ballegooijen M, van Roon AH, Stegeman I, Kraaijenhagen RA, Fockens P, van Leerdam ME. Immunochemical fecal occult blood testing is equally sensitive for proximal and distal advanced neoplasia. Am J Gastroenterol. 2012;107:1570-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 23. | Meucci G, Tatarella M, Vecchi M, Ranzi ML, Biguzzi E, Beccari G, Clerici E, de Franchis R. High prevalence of Helicobacter pylori infection in patients with colonic adenomas and carcinomas. J Clin Gastroenterol. 1997;25:605-607. [PubMed] |
| 24. | Nakatsuru S, Yanagisawa A, Ichii S, Tahara E, Kato Y, Nakamura Y, Horii A. Somatic mutation of the APC gene in gastric cancer: frequent mutations in very well differentiated adenocarcinoma and signet-ring cell carcinoma. Hum Mol Genet. 1992;1:559-563. [PubMed] |
| 25. | Shmuely H, Passaro D, Figer A, Niv Y, Pitlik S, Samra Z, Koren R, Yahav J. Relationship between Helicobacter pylori CagA status and colorectal cancer. Am J Gastroenterol. 2001;96:3406-3410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 108] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Uchino S, Noguchi M, Ochiai A, Saito T, Kobayashi M, Hirohashi S. p53 mutation in gastric cancer: a genetic model for carcinogenesis is common to gastric and colorectal cancer. Int J Cancer. 1993;54:759-764. [PubMed] |
| 27. | Uchino S, Tsuda H, Noguchi M, Yokota J, Terada M, Saito T, Kobayashi M, Sugimura T, Hirohashi S. Frequent loss of heterozygosity at the DCC locus in gastric cancer. Cancer Res. 1992;52:3099-3102. [PubMed] |
| 28. | Robertson DJ, Sandler RS, Ahnen DJ, Greenberg ER, Mott LA, Cole BF, Baron JA. Gastrin, Helicobacter pylori, and colorectal adenomas. Clin Gastroenterol Hepatol. 2009;7:163-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Ohtani H, Yashiro M, Onoda N, Nishioka N, Kato Y, Yamamoto S, Fukushima S, Hirakawa-Ys Chung K. Synchronous multiple primary gastrointestinal cancer exhibits frequent microsatellite instability. Int J Cancer. 2000;86:678-683. [PubMed] |
| 30. | Schoenfeld P, Cash B, Flood A, Dobhan R, Eastone J, Coyle W, Kikendall JW, Kim HM, Weiss DG, Emory T. Colonoscopic screening of average-risk women for colorectal neoplasia. N Engl J Med. 2005;352:2061-2068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 343] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 31. | Rex DK. Colonoscopy: a review of its yield for cancers and adenomas by indication. Am J Gastroenterol. 1995;90:353-365. [PubMed] |
| 32. | Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Risk of advanced proximal neoplasms in asymptomatic adults according to the distal colorectal findings. N Engl J Med. 2000;343:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 668] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 33. | Regula J, Rupinski M, Kraszewska E, Polkowski M, Pachlewski J, Orlowska J, Nowacki MP, Butruk E. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355:1863-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 542] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 34. | Jinjuvadia R, Lohia P, Jinjuvadia C, Montoya S, Liangpunsakul S. The association between metabolic syndrome and colorectal neoplasm: systemic review and meta-analysis. J Clin Gastroenterol. 2013;47:33-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 35. | Park W, Lee H, Kim EH, Yoon JY, Park JC, Shin SK, Lee SK, Lee YC, Kim WH, Noh SH. Metabolic syndrome is an independent risk factor for synchronous colorectal neoplasm in patients with gastric neoplasm. J Gastroenterol Hepatol. 2012;27:1490-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Heitman SJ, Ronksley PE, Hilsden RJ, Manns BJ, Rostom A, Hemmelgarn BR. Prevalence of adenomas and colorectal cancer in average risk individuals: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2009;7:1272-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 175] [Article Influence: 10.9] [Reference Citation Analysis (0)] |