Published online Jun 21, 2017. doi: 10.3748/wjg.v23.i23.4181
Peer-review started: November 21, 2016
First decision: January 19, 2017
Revised: February 4, 2017
Accepted: April 13, 2017
Article in press: April 13, 2017
Published online: June 21, 2017
Processing time: 214 Days and 11.7 Hours
To evaluate a calcium activated potassium channel (KCa3.1) inhibitor attenuates liver disease in models of non-alcoholic fatty liver disease (NAFLD).
We have performed a series of in vitro and in vivo studies using the KCa3.1 channel inhibitor, Senicapoc. Efficacy studies of Senicapoc were conducted in toxin-, thioacetamide (TAA) and high fat diet (HFD)-induced models of liver fibrosis in rats. Efficacy and pharmacodynamic effects of Senicapoc was determined through biomarkers of apoptosis, inflammation, steatosis and fibrosis.
Upregulation of KCa3.1 expression was recorded in TAA-induced and high fat diet-induced liver disease. Treatment with Senicapoc decreased palmitic acid-driven HepG2 cell death. (P < 0.05 vs control) supporting the finding that Senicapoc reduces lipid-driven apoptosis in HepG2 cell cultures. In animals fed a HFD for 6 wk, co-treatment with Senicapoc, (1) reduced non-alcoholic fatty liver disease (NAFLD) activity score (NAS) (0-8 scale), (2) decreased steatosis and (3) decreased hepatic lipid content (Oil Red O, P < 0.05 vs vehicle). Randomization of TAA animals and HFD fed animals to Senicapoc was associated with a decrease in liver fibrosis as evidenced by hydroxyproline and Masson’s trichrome staining (P < 0.05 vs vehicle). These results demonstrated that Senicapoc mitigates both steatosis and fibrosis in liver fibrosis models.
These data suggest that Senicapoc interrupts more than one node in progressive fatty liver disease by its anti-steatotic and anti-fibrotic activities, serving as a double-edged therapeutic sword.
Core tip: Given the large numbers of people with fatty livers and even relatively indolent steatosis can result in a significant population progressing to non-alcoholic steatohepatitis (NASH), NASH with fibrosis and cirrhosis. We report for the first time that a KCa3.1 channel inhibitor exerts an anti-steatotic effect in the setting of fatty liver disease which can be harnessed for the treatment of liver fibrosis. Second, we are the first to report that Senicapoc, a drug that has been through Phase III clinical trials, can be repurposed for the treatment of fatty liver disease and potentially for the treatment of other lipid disorders.
- Citation: Paka L, Smith DE, Jung D, McCormack S, Zhou P, Duan B, Li JS, Shi J, Hao YJ, Jiang K, Yamin M, Goldberg ID, Narayan P. Anti-steatotic and anti-fibrotic effects of the KCa3.1 channel inhibitor, Senicapoc, in non-alcoholic liver disease. World J Gastroenterol 2017; 23(23): 4181-4190
- URL: https://www.wjgnet.com/1007-9327/full/v23/i23/4181.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i23.4181
Obesity, diabetes and metabolic syndrome drive non-alcoholic fatty liver disease (NAFLD), the accumulation of fat in hepatocytes not caused by excessive consumption of alcohol. Twenty-five percent of the adult United States population is thought to suffer from NAFLD, which can progress to nonalcoholic steatohepatitis (NASH) or accumulation of fat within the liver accompanied by inflammation[1]. Left untreated, NASH, which affects several million persons in the United States alone, can progress to liver fibrosis, the precursor to cirrhosis and decompensated organ failure[2]. The development of new therapies that prevent the transition from steatosis and inflammation to fibrosis would have substantial clinical value.
The intermediate-conductance Ca2+-activated K+ channel KCa3.1/KCNN4 is expressed in non-excitable tissues affecting proliferation, migration and vascular resistance and plays an important role in the modulation of Ca2+ signaling and cell function[3]. Modulators of this channel have been reported to confer therapeutic benefit in models of inflammatory bowel disease and chronic kidney disease[4-6]. In activated hepatic stellate cells (HSC) and fibrotic livers, KCa3.1 channel expression is upregulated and in liver disease the KCa3.1 channel inhibitor TRAM-34 downregulated fibrosis-associated gene expression and reduced portal perfusion pressure[7]. These results suggest that the KCa3.1 channel might represent a novel target for the treatment of hepatic fibrosis. Interestingly, long-term KCa3.1 blockade therapy with clotrimazole or TRAM-34 reduced atherosclerosis in the murine aorta and carotid arteries[8]. These data suggest that inhibition of KCa3.1 might impact events upstream of and leading to extracellular matrix accumulation.
Senicapoc (C20H15F2NO; 323.34 Da) is an orally bioavailable, potent and selective blocker of the KCa3.1 channel[9]. A clinical stage compound, Senicapoc was deemed safe and generally well-tolerated in Phase I, II and III clinical trials[9-13]. We herein test the hypothesis that in NASH with fibrosis, the KCa3.1 inhibitor Senicapoc mitigates interstitial collagen accumulation via a reduction in steatosis.
HepG2 cells (ATCC) were maintained in DMEM/10% FCS supplemented with L-glutamine. For the experiment, cells were seeded onto a 96 well plate using 10K cells/well. Cells were allowed to attach overnight. On the day of the study, cells were incubated with DMEM/0.2%FCS/L-glutamine. Senicapoc (OX-CHEM Corp., CA, United States) was added to final concentrations of 0, 0.1, 1.0 or 10 μmol/L and incubated for 30 min following which palmitic acid (hexadecanoic acid; C16H32O2; Sigma) coupled to fatty acid-free BSA (Sigma) was added to a final concentration of 0, 75 or 150 μmol/L[14,15]. Cells were incubated overnight and apoptosis measured using the Caspase Glo 3/7 reagent (Promega).
All studies relating to animals were approved by our institutional animal use and care committee. Animals had access to drinking water ad libitum throughout the experimental protocols.
Biliary obstruction: Adult male Wistar rats (175-200 g; n = 3) were submitted to biliary occlusion using a previously reported method[16]. Briefly, after general anaesthesia (25/5 m/kg ketamine/xylazine, ip), laparotomy was performed by using midline abdominal skin and muscle incisions. The common bile duct was exposed and ligated with 6-0 silk sutures, the abdominal wall sutured closed and the animal returned to its cage. Twenty-eight days after surgery, animals were anesthetized and the livers removed. Sham-operated rats (n = 3) were used as control.
Thioacetamide administration: Adult male Wistar-Furth rats (225-250 g) were administered thioacetamide (TAA) (150 mg/kg; n = 3) twice a week for 8 wk[17] after which some animals were sacrificed and the livers removed. Sham (saline-administered; n = 3) rats were used as control.
Intervention with senicapoc in TAA model: Adult male Wistar-Furth rats (225-250 g) were administered TAA (150 mg/kg, IP) or saline (0.5 mL, IP) twice a week for 8 wk after which some animals (n = 6/group) were sacrificed and the livers retrieved to confirm disease. TAA-administered animals were then randomized to vehicle (Cremaphor 10% and PEG400 10% in water; n = 12) or Senicapoc (50 mg/kg, PO, BID; n = 12) for 8 wk. Animals were sacrificed 16 wk into TAA administration following which livers were retrieved for analysis.
Diet-induced liver disease: Adult male C57BL/6 mice (18-22 g) were randomized to standard laboratory rodent diet (5001 LabDiet, MO; sham group) or a modified HFD - an L-amino acid diet with 60 kcal% fat with 0.1% methionine and no added choline (CDAHFD; Research Diets, NJ, United States)[18] - for 8 wk after which animals (n = 3/group) were sacrificed and livers retrieved for analysis.
Intervention with Senicapoc in CDAHFD model: Adult male C57BL/6 mice (18-22 g) were randomized to standard laboratory rodent diet (sham group) or CDAHFD. After 4 wk on these diets, animals (n = 6/group) were sacrificed and the livers retrieved for confirmation of disease. Animals on the special diet were randomized immediately to vehicle (n = 12) or Senicapoc (10 mg/kg, PO, BID; n = 12) for 4 wk following which they were sacrificed and their livers harvested.
Pretreatment with Senicapoc in CDAHFD model: Adult male C57BL/6 mice (18-22 g) were randomized to standard laboratory rodent diet (sham group; n = 8) or CDAHFD (n = 16) for 7 d after which animals were sacrificed and livers retrieved for analysis. Treatment with vehicle (n = 8) or Seniapoc (10 mg/kg, PO, BID; n = 8) was started on day 1 of CDAHFD and continued until sacrifice.
Pretreatment with Senicapoc in HFD model: Adult male C57BL/6 mice were randomized to standard laboratory rodent diet or a high fat diet fat (HFD) containing 10% lard, 5% corn oil and 2% cholesterol (Research Diets, NJ, United States)[19]. Animals on the special diet were randomized immediately to vehicle or Senicapoc (10 mg/kg, PO, BID). After 6 wk days on this diet, animals were sacrificed and livers retrieved for analysis.
As described by Freise et al[7] KCa3.1 protein expression (normalized to GAPDH) was determined in liver homogenates using anti-KCa3.1 (12 hr incubation, K4, IKCa1, #APC-06, Alomone Labs) antibody followed by incubation with anti-rabbit secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, United States). The signal was detected by enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ, United States) and densitometry was performed using Bioquant. Immunohistochemical staining of liver sections were performed with the same antibody as described previously.
Liver hydroxyproline: Liver tissues (approximately 200 mg) were prepared and analyzed for hydroxyproline as described by Zhang et al[20]. The total hydroxyproline within each liver tissue was determined by total wet liver mass.
Fibrosis was detected histologically by Masson’s trichrome and picrosirius red staining of collagen by using digital slide scanning and associated image analysis software as described previously[21].
An observed blinded to the treatment groups assigned NAFLD activity score (NAS) (0-8 scale), steatosis (0-3 scale) and inflammation (0-3 scale) values to H&E-stained liver sections as described previously[22].
Liver triglycerides: Triglyceride content in liver homogenates was measured using a commercial enzyme-linked immunosorbent assay kit (ELI Tech, Seoul, South Korea).
Liver histopathology: For hematoxylin and eosin (H&E) staining, formalin-fixed liver was embedded into paraffin and cut into 4 μm sections. For Oil Red-O staining, liver tissue was dehydrated before embedded into OCT compound (Sakura, Tokyo, Japan), and cut into 4 μm frozen sections. Commercially available kits (Beyotime, Shanghai, China) were used to stain sections. Analysis was performed by an observer blinded to the treatment groups. Lipid vacuoles in H&E sections and lipid content in Oil Red O sections were semi-quantified using Bioquant Image analysis software program (Nashville, TN, United States).
Hepatic inflammation (F4/80) and lipid peroxidation (4-hydroxynonenal): Five-μm-thick paraffin sections of liver tissue were prepared from the center of each hepatic lobe. Sections were subsequently stained with antibodies against F4/80 (anti-F4/80; sc-59171, Santa Cruz, CA, United States) and 4-hydroxynonenal (4-HNE) (anti-4HNE antibody; JaICA, Shizuoka, Japan). Staining was quantified (Bioquant) by an observer blinded to the treatment groups.
Data are expressed as mean ± SEM. Differences between groups were measured by one-way ANOVA with Tukey’s posthoc test for multiple comparisons. P values less than 0.05 were considered significant.
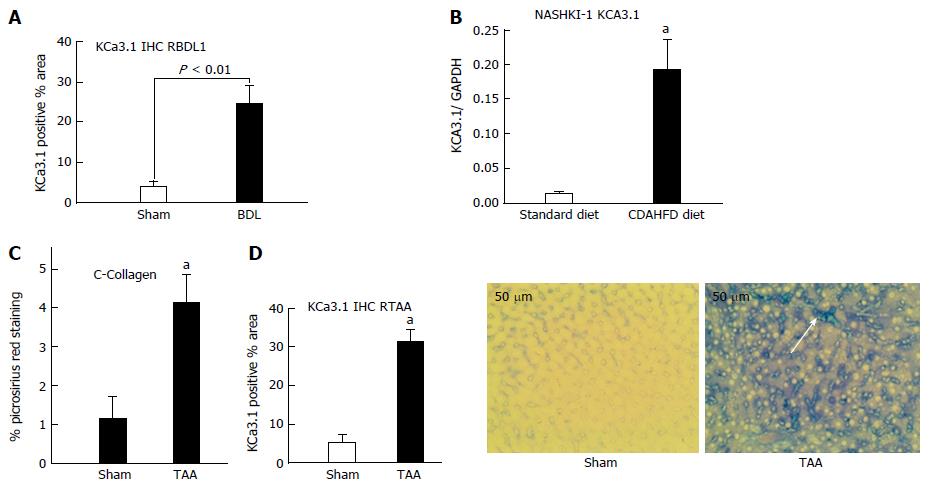
Upregulation of KCa3.1 has been reported in fibrotic liver[18]. We evaluated hepatic KCa3.1 expression in 3 distinct rodent models of liver fibrosis. As seen in Figure 1, hepatic KCa3.1 expression was elevated in livers from animals submitted to biliary occlusion for 4 wk (Figure 1A), in livers from animals fed CDAHFD for 8 wk (Figure 1B) and in fibrotic livers from animals administered TAA for 8 wk (Figure 1C and D).
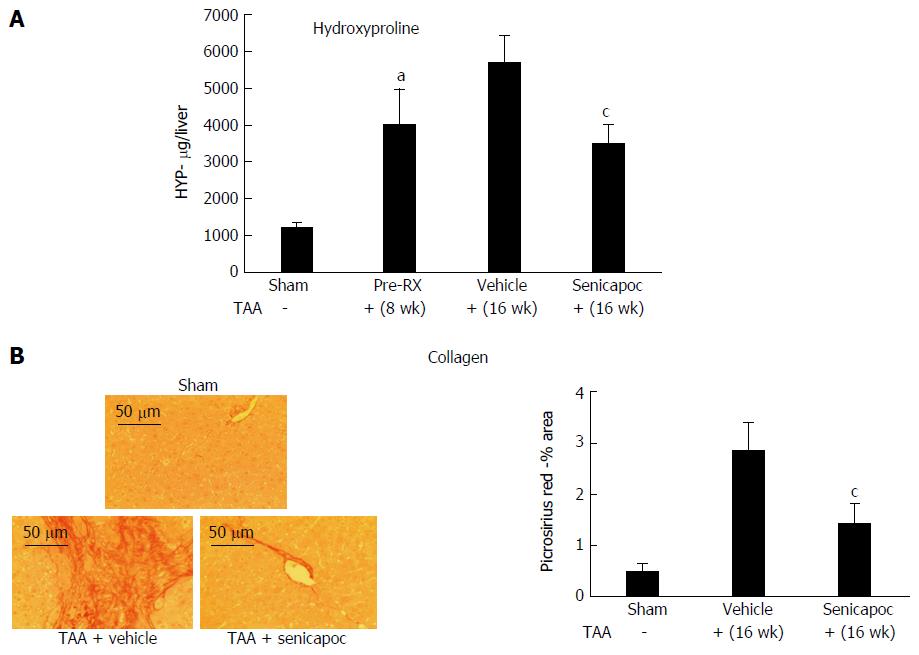
Consistent with published literature[3,7] and having confirmed a potential role for KCa3.1 channels in liver disease we evaluated whether intervention with Senicapoc decreases hepatic collagen accumulation. Drug effects were evaluated in 2 etiologically distinct models of liver fibrosis. Rats administered TAA for 8 wk exhibited significant increase in liver collagen (hydroxyproline, Figure 2A) at which time they were randomized to Senicapoc or vehicle. As seen in Figure 2A and B, treatment with Senicapoc with continued administration of TAA, blocked the increase in liver collagen from weeks 8 through 16.
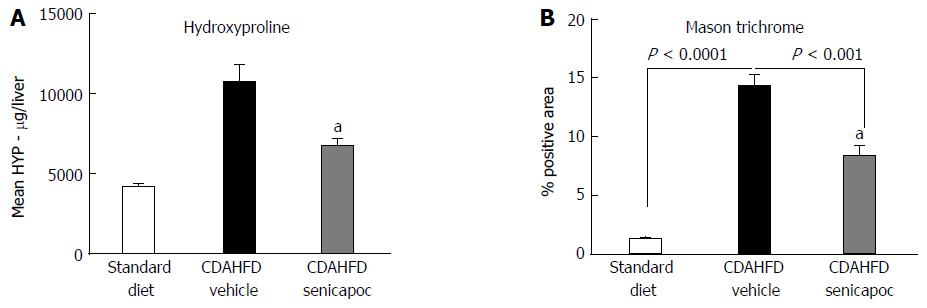
Animals fed CDAHFD for 4 wk exhibited hepatomegaly evidenced by increased liver to body mass ratio (7.5% ± 0.2% vs 4.8% ± 0.1%, standard diet; P < 0.05). Furthermore, animals on this diet exhibited a NAS (0 to 8 scale) of 5.1 ± 0.9 vs 1 ± 0.2, standard diet; P < 0.05. Randomization of animals on CDAHFD to Senicapoc for 4 wk was associated with a decrease in liver fibrosis as evidenced by hydroxyproline (Figure 3A) and Masson’s trichrome staining (Figure 3B).
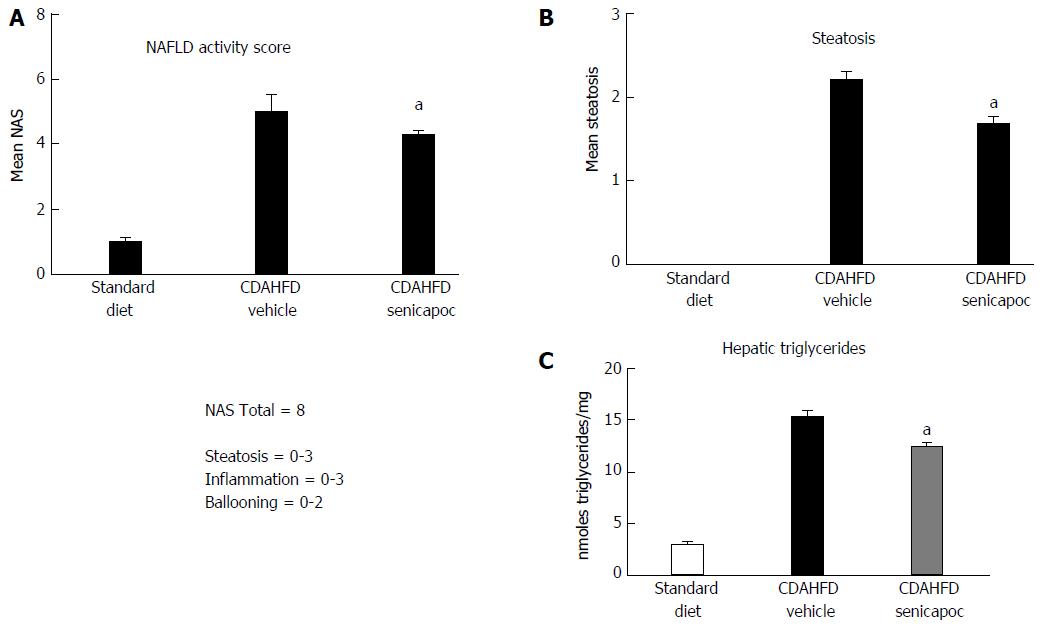
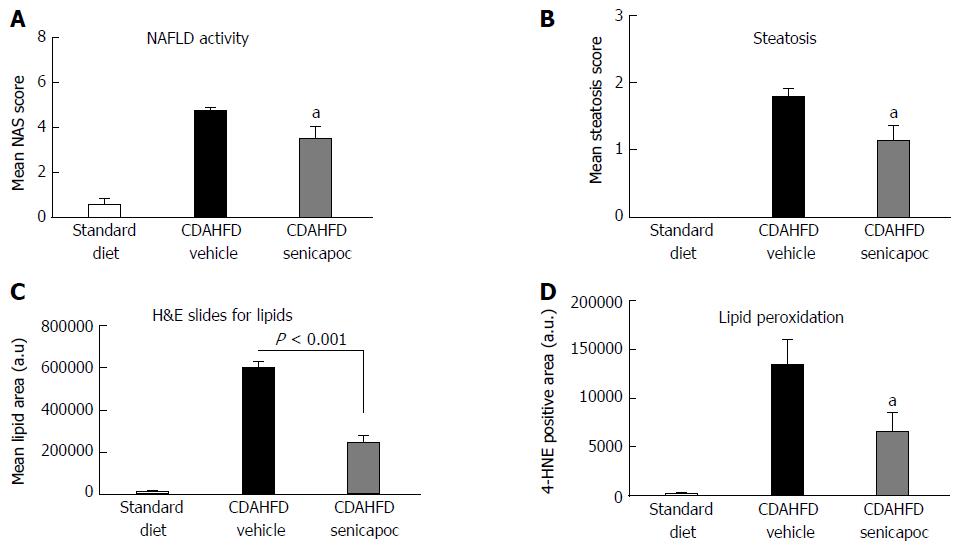
We sought to evaluate whether the anti-fibrotic effects of Senicapoc in diet-induced liver disease, is mediated, at least, in part, via a reduction in liver lipid levels. To this end, we first compared NAS and steatosis (0 to 3 scale) in CDAHFD (8 wk) animals randomized to vehicle or Senicapoc (weeks 4 through 8). Intervention with Senicapoc, reduced both NAS (Figure 4A) and steatosis (Figure 4B). Next, we evaluated the effect of Senicapoc on liver triglycerides, the main form of fat stored in the liver. Intervention with Senicapoc reduced hepatic triglyceride content (Figure 4C) in the CDAHFD model.
Lipid accumulation occurring in NAFLD can trigger a cascade including hepatocyte apoptosis, hepatic inflammation and extracellular matrix deposition. The effect of Senicapoc on steatosis was further investigated in models of diet-induced liver disease. In animals fed CDAHFD for 7 d and concomitantly treated with Senicapoc, a reduction in NAS was observed in comparison to the CDAHFD+vehicle cohort (Figure 5A). Again, Senicapoc reduced steatosis (Figure 5B). To further verify this effect of Senicapoc on hepatic lipid homeostasis, we analyzed the lipid vacuole area in hepatic sections stained with H&E from vehicle and drug-treated animals. As seen in Figure 5C, treatment with Senicapoc was associated with decreased hepatic lipid vacuole area. Furthermore, Senicapoc reduced hepatic lipid peroxidation, a measure of macrophage oxidative burst and early inflammation as evidenced by 4-HNE staining (Figure 5D).
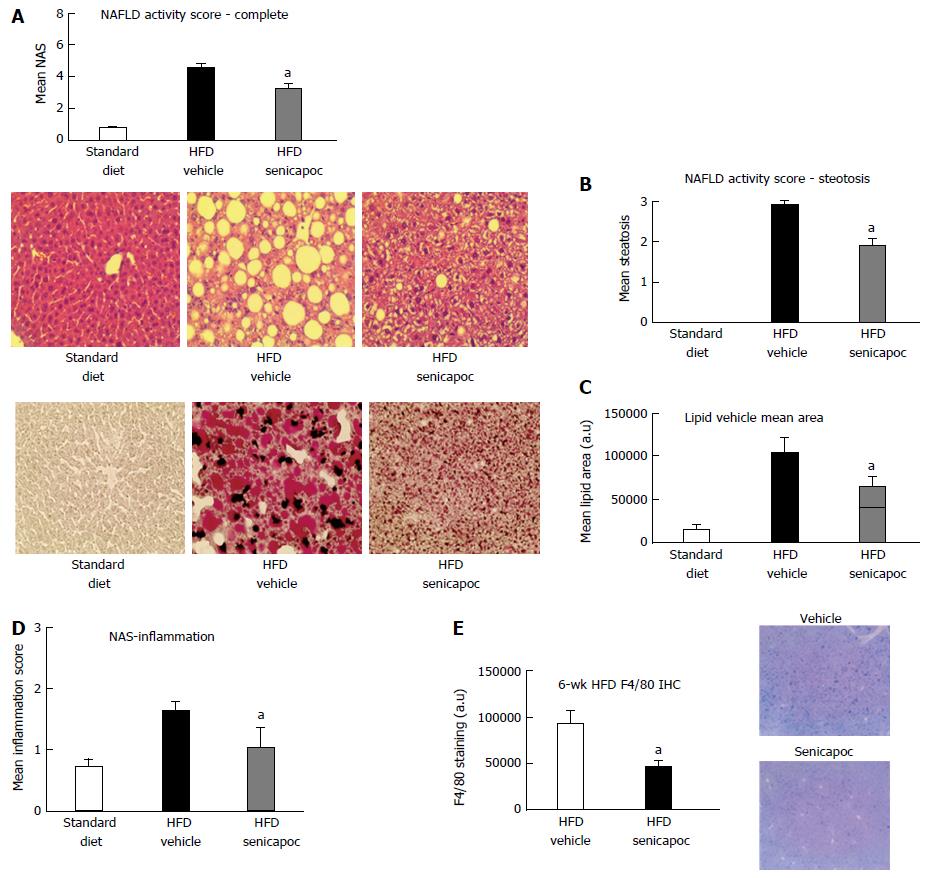
To confirm that the anti-steatotic effect of Senicapoc was not unique to the CDAHFD model, we evaluated use of this drug in the HFD model of NAFLD-NASH[18,19]. As seen in Figure 6A, treatment with Senicapoc reduced NAS observed in this model of metabolic insult. Senicapoc reduced steatosis (Figure 6B) which was also evident in the extent of Oil Red O staining of the liver (Figure 6C). Since 6 wk on this diet is sufficient to trigger an inflammatory response in the liver, we investigated whether the anti-steatotic effect of Senicapoc translates to a reduction in hepatic inflammation. Senicapoc reduced inflammation (Figure 6D, 0-3 scale) which was also evident by decreased hepatic F4/80 staining (Figure 6E), a marker of active macrophage population.
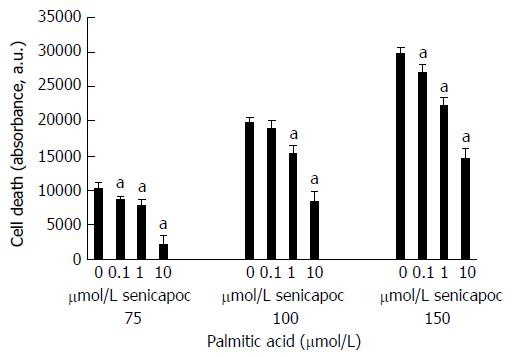
While lipid accumulation in hepatocytes is a critical event in the genesis and progression of fatty liver disease, hepatocytes overloaded with saturated free fatty acids such as palmitic acid undergo apoptosis[14,15]. Cell death is therefore a surrogate marker of hepatocyte lipid accumulation. We determined whether the anti-steatotic effect of Senicapoc translates to reduced apoptosis in eHpG2 cells challenged with increasing concentrations of palmitic acid. Senicapoc was without effect on cells alone (without palmitic acid; data not shown). As seen in Figure 7, at each concentration of palmitic acid tested, Senicapoc dose-dependently decreased HepG2 apoptosis.
Left untreated, NAFLD can progress to NASH, fibrosis, cirrhosis and decompensated liver failure. The continuum between steatosis, hepatitis and fibrosis lends itself to therapeutic intervention at any one of these nodes. This study is the first to demonstrate that Senicapoc, a KCa3.1 channel inhibitor, exerts an anti-fibrotic effect in the liver and that in diet-induced liver disease this anti-fibrotic effect is mediated via a reduction in steatosis.
Given the obesity, diabetes and metabolic syndrome epidemics, in the United States alone approximately 81 million people are suspected to have some underlying steatosis; approximately 16 million people are suspected to have NASH of which approximately 5 million are suspected to have NASH with fibrosis[1]. Left untreated, the disease can progress to cirrhosis and decompensated liver failure. Non-clinical and clinical reports[2,3] indicate that in this form of liver disease there is continuum from steatosis to inflammation to fibrosis. In an elegant proof-of-concept study[22], mice fed a fatty diet for 49 wk, presented with steatosis but not inflammation at 8 wk, progressed to steatohepatitis and early fibrosis at 27 wk and then to steatohepatitis, fibrosis and hepatocellular carcinoma by week 49. The investigators hypothesized that the gradual progression to fibrosis associated with prolonged metabolic insult results from “multiple hits” including steatosis and activation of pathways involving inflammatory cytokines and inflammatory cells. In a clinical sample set, Leandro et al[23] reported that the association between steatosis and fibrosis invariably was dependent on a simultaneous association between steatosis and hepatic inflammation. Together, these findings suggest that the fibrotic program secondary to metabolic insult can be interrupted at a number of nodes including steatosis.
There is increasing recognition of the role ion channels play in both health and disease (channelopathies) and in the modulation of these channels to therapeutic benefit[2,3,24]. Freise et al[7] was the first to report that the KCa3.1 channel is upregulated in activated HSCs and the fibrotic liver and that the KCa3.1 inhibitor TRAM-34 blocks proliferation and TGF-β1-induced activation of HSCs and reduces portal hypertension in cholestatic liver disease. This group of investigators argued that KCa3.1 inhibition may provide a novel therapeutic rationale for treatment of progressive hepatic fibrosis and recommended further study of the therapeutic potential of this target in other models of hepatic fibrosis. Consistent with these findings, we found that KCa3.1 channel expression is indeed upregulated in liver disease. In fact, this phenomenon is independent of disease etiology as channel upregulation was observed in 3 distinct models of liver disease viz. diet-, cholestasis- and toxin-induced. To the best of our knowledge, this study is the first to report hepatic KCa3.1 channel expression in metabolic insult-induced liver disease.
Unlike clotrimazole or TRAM-34 that are accompanied by severe cytochrome P450 liability[1], Senicapoc is a clinical trial ready, potent (IC50 6-20 nmol/L) and selective (IC50 > 1 μmol/L for Kv1.5, hERG, Na (tetrodotoxin-sensitive), IKs, KvLQT and h-H1) blocker of the KCa3.1 channel[24]. Senicapoc was safe and generally well tolerated in multiple clinical trials involving healthy volunteers or patients with sickle cell anemia or asthma over a dosing period of up to 1 year[10-13]. It has a half-life of approximately 13 d in human. Clinical development of this compound for sickle cell anemia was discontinued for lack of efficacy and not safety[9].
Based on the afore-referenced findings of KCa3.1 channel upregulation in liver disease and successful intervention with a KCa3.1 inhibitor, the objective of the present study was to evaluate the effects of Senicapoc on the progression of fatty liver disease. Specifically, we sought to determine whether Senicapoc mitigates liver disease, at least, in part, via reducing steatosis. Our findings from both the cell culture studies and the in vivo studies support this notion. Palmitic acid is a major form of fatty acid stored in the body. Multiple studies have shown a direct relation between uptake of palmitic acid and hepatocyte and HeG2 cell death[14,25]. While we did not measure lipid accumulation in HepG2 cells, their protection from palmitic acid-induced death by.
Senicapoc is a surrogate for the anti-steatotic activity of this drug. Our results from diet-induced, but mechanistically distinct, models of fatty liver disease suggest that Senicapoc decreases lipid accumulation within the liver. The aggressive CDAHFD model has been demonstrated to mimic human NASH in rodents by sequentially producing steatohepatitis and liver fibrosis[22]. Steatosis in this model is more than likely a defect in the export of triglycerides from the liver to peripheral tissue[22]. The HFD model by contrast is associated primarily with a more gradual accumulation of lipid within the hepatocytes followed by inflammation. Liver fibrosis in this model takes months to appear and is manifest with picrosirius red staining as a fine filigree network rather than a bridging phenotype[19]. Senicapoc’s effect on lipid accumulation in both models appears to be very consistent as evinced by multiple different readouts including cell apoptosis, NAS, lipid vacuole measurement (H&E), lipid content measurement (Oil Red O) and liver triglycerides across the diet-induced models studied. In fact, these anti-steatotic effects are consistent with similar effects of TRAM-34 on the development of lipid-rich lesions within the vascular bed of atherosclerosis-prone mice[8]. Furthermore, associated with this reduction in hepatic steatosis by Senicapoc was a reduction in markers of hepatic inflammation - the inflammatory component of NAS, 4-HNE and F4/80. While the present study did not seek to determine whether this anti-inflammatory effect derived from the upstream anti-steatotic effect or was a direct effect of KCa3.1 inhibition on Kupffer cells as reported previously, our results with Senicapoc are in line with that of TRAM-34 in a model of murine airway inflammation and remodeling[26]. In that study, TRAM-34 inhibited the generation and maintenance of inflammation and subepithelial extracellular matrix deposition.
The other major finding in this study was that Senicapoc mitigates hepatic collagen accumulation evidenced in two etiologically distinct models of liver fibrosis viz. diet- and toxin-induced. Importantly, these effects of Senicapoc were interventional since animals on the CDAHFD for 4 wk exhibited significant liver pathology prior to initiation of therapy and livers from TAA-treated animals exhibited fibrosis prior to randomization. This anti-fibrotic effect of Senicapoc appears to derive from both its anti-steatotic effect, evidenced in the CDAHFD model as well as a direct anti-fibrotic effect, evidenced in the TAA study. While association between steatosis, inflammation and fibrosis has been documented and observed in the present study, the direct anti-fibrotic effects of Senicapoc might relate to an effect of KCa3.1 channel inhibitors on hepatic stellate cells (HSCs). Freise et al[25] reported that KCa3.1 inhibition with TRAM-34 reduced HSC proliferation by induction of cell cycle arrest and reduced TGF-β1- induced gene expression of collagen I, α-SMA and TGF-β1 itself. Furthermore, TRAM-34 blocked TGF-β1-induced activation of TGF-β1 signaling in HSCs.
In conclusion, together, our study and data suggests that Senicapoc interrupts more than one node in progressive fatty liver disease, perhaps serving as a double-edged therapeutic sword.
Nonalcoholic steatohepatitis (NASH), a potentially serious form of the disease due to liver inflammation, which may progress to scarring and irreversible damage. This damage is similar to the damage caused by heavy alcohol use. In severe cases NASH can progress to cirrhosis and liver failure risk factors being obesity, diabetes and aging. Nonalcoholic fatty liver disease (NAFLD) is increasingly common around the world, especially in Western nations. In the United States, it is the most common form of chronic liver disease, affecting an estimated 80 to 100 million people.
Given the large numbers of people with fatty livers and the generally increased life expectancy even relatively indolent steatosis can result in a significant population progressing to NASH, NASH with fibrosis and finally cirrhosis. The development of new therapies that prevent the transition from steatosis and inflammation to fibrosis would have substantial clinical value.
The authors report for the first time that a KCa3.1 channel inhibitor exerts an anti-steatotic effect in the setting of fatty liver disease which can be harnessed for the treatment of liver fibrosis. Second, we are the first to report that Senicapoc, a drug that has been through Phase III clinical trials, can be repurposed for the treatment of fatty liver disease and potentially for the treatment of other lipid disorders.
Based on its selectivity for the KCa3.1 channel, its half-life in humans, it’s highly favorable safety profile and its activity against steatosis, Senicapoc is an ideal candidate to be repurposed for NASH or other lipid-related disorders.
The continuum between steatosis, hepatitis, fibrosis and cirrhosis lends itself to therapeutic intervention at any one of these nodes. This study is the first to demonstrate that Senicapoc, a KCa3.1 channel inhibitor, exerts an anti-fibrotic effect in the liver and that in diet-induced liver disease this anti-fibrotic effect is mediated via a reduction in steatosis.
The authors aimed to determine the anti-fibrotic effects of Senicapoc (a KCa3.1 channel inhibitor) in different animal models. Senicapoc therapeutic effect seems to be mediated by a reduction in steatosis suggesting that fat accumulation in the hepatocytes might be directly related to the development of inflammation and in turn of fibrosis. The authors conclude that Senicapoc could be a good candidate for the treatment of NASH for the improvement of both steastosis and fibrosis.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Rosso C S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
| 1. | Sanyal AJ, Friedman SL, McCullough AJ, Dimick-Santos L; American Association for the Study of Liver Diseases; United States Food and Drug Administration. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American Association for the Study of Liver Diseases-U.S. Food and Drug Administration Joint Workshop. Hepatology. 2015;61:1392-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 271] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 2. | Spengler EK, Loomba R. Recommendations for Diagnosis, Referral for Liver Biopsy, and Treatment of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Mayo Clin Proc. 2015;90:1233-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 207] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 3. | Wulff H, Castle NA. Therapeutic potential of KCa3.1 blockers: recent advances and promising trends. Expert Rev Clin Pharmacol. 2010;3:385-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 4. | Wulff H, Kolski-Andreaco A, Sankaranarayanan A, Sabatier JM, Shakkottai V. Modulators of small- and intermediate-conductance calcium-activated potassium channels and their therapeutic indications. Curr Med Chem. 2007;14:1437-1457. [PubMed] |
| 5. | Di L, Srivastava S, Zhdanova O, Ding Y, Li Z, Wulff H, Lafaille M, Skolnik EY. Inhibition of the K+ channel KCa3.1 ameliorates T cell-mediated colitis. Proc Natl Acad Sci USA. 2010;107:1541-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Huang C, Shen S, Ma Q, Chen J, Gill A, Pollock CA, Chen XM. Blockade of KCa3.1 ameliorates renal fibrosis through the TGF-β1/Smad pathway in diabetic mice. Diabetes. 2013;62:2923-2934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Freise C, Heldwein S, Erben U, Hoyer J, Köhler R, Jöhrens K, Patsenker E, Ruehl M, Seehofer D, Stickel F. K+ -channel inhibition reduces portal perfusion pressure in fibrotic rats and fibrosis associated characteristics of hepatic stellate cells. Liver Int. 2015;35:1244-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Toyama K, Wulff H, Chandy KG, Azam P, Raman G, Saito T, Fujiwara Y, Mattson DL, Das S, Melvin JE. The intermediate-conductance calcium-activated potassium channel KCa3.1 contributes to atherogenesis in mice and humans. J Clin Invest. 2008;118:3025-3037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 191] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 9. | PF-05416266 (senicapoc; ICA-17043). Available from: https://ncats.nih.gov/files/PF-05416266.pdf. |
| 10. | Ataga KI, Reid M, Ballas SK, Yasin Z, Bigelow C, James LS, Smith WR, Galacteros F, Kutlar A, Hull JH. Improvements in haemolysis and indicators of erythrocyte survival do not correlate with acute vaso-occlusive crises in patients with sickle cell disease: a phase III randomized, placebo-controlled, double-blind study of the Gardos channel blocker senicapoc (ICA-17043). Br J Haematol. 2011;153:92-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 173] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 11. | Ataga KI, Smith WR, De Castro LM, Swerdlow P, Saunthararajah Y, Castro O, Vichinsky E, Kutlar A, Orringer EP, Rigdon GC. Efficacy and safety of the Gardos channel blocker, senicapoc (ICA-17043), in patients with sickle cell anemia. Blood. 2008;111:3991-3997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 173] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 12. | Ataga KI, Stocker J. Senicapoc (ICA-17043): a potential therapy for the prevention and treatment of hemolysis-associated complications in sickle cell anemia. Expert Opin Investig Drugs. 2009;18:231-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Minniti CP, Wilson J, Mendelsohn L, Rigdon GC, Stocker JW, Remaley AT, Kato GJ. Anti-haemolytic effect of senicapoc and decrease in NT-proBNP in adults with sickle cell anaemia. Br J Haematol. 2011;155:634-636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Shan X, Miao Y, Fan R, Song C, Wu G, Wan Z, Zhu J, Sun G, Zha W, Mu X. Suppression of Grb2 expression improved hepatic steatosis, oxidative stress, and apoptosis induced by palmitic acid in vitro partly through insulin signaling alteration. In Vitro Cell Dev Biol Anim. 2013;49:576-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Yao HR, Liu J, Plumeri D, Cao YB, He T, Lin L, Li Y, Jiang YY, Li J, Shang J. Lipotoxicity in HepG2 cells triggered by free fatty acids. Am J Transl Res. 2011;3:284-291. [PubMed] |
| 16. | Kawazoe Y, Miyauchi M, Nagasaki A, Furusho H, Yanagisawa S, Chanbora C, Inubushi T, Hyogo H, Nakamoto T, Suzuki K. Osteodystrophy in Cholestatic Liver Diseases Is Attenuated by Anti-γ-Glutamyl Transpeptidase Antibody. PLoS One. 2015;10:e0139620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Traber PG, Chou H, Zomer E, Hong F, Klyosov A, Fiel MI, Friedman SL. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PLoS One. 2013;8:e75361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 166] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 18. | Ganz M, Bukong TN, Csak T, Saha B, Park JK, Ambade A, Kodys K, Szabo G. Progression of non-alcoholic steatosis to steatohepatitis and fibrosis parallels cumulative accumulation of danger signals that promote inflammation and liver tumors in a high fat-cholesterol-sugar diet model in mice. J Transl Med. 2015;13:193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 19. | Huang B, Abbott NAE, Dacon L, McCormack ES, Zhou P, Zhang L, Duan B, Li J, Zhang B, Yamin M. Acute Injury in Natural Diet-Induced Fatty Livers - A Model for Therapy Development. Recent Patents on Biomarkers. 2015;5:101-107. |
| 20. | Zhang GB, Song YN, Chen QL, Dong S, Lu YY, Su MY, Liu P, Su SB. Actions of Huangqi decoction against rat liver fibrosis: a gene expression profiling analysis. Chin Med. 2015;10:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Ronis MJ, Hennings L, Stewart B, Basnakian AG, Apostolov EO, Albano E, Badger TM, Petersen DR. Effects of long-term ethanol administration in a rat total enteral nutrition model of alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2011;300:G109-G119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Matsumoto M, Hada N, Sakamaki Y, Uno A, Shiga T, Tanaka C, Ito T, Katsume A, Sudoh M. An improved mouse model that rapidly develops fibrosis in non-alcoholic steatohepatitis. Int J Exp Pathol. 2013;94:93-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 375] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 23. | Leandro G, Mangia A, Hui J, Fabris P, Rubbia-Brandt L, Colloredo G, Adinolfi LE, Asselah T, Jonsson JR, Smedile A, Terrault N, Pazienza V, Giordani MT, Giostra E, Sonzogni A, Ruggiero G, Marcellin P, Powell EE, George J, Negro F; HCV Meta-Analysis (on) Individual Patients' Data Study Group. Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology. 2006;130:1636-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 408] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 24. | Stocker JW, De Franceschi L, McNaughton-Smith GA, Corrocher R, Beuzard Y, Brugnara C. ICA-17043, a novel Gardos channel blocker, prevents sickled red blood cell dehydration in vitro and in vivo in SAD mice. Blood. 2003;101:2412-2418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 154] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Alkhouri N, Dixon LJ, Feldstein AE. Lipotoxicity in nonalcoholic fatty liver disease: not all lipids are created equal. Expert Rev Gastroenterol Hepatol. 2009;3:445-451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 302] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 26. | Yu ZH, Xu JR, Wang YX, Xu GN, Xu ZP, Yang K, Wu DZ, Cui YY, Chen HZ. Targeted inhibition of KCa3.1 channel attenuates airway inflammation and remodeling in allergic asthma. Am J Respir Cell Mol Biol. 2013;48:685-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |