Published online Jun 14, 2017. doi: 10.3748/wjg.v23.i22.3954
Peer-review started: October 28, 2016
First decision: December 19, 2016
Revised: January 19, 2017
Accepted: February 7, 2017
Article in press: February 7, 2017
Published online: June 14, 2017
Processing time: 230 Days and 14.1 Hours
The use of non-steroidal anti-inflammatory drugs (NSAIDs) is widespread worldwide thanks to their analgesic, anti-inflammatory and antipyretic effects. However, even more attention is placed upon the recurrence of digestive system complications in the course of their use. Recent data suggests that the complications of the lower gastro-intestinal tract may be as frequent and severe as those of the upper tract. NSAIDs enteropathy is due to enterohepatic recycling of the drugs resulting in a prolonged and repeated exposure of the intestinal mucosa to the compound and its metabolites. Thus leading to so-called topical effects, which, in turn, lead to an impairment of the intestinal barrier. This process determines bacterial translocation and toxic substances of intestinal origin in the portal circulation, leading to an endotoxaemia. This condition could determine a liver inflammatory response and might promote the development of non-alcoholic steatohepatitis, mostly in patients with risk factors such as obesity, metabolic syndrome and a high fat diet, which may induce a small intestinal bacterial overgrowth and dysbiosis. This alteration of gut microbiota may contribute to nonalcoholic fatty liver disease and its related disorders in two ways: firstly causing a malfunction of the tight junctions that play a critical role in the increase of intestinal permeability, and then secondly leading to the development of insulin resistance, body weight gain, lipogenesis, fibrogenesis and hepatic oxidative stress.
Core tip: Among the gastro-intestinal effects, in non-steroidal anti-inflammatory drugs (NSAIDs) users, those of the lower tract seem to be rising. NSAIDs enteropathy is due to the enterohepatic recycling of drugs, resulting in a prolonged and repeated exposure of the intestinal mucosa to the compound and its metabolites, leading to so called topical effects. The impairment of the intestinal barrier represents the initial damage of NSAIDs enteropathy that leads to the translocation of bacteria and toxic substances of intestinal origin in the portal circulation, promoting an endotoxaemia. This condition, mostly in patients with risk factors for nonalcoholic fatty liver diseas, such as obesity and metabolic syndrome, might lead to liver inflammatory response that could promote the development of nonalcoholic steatohepatitis.
- Citation: Utzeri E, Usai P. Role of non-steroidal anti-inflammatory drugs on intestinal permeability and nonalcoholic fatty liver disease. World J Gastroenterol 2017; 23(22): 3954-3963
- URL: https://www.wjgnet.com/1007-9327/full/v23/i22/3954.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i22.3954
Nonsteroidal anti-inflammatory drugs (NSAIDs) are among the most consumed drugs in the world thanks to their benefits as analgesic, anti-inflammatory and antipyretic agents[1]. However, these benefits are, in part, overshadowed by the recurrence of digestive system complications which may arise during the course of therapy and can, at times, be severe.
Nevertheless, NSAIDs can cause a variety of functional and structural abnormalities, even in the small and large intestine for patients who make long-term use of the said drugs. About 60%-70% of patients on long-term NSAIDs develop mucosal damage[2-4], including an increase in intestinal permeability, intestinal inflammation, erosions and protein loss, but also more serious complications such as anemia, bleeding, ulcers, perforations, obstruction, diverticulitis, ileal dysfunction and diaphragm-like strictures[5-7] (Table 1). It is estimated that about one third of all complications associated with the use of NSAIDs is represented by severe injuries of the small bowel[8]. Several videoenterocapsule studies have shown that the use of NSAIDs [both nonselective and selective cyclooxygenase-2 (COX-2) inhibitors] may be associated with a high incidence of small-bowel erosion and ulceration (55%-75%)[9-12]; the chronic use of low dose aspirin has also been shown to be associated with the presence of similar small-bowel lesions[13,14]. Recent data has shown that the incidence of complications of the lower gastrointestinal tract, many of these due to the use of NSAIDs and ASA, is on the rise while the incidence of upper gastrointestinal lesions is declining[15].
| Adverse effect | Frequency |
| Increased gut permeability | 44%-70% |
| Gut inflammation | 60%-70% |
| Blood loss and anemia | 30% |
| Malabsorption | 40%-70% |
| Mucosal ulceration | 30%-40% |
| Protein loss | 10% |
| Mucosal ulceration | 30%-40% |
| Complications requiring hospitalizations | 0.3%-0.9% |
| Diaphragms of the small bowel | < 1% |
At the core of this broad spectrum of lesions there is a multifactorial pathogenesis with structural and functional alterations of different components, which make up the intestinal barrier. Additionally, the appearance of factors that interfere with the maintenance and homeostasis of normal bowel functionality can be found.
Due to the intestinal barrier and its constituent elements alteration, in particular intestinal permeability, luminal substances including toxins, microorganisms and their components can access the portal circulation causing toxemia with pathological effects also in the long term. In this regard, there is strong evidence to confirm the liver as one of the main targets of toxemia resulting from the alteration of intestinal permeability. As a consequence, a process of inflammation, as well as an alteration in the metabolic processes can occur in the liver, contributing to the pathogenesis of Non Alcoholic Fatty Liver Disease and to its various manifestations[16], which, are beyond the scope of this paper.
The NSAIDs pharmacological target is the inhibition of cyclooxygenase (COX or prostaglandin endoperoxide synthetase) with consequent reduction in the production of prostaglandins (PGE)[17]. The PGE are implicated in a significant number of critical functions in the bowel. The cyclooxygenase consists of two isoforms with distinct functions but both inhibited by NSAIDs: (1) the COX-1, which is constitutively expressed in many tissues, in the gut catalyzes the formation of many cytoprotective PGE involved in the synthesis of mucus, bicarbonate, maintenance of blood flow, the turnover of epithelial cells and the resolution of inflammatory processes; and (2) COX-2 is an inducible form implicated in the resolution of inflammation processes. It is responsible for the production of a variety of PGE that may cause or protect against inflammatory processes[18].
The concept that the inhibition of both isoforms causes enteropathy is strengthened by several studies. Unlike traditional NSAIDs, selective COX-2 NSAIDs results in lower adverse gastrointestinal effects[19], even if this beneficial effect may be lost with long-term use[14].
Topical effects: Multi - hit concept: The mechanisms underlying NSAIDs enteropathy are primarily represented by the so-called "topical effects", i.e., those adverse effects coming from the high local concentration of NSAIDs in the intestinal lumen[20] and their enterohepatic circulation. The topical effects are by definition independent from the impact of NSAIDs on COX[21].
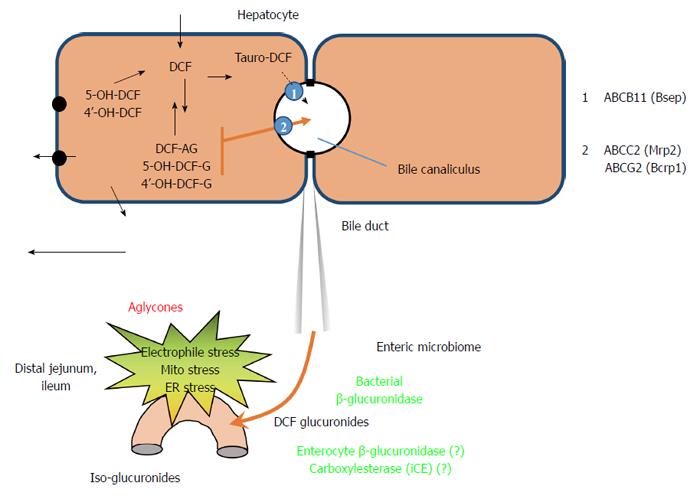
From a pharmacokinetic point of view NSAIDs are weak acids (pKa 3-6) which are protonated and then absorbed in the stomach according to their lipophilicity[22,23]. Subsequently, the NSAID-containing carboxylic acids, after their introduction either orally or intraperitoneally, reach the liver via the portal system where the drug is glucuronidated: it is conjugated to glucuronic acid[24,25] or taurine[26] or sulfate, and excreted into the bile in large quantities. Specifically, it is exported inside the bile canaliculi against a concentration gradient through the ATP-dependent transporters present on the apical membrane of the hepatocyte, the MRP2 (ABCC2)[27] or Bcrp1 (ABCG2)[28]; the specific carrier of tauro-conjugates is less defined.
At this point the small intestine is exposed to the drug and to its oxidative conjugated metabolites that reach the most distal part where the glucuronide is cleaved by bacterial beta-glucuronidase, forming aglycones, which are free derivatives of NSAIDs or oxidative metabolites[29]. At this point the drug is transferred again into the enterohepatic circulation (Figure 1).
An initial increase in small intestine permeability is a prerequisite of the subsequent development of small intestine inflammation, which is associated with blood and protein loss but is often silent[30].
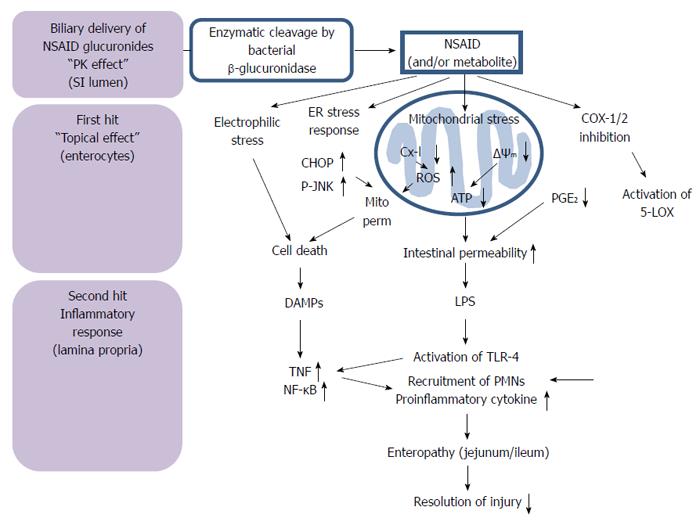
It would appear that the enterohepatic recycling results in a prolonged and repeated exposure of the intestinal mucosa to the compound[31]. They include the uptake of the drug and its metabolites in the enterocytes where they are metabolized by cytochrome P450 (CYP450) in order to potentially reactivate intermediates with possible bioactivation and the induction of mitochondrial[32-34] and endoplasmic reticulum stress[35,36] (Figure 2). Therefore, the production of reactive metabolites occurs through CYPs of enterocytes, ER stress, oxidative stress and mitochondrial damage[21]. In humans it is mainly CYP2C8/9/19 to be involved in the oxidative biotransformation of many FANS[37]. This step is called the “first hit”. After this initial insult of enterocytes, the mucosal epithelium becomes more permeable and the LPS present in the lumen can penetrate deeply into the mucosa and activate the toll-like receptor 4 (TLR4) of macrophages in the lamina propria. This can cause cell damage mediated by the tumor necrosis factor, and subsequently the activation of the innate immune system with the recruitment of inflammatory cells into the injury site. The inflammatory response that follows is the “second hit”[38].
Mitochondrial damage: most NSAIDs cause a decoupling of oxidative phosphorylation in the mitochondria both in vivo and in vitro, dissociating the breathing from the production of energy and dissipating the inner transmembrane potential of mitochondria[39]. During the absorption of the NSAIDs there is an intracellular accumulation of the drug proportional to its acidity, even at micromolar concentrations: this is able to uncouple oxidative phosphorylation at the mitochondrial level. This event can have two effects on enterocytes. Firstly, an attenuation of ATP production with gradual depletion of cellular ATP; secondly, a collapse of the gradient can determine the opening of the mitochondrial permeability transition pore (mPT) leading to cell death[20]. This ability is due to the structure of NSAIDs. In fact, NSAIDs are weak and lipophilic acids which induce enteropathy through mitochondrial energetic depletion[20]. Some NSAIDs inhibit several complexes of the electron transport chain. Other NSAIDs, such as indomethacin and diclofenac, inhibit the activity of rotenone-sensitive complex I in mitochondria and therefore increase the production of superoxide. This inhibition is reversible with the administration of quercetin, an ubiquinone-mimetic (coenzyme Q)[40].
Interaction with biomembranes: is due to the direct effect of NSAIDs on cell membranes by altering the biophysical properties. One example is the electrostatic interaction between the NSAID and hydrophobic anions and the positive charged nitrogen of phosphatidylcholine, which alters the biophysical properties of the membrane, its fluidity and finally increases the permeability to protons and to bacterial toxins[41].
Detergents properties: NSAIDs are invariably lipophilic weak acids and this makes them the detergents for phospholipid components of the brush border. This causes direct damage to the epithelial surface.
Mitochondrial permeability: NSAIDs can induce mitochondrial permeabilization followed by the release of apoptotic factors from the intermembrane space inside the cytosol. This mechanism is mediated by the opening of the MPT pore, involving both the internal and external membrane, and can be triggered by an increase of Calcium (a mechanism introduced by many NSAIDs), oxidative stress or by the collapse of mitochondrial membrane potential[42,43].
Intestinal permeability: this effect also affects TJ, which are under the control of the actin-myosin ATP-dependent complex. The consequence is an increase in the intestinal permeability[44]. The reduction of mitochondrial ATP production causes a loss of the intestinal barrier function and this can be tested quantitatively by the oral administration of dextran[45].
Oxidative Stress: there is only indirect evidence of its involvement in NSAIDs enteropathy. For example, indomethacin raises the expression of heme oxygenase, an antioxidant enzyme induced by oxidative stress[46]. Another pathway activated by oxidative stress is that of the MAPK (via phosphorylation of JNK). These effects can be induced by mitochondrial dysfunction that increase oxidative stress. This last event may be a side effect triggered by the inflammatory response of the innate immune system cells[32].
ER stress: According to some studies performed on patients taking diclofenac, there is an increase of markers of endoplasmic reticulum stress proteins, like GRP78 and CHOP. CHOP is a transcription factor that induces cell death mediated by mitochondria[47].
It consists of the innate immune system and the inflammatory response. The innate response is triggered by bacteria and proinflammatory mediators coming from bacteria that invade the mucous layer over the epithelium. As a result, the signaling pathway TLR-mediated is activated and the neutrophils infiltrate the damaged areas. On the other hand, the adaptive immune system does not seem to play a critical role in NSAIDs enteropathy[21].
TLR and LPS: TLRs recognize specific molecular patterns associated with pathogens, and trigger the inflammatory response. In particular, TLR4 is the LPS receptor and it is expressed in monocytes and macrophages of the lamina propria as an extracellular domain rich in leucinic repetitions and an IL-1R signal intracellular domain[48]. So, the TLR4 activates the NF-kB with consequent production of proinflammatory cytokines including TNF and IL-1 beta[49].
TNF: prostaglandins, and in particular PGE2, inhibit TNF synthesis, while the reduced levels of prostaglandins induced by NSAIDs lead to an increase of its synthesis[50]. TNF is implicated in the apoptosis of enterocytes and in the inflammatory response in the intestine. However, according to some studies, TNF appears to have cytoprotective effects on the intestinal mucosa by inducing the expression of COX2, mediated by EGFR transactivation[51]. Also IL-17A should be mentioned. It is produced by T cells of the lamina propria and regulates the production of proinflammatory cytokines and chemokines[52].
Neutrophils: the NSAID enteropathy is characterized by a massive infiltration of neutrophils in the ulcerated areas, which aggravate the damage through the production of ROS or protease. The biomarkers used for their study is the time-dependent increase in the activity of myeloperoxidase[38].
Bile acid metabolism: the critical role of bile in the pathogenesis of NSAID enteropathy is evident from studies showing that bile duct ligation prevented NSAID-induced intestinal damage in rats[20,53-55]. According to some animal models, the NSAIDs, especially indomethacin, are rapidly excreted via the bile and then enter the enterohepatic circulation through the small intestine, resulting in a high concentration of the drugs in the liver and bile. Bile acids are cytotoxic because of their cleansing effect, by binding to phospholipids and directly altering the integrity of the membrane. The phospholipids and cholesterol are considered luminary factors with direct effects that appear to be involved in protective mechanisms on gastrointestinal and liver cells through a cleansing effect on the bile acids. NSAIDs are highly amphiphilic molecules and create stronger links with the phospholipids. Animal and laboratory studies show that NSAIDs reduce the hydrophobic properties of the upper GI barrier, partly determined by the active surface phospholipids. Therefore, NSAIDs secreted in bile interact with its amphipathic components, such as phosphatidylcholine and bile acids; this leads to an alteration of the structure and the stability of these components, and consequently the toxicity of bile in the small intestine is modified[56]. Dial et al[57] examined the biliary phosphatidylcholine (PC), which appears to have protective effects on enterocytes, cholangiocytes and erythrocytes against damage induced by bile salts. NSAIDs appear to determine intestinal damage in proportion to their ability to be secreted via the bile because of their capacity to chemically bind with the micelles and with the PC, lowering their effects. As previously mentioned, NSAIDs bind the PC and this process takes place in the gastrointestinal tract, where the drug-induced loss of PC, which protects the mucosa, causes mucosal damage. The PC would protect it from both damage induced by bile salts and by NSAIDs[57,58].
The intestinal micro-organisms and their degradation products are necessary for the development of NSAIDs enteropathy, as ‘‘germ-free’’ animals were found to be resistant to indomethacin injuries[14,59]. Therefore, the role of enteric bacteria in determining the NSAID enteropathy is twofold: (1) the toxic insult on the tight junctions determines an increase in intestinal permeability with a subsequent bacterial invasion of the mucous membrane that activates the TLRs essential for the development of NSAID-induced small bowel lesions; and (2) they can metabolically convert NSAIDs glucuronide in aglycones by activation of beta- glucuronidase. Such enzymatic activity would seem greater in the distal part of the small intestine than in the other parts[60]. However, the gus gene, which codes for this enzyme, is not present in all bacterial strains, but only in 50% of the human gut symbiotic bacteria[61].
It has also been shown that treatment with NSAIDs may disrupt the homeostasis of intestinal flora and has been associated with an overgrowth of Gram-negative and anaerobic bacterial species in the small intestine, secreting LPS, which are able to exacerbate the NSAID-induced intestinal injury[38,62-64]. This mechanism of unbalanced increase of Gram-negative bacteria in NSAID users has not, as yet, been elucidated, however, it seems that the bacteria might easily penetrate into the mucosa when mucosal permeability is enhanced by NSAIDs[65].
Recently more importance has been placed on the co-administration of NSAIDs and the inhibitors of gastric acid secretion, such as proton pump inhibitors and histamine H2 receptor antagonists, in determining a significant alteration of intestinal microbiota composition and exacerbating NSAIDs enteropathy[21,66]. PPI determine hypochloremia causing abnormal growth of bacteria that can colonize the small intestine causing SIBO (Small Intestinal Bacterial Overgrowth) with increased bacterial translocation[67,68]. Wallace et al[69] in fact reported that PPIs, in particular omeprazole resulted in significant dysbiosis, with both a substantial increase in Gram-negative bacteria and a significant reduction in the proportion of Actinobacteria (mainly Bifidobacter ssp.) in the jejunum.
There is growing evidence that an alterated interaction between gut microbiota and the host at the intestinal mucosa level determine an impairment of gut-liver axis and contribute to a state of low-grade inflammation, endotoxemia, obesity and metabolic liver disorders like nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH)[70-72]. Through the alteration of the intestinal barrier, as happens in the NSAID enteropathy, a translocation of bacteria and toxic substances of intestinal origin can occur: entering the portal circulation, they can reach the liver and can lead to a large number of pathological alterations such as steatosis, steatohepatitis and liver fibrosis. It can be assumed that this condition promotes the development of NASH in patients with predisposition factors towards the development of NAFLD, such as obesity and metabolic syndrome. Although NAFLD is a multifactorial disease, in recent years more and more importance has been given to the role of microbiota, which seems to be crucial in determining its development, starting from the accumulation of fat in the liver through to the triggering of liver inflammation.
In this regard, several studies have shown that in obese patients there is an alteration of the specific individual bacterial composition, with a reversal of the Bacteroides and Firmicutes ratio, with Bifidobaceri reduction, resulting in an increase of bacteria able to metabolize carbohydrates and assume energy, thus increasing adiposity[73]. These studies also indicate that obese patients do not have a predetermined microbial composition, but it is rather the specific Western diet, which is high in fat, that influences this composition by increasing the Firmicutes. It is also possible to observe a similar prevalence of Firmicutes in patients with NAFLD[74].
Specifically, NSAIDs would act at colic level exposing the patients with NASH to a susceptibility to gut leakiness. In this case, the endotoxemia represents the stimulus required to trigger the necro-inflammatory cascade in hepatocytes, already affected by alteration in lipid homeostasis induced by obesity. According to a study by Farhadi et al[75] 2008, the intestinal permeability was measured in patients with steatosis or with NASH and in healthy patients, before and after the administration of aspirin, through the urinary excretion: the lactulose/mannitol (L/M) ratio was evaluated after 5 h, while the sucralose after 24 h. It was observed that the aspirin increases the urinary excretion L/M in the majority of patients, but, especially, it significantly increases the intestinal permeability in patients with NASH. According to this model, patients with NASH would not have a constantly altered intestinal permeability, but this situation could occur due to stress factors such as aspirin, NSAIDs, psychological or physical stress or other. For this reason it would be reasonable that patients with a particular susceptibility to oxidative stress, such as those with metabolic syndrome (obesity, diabetes, NAFLD and insulin resistance) and altered metabolism of fatty acids, avoided agents such as alcohol and NSAIDs that increase intestinal permeability.
The direct effects of NSAIDs in determining liver damage should be emphasized as this is a predisposing factor for the development of nonalcoholic hepatic steatosis (micro or macrovesicular type) and steatohepatitis. In fact, these drugs are implicated in the pathogenesis of the so-called drug-induced liver injury[76], which is diagnosed when the worsening of liver function is given by prescribed medications or not. Steatohepatitis caused by drugs can occur many months after their use and cannot be resolved within 15 d. However, it can be possible that the drugs exacerbate a pre-existing NAFLD[77-80].
The drugs that determine steatosis and NASH interfere firstly with the mitochondrial respiration, beta-oxidation, or both, as shown in one of the first studies performed on Pirprofen[81]. When the hepatic mitochondrial beta oxidation is severely inhibited, the damage of the beta-CoA oxidation increases the levels of non-esterified fatty acids, which are converted into triglycerides determining hepatic steatosis[82]. An increased production of ROS is the result of this process, and, in the most severe cases, this increase leads to liver necrosis[83,84].
Currently, there are no approved pharmacological strategies that can treat or completely prevent NSAID-mediated enteropathy. Some compounds suitable to reduce the inflammatory response or to stimulate the effects mediated by prostaglandins were used, but with limited effectiveness or adverse effects[85,86]. Most of the experiments and therapeutic approaches are focused on the inflammatory component that constitutes the second hit, while approaches to protect against the damage of the first hit (mitochondrial stress, endoplasmic reticulum stress, electrophilic stress) or acting on the release of glucuronide and aglicoles, have not yet been fully explored. Among the various approaches, there is the use of NO or H2S -releasing NSAIDs, because, as is well known, nitric oxide and hydrogen sulfide are powerful vasodilatory molecules that protect the mucous membrane and maintain its integrity[87]. Therefore, it could be reasonable to assume that therapeutic strategies that aim to restore the intestinal microbiota firstly with dietary interventions, antibiotics and probiotics could be sound practice.
Since there are multiple mechanisms involved in NSAIDs enteropathy and in the subsequent development of NAFLD, also the therapeutic approach has to aim at applying multiple strategies simultaneously.
NAFLD is a rising disease in the Western world due to the increased predisposing diet and lifestyle, and its incidence grows along with that of obesity and metabolic syndrome. Moreover, the simultaneous use of NSAIDs such as analgesics, anti-inflammatory and antipyretic, and the growing prospect of new therapeutic uses also as anti - cancer drugs or in the treatment of Alzheimer's, make them widespread drugs. Despite being beyond the scope of the present work, the many positive effects of NSAIDs cannot be overlooked, primarily their role in malignant transformation. The long-term use of aspirin and other NSAIDs has been shown to reduce the risk of colon cancer and other gastrointestinal organs in addition to cancer of the breast, prostate, lung and skin. NSAIDs restore normal apoptosis and reduce cell proliferation in human adenomatous colorectal polyps. Moreover, NSAIDs, particularly selective COX-2 inhibitors, have been shown to inhibit angiogenesis in cell culture and in rodent models of angiogenesis[88].
The magnitude of serious outcomes from the lower GI tract is not well defined, but recent data suggests that they may be as frequent and severe as upper GI complications. Contrary to what happens in the upper GI tract, treatment and prevention of NSAID enteropathy is difficult, since the pathogenic mechanisms are different and not well understood. Therefore attention should be paid to the administration of NSAIDs or aspirin in patients with particular susceptibility to oxidative stress such as those with metabolic syndrome (obesity, diabetes and insulin resistance) and NAFLD and to the co-administration of antisecretory agents which may exacerbate NSAID-induced intestinal damage. The impairment of the intestinal barrier, represents, the initial damage of NSAIDs enteropathy leading to an endotoxaemia, due to the translocation of bacteria and toxic substances of intestinal origin in the portal circulation. This condition could determine a liver inflammatory response and might promote the development of NASH. Nevertheless, it is necessary to consider the incessant increase of the NSAID enteropathy, and, therefore, research into new therapeutic strategies is needed to prevent or reduce the incidence of this complication and consequent systemic diseases, like NAFLD.
Since the NSAID-induced enteropathy that may accelerate NAFLD/NASH seems at the moment to be an interesting pathogenetic hypothesis, further prospective studies will be necessary in order to definitely confirm such theory.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Balaban Y, Mattner J, Sazci A S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Sigthorsson G, Tibble J, Hayllar J, Menzies I, Macpherson A, Moots R, Scott D, Gumpel MJ, Bjarnason I. Intestinal permeability and inflammation in patients on NSAIDs. Gut. 1998;43:506-511. [PubMed] |
| 2. | Adebayo D, Bjarnason I. Is non-steroidal anti-inflammaory drug (NSAID) enteropathy clinically more important than NSAID gastropathy? Postgrad Med J. 2006;82:186-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 111] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 3. | Lanas A, Panés J, Piqué JM. Clinical implications of COX-1 and/or COX-2 inhibition for the distal gastrointestinal tract. Curr Pharm Des. 2003;9:2253-2266. [PubMed] |
| 4. | Graham DY, Opekun AR, Willingham FF, Qureshi WA. Visible small-intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol. 2005;3:55-59. [PubMed] |
| 5. | Lanas A, Sopeña F. Nonsteroidal anti-inflammatory drugs and lower gastrointestinal complications. Gastroenterol Clin North Am. 2009;38:333-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Bjarnason I, Hayllar J, MacPherson AJ, Russell AS. Side effects of nonsteroidal anti-inflammatory drugs on the small and large intestine in humans. Gastroenterology. 1993;104:1832-1847. [PubMed] |
| 7. | Davies NM, Saleh JY, Skjodt NM. Detection and prevention of NSAID-induced enteropathy. J Pharm Pharm Sci. 2000;3:137-155. [PubMed] |
| 8. | Scarpignato C, Hunt RH. Nonsteroidal antiinflammatory drug-related injury to the gastrointestinal tract: clinical picture, pathogenesis, and prevention. Gastroenterol Clin North Am. 2010;39:433-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 9. | Goldstein JL, Eisen GM, Lewis B, Gralnek IM, Zlotnick S, Fort JG. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol. 2005;3:133-141. [PubMed] |
| 10. | Maiden L, Thjodleifsson B, Theodors A, Gonzalez J, Bjarnason I. A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology. 2005;128:1172-1178. [PubMed] |
| 11. | Maiden L. Capsule endoscopic diagnosis of nonsteroidal antiinflammatory drug-induced enteropathy. J Gastroenterol. 2009;44 Suppl 19:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Fujimori S, Gudis K, Takahashi Y, Seo T, Yamada Y, Ehara A, Kobayashi T, Mitsui K, Yonezawa M, Tanaka S. Distribution of small intestinal mucosal injuries as a result of NSAID administration. Eur J Clin Invest. 2010;40:504-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Leung WK, Bjarnason I, Wong VW, Sung JJ, Chan FK. Small bowel enteropathy associated with chronic low-dose aspirin therapy. Lancet. 2007;369:614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Higuchi K, Umegaki E, Watanabe T, Yoda Y, Morita E, Murano M, Tokioka S, Arakawa T. Present status and strategy of NSAIDs-induced small bowel injury. J Gastroenterol. 2009;44:879-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 187] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 15. | Lanas A, García-Rodríguez LA, Polo-Tomás M, Ponce M, Alonso-Abreu I, Perez-Aisa MA, Perez-Gisbert J, Bujanda L, Castro M, Muñoz M. Time trends and impact of upper and lower gastrointestinal bleeding and perforation in clinical practice. Am J Gastroenterol. 2009;104:1633-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 414] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 16. | Minemura M, Shimizu Y. Gut microbiota and liver diseases. World J Gastroenterol. 2015;21:1691-1702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 135] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (1)] |
| 17. | Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232-235. [PubMed] |
| 18. | Bjarnason I, Takeuchi K. Intestinal permeability in the pathogenesis of NSAID-induced enteropathy. J Gastroenterol. 2009;44 Suppl 19:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Laine L, Connors LG, Reicin A, Hawkey CJ, Burgos-Vargas R, Schnitzer TJ, Yu Q, Bombardier C. Serious lower gastrointestinal clinical events with nonselective NSAID or coxib use. Gastroenterology. 2003;124:288-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 236] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 20. | Somasundaram S, Rafi S, Hayllar J, Sigthorsson G, Jacob M, Price AB, Macpherson A, Mahmod T, Scott D, Wrigglesworth JM. Mitochondrial damage: a possible mechanism of the “topical” phase of NSAID induced injury to the rat intestine. Gut. 1997;41:344-353. [PubMed] |
| 21. | Boelsterli UA, Redinbo MR, Saitta KS. Multiple NSAID-induced hits injure the small intestine: underlying mechanisms and novel strategies. Toxicol Sci. 2013;131:654-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 22. | McCormack K, Brune K. Classical absorption theory and the development of gastric mucosal damage associated with the non-steroidal anti-inflammatory drugs. Arch Toxicol. 1987;60:261-269. [PubMed] |
| 23. | Rainsford KD, Bjarnason I. NSAIDs: take with food or after fasting? J Pharm Pharmacol. 2012;64:465-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Peris-Ribera JE, Torres-Molina F, Garcia-Carbonell MC, Aristorena JC, Pla-Delfina JM. Pharmacokinetics and bioavailability of diclofenac in the rat. J Pharmacokinet Biopharm. 1991;19:647-665. [PubMed] |
| 25. | King C, Tang W, Ngui J, Tephly T, Braun M. Characterization of rat and human UDP-glucuronosyltransferases responsible for the in vitro glucuronidation of diclofenac. Toxicol Sci. 2001;61:49-53. [PubMed] |
| 26. | Mohri K, Okada K, Benet LZ. Stereoselective metabolism of benoxaprofen in rats. Biliary excretion of benoxaprofen taurine conjugate and glucuronide. Drug Metab Dispos. 1998;26:332-337. [PubMed] |
| 27. | Seitz S, Boelsterli UA. Diclofenac acyl glucuronide, a major biliary metabolite, is directly involved in small intestinal injury in rats. Gastroenterology. 1998;115:1476-1482. [PubMed] |
| 28. | Lagas JS, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH. Hepatic clearance of reactive glucuronide metabolites of diclofenac in the mouse is dependent on multiple ATP-binding cassette efflux transporters. Mol Pharmacol. 2010;77:687-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Boelsterli UA, Ramirez-Alcantara V. NSAID acyl glucuronides and enteropathy. Curr Drug Metab. 2011;12:245-252. [PubMed] |
| 30. | Bjarnason I, Smethurst P, Macpherson A, Walker F, McElnay JC, Passmore AP, Menzies IS. Glucose and citrate reduce the permeability changes caused by indomethacin in humans. Gastroenterology. 1992;102:1546-1550. [PubMed] |
| 31. | Bhatia LS, Curzen NP, Calder PC, Byrne CD. Non-alcoholic fatty liver disease: a new and important cardiovascular risk factor? Eur Heart J. 2012;33:1190-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 345] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 32. | Ramirez-Alcantara V, LoGuidice A, Boelsterli UA. Protection from diclofenac-induced small intestinal injury by the JNK inhibitor SP600125 in a mouse model of NSAID-associated enteropathy. Am J Physiol Gastrointest Liver Physiol. 2009;297:G990-G998. [PubMed] |
| 33. | LoGuidice A, Ramirez-Alcantara V, Proli A, Gavillet B, Boelsterli UA. Pharmacologic targeting or genetic deletion of mitochondrial cyclophilin D protects from NSAID-induced small intestinal ulceration in mice. Toxicol Sci. 2010;118:276-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Watanabe T, Tanigawa T, Nadatani Y, Otani K, Machida H, Okazaki H, Yamagami H, Watanabe K, Tominaga K, Fujiwara Y. Mitochondrial disorders in NSAIDs-induced small bowel injury. J Clin Biochem Nutr. 2011;48:117-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Tsutsumi S, Gotoh T, Tomisato W, Mima S, Hoshino T, Hwang HJ, Takenaka H, Tsuchiya T, Mori M, Mizushima T. Endoplasmic reticulum stress response is involved in nonsteroidal anti-inflammatory drug-induced apoptosis. Cell Death Differ. 2004;11:1009-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 203] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 36. | Tanaka K, Tomisato W, Hoshino T, Ishihara T, Namba T, Aburaya M, Katsu T, Suzuki K, Tsutsumi S, Mizushima T. Involvement of intracellular Ca2+ levels in nonsteroidal anti-inflammatory drug-induced apoptosis. J Biol Chem. 2005;280:31059-31067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 37. | Nakanishi Y, Matsushita A, Matsuno K, Iwasaki K, Utoh M, Nakamura C, Uno Y. Regional distribution of cytochrome p450 mRNA expression in the liver and small intestine of cynomolgus monkeys. Drug Metab Pharmacokinet. 2010;25:290-297. [PubMed] |
| 38. | Watanabe T, Higuchi K, Kobata A, Nishio H, Tanigawa T, Shiba M, Tominaga K, Fujiwara Y, Oshitani N, Asahara T. Non-steroidal anti-inflammatory drug-induced small intestinal damage is Toll-like receptor 4 dependent. Gut. 2008;57:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 170] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 39. | Mahmud T, Scott DL, Bjarnason I. A unifying hypothesis for the mechanism of NSAID related gastrointestinal toxicity. Ann Rheum Dis. 1996;55:211-213. [PubMed] |
| 40. | Sandoval-Acuña C, Lopez-Alarcón C, Aliaga ME, Speisky H. Inhibition of mitochondrial complex I by various non-steroidal anti-inflammatory drugs and its protection by quercetin via a coenzyme Q-like action. Chem Biol Interact. 2012;199:18-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 41. | Lichtenberger LM, Zhou Y, Jayaraman V, Doyen JR, O’Neil RG, Dial EJ, Volk DE, Gorenstein DG, Boggara MB, Krishnamoorti R. Insight into NSAID-induced membrane alterations, pathogenesis and therapeutics: characterization of interaction of NSAIDs with phosphatidylcholine. Biochim Biophys Acta. 2012;1821:994-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 42. | Baines CP. The molecular composition of the mitochondrial permeability transition pore. J Mol Cell Cardiol. 2009;46:850-857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 43. | Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol. 2009;46:821-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 759] [Cited by in RCA: 707] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 44. | Nazli A, Yang PC, Jury J, Howe K, Watson JL, Söderholm JD, Sherman PM, Perdue MH, McKay DM. Epithelia under metabolic stress perceive commensal bacteria as a threat. Am J Pathol. 2004;164:947-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 140] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 45. | Bruyère A, Declevès X, Bouzom F, Proust L, Martinet M, Walther B, Parmentier Y. Development of an optimized procedure for the preparation of rat intestinal microsomes: comparison of hepatic and intestinal microsomal cytochrome P450 enzyme activities in two rat strains. Xenobiotica. 2009;39:22-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Harusato A, Naito Y, Takagi T, Uchiyama K, Mizushima K, Hirai Y, Yamada S, Tuji T, Yoriki H, Horie R. Suppression of indomethacin-induced apoptosis in the small intestine due to Bach1 deficiency. Free Radic Res. 2011;45:717-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 47. | Ohyama K, Shiokawa A, Ito K, Masuyama R, Ichibangase T, Kishikawa N, Imai K, Kuroda N. Toxicoproteomic analysis of a mouse model of nonsteroidal anti-inflammatory drug-induced gastric ulcers. Biochem Biophys Res Commun. 2012;420:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 48. | Medzhitov R, Preston-Hurlburt P, Janeway CA. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3900] [Cited by in RCA: 3802] [Article Influence: 135.8] [Reference Citation Analysis (0)] |
| 49. | Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CA. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253-258. [PubMed] |
| 50. | Bertrand V, Guimbaud R, Tulliez M, Mauprivez C, Sogni P, Couturier D, Giroud JP, Chaussade S, Chauvelot-Moachon L. Increase in tumor necrosis factor-alpha production linked to the toxicity of indomethacin for the rat small intestine. Br J Pharmacol. 1998;124:1385-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 51. | Hobbs SS, Goettel JA, Liang D, Yan F, Edelblum KL, Frey MR, Mullane MT, Polk DB. TNF transactivation of EGFR stimulates cytoprotective COX-2 expression in gastrointestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2011;301:G220-G229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 52. | Yamada S, Naito Y, Takagi T, Mizushima K, Hirai Y, Horie R, Fukumoto K, Inoue K, Harusato A, Yoshida N. Reduced small-intestinal injury induced by indomethacin in interleukin-17A-deficient mice. J Gastroenterol Hepatol. 2011;26:398-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 53. | Jacob M, Foster R, Sigthorsson G, Simpson R, Bjarnason I. Role of bile in pathogenesis of indomethacin-induced enteropathy. Arch Toxicol. 2007;81:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 54. | Wax J, Clinger WA, Varner P, Bass P, Winder CV. Relationship of the enterohepatic cycle to ulcerogenesis in the rat small bowel with flufenamic acid. Gastroenterology. 1970;58:772-780. [PubMed] |
| 55. | Lichtenberger LM, Phan T, Okabe S. Aspirin’s ability to induce intestinal injury in rats is dependent on bile and can be reversed if pre-associated with phosphatidylcholine. J Physiol Pharmacol. 2011;62:491-496. [PubMed] |
| 56. | Zhou Y, Dial EJ, Doyen R, Lichtenberger LM. Effect of indomethacin on bile acid-phospholipid interactions: implication for small intestinal injury induced by nonsteroidal anti-inflammatory drugs. Am J Physiol Gastrointest Liver Physiol. 2010;298:G722-G731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 57. | Dial EJ, Darling RL, Lichtenberger LM. Importance of biliary excretion of indomethacin in gastrointestinal and hepatic injury. J Gastroenterol Hepatol. 2008;23:e384-e389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Barrios JM, Lichtenberger LM. Role of biliary phosphatidylcholine in bile acid protection and NSAID injury of the ileal mucosa in rats. Gastroenterology. 2000;118:1179-1186. [PubMed] |
| 59. | Robert A, Asano T. Resistance of germfree rats to indomethacin-induced intestinal lesions. Prostaglandins. 1977;14:333-341. [PubMed] |
| 60. | Hawksworth G, Drasar BS, Hill MJ. Intestinal bacteria and the hydrolysis of glycosidic bonds. J Med Microbiol. 1971;4:451-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 218] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 61. | Wallace BD, Wang H, Lane KT, Scott JE, Orans J, Koo JS, Venkatesh M, Jobin C, Yeh LA, Mani S. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science. 2010;330:831-835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 863] [Cited by in RCA: 799] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 62. | Lanas A, Scarpignato C. Microbial flora in NSAID-induced intestinal damage: a role for antibiotics? Digestion. 2006;73 Suppl 1:136-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 63. | Zwolinska-Wcislo M, Krzysiek-Maczka G, Ptak-Belowska A, Karczewska E, Pajdo R, Sliwowski Z, Urbanczyk K, Drozdowicz D, Konturek SJ, Pawlik WW. Antibiotic treatment with ampicillin accelerates the healing of colonic damage impaired by aspirin and coxib in the experimental colitis. Importance of intestinal bacteria, colonic microcirculation and proinflammatory cytokines. J Physiol Pharmacol. 2011;62:357-368. [PubMed] |
| 64. | Montalto M, Gallo A, Curigliano V, D’Onofrio F, Santoro L, Covino M, Dalvai S, Gasbarrini A, Gasbarrini G. Clinical trial: the effects of a probiotic mixture on non-steroidal anti-inflammatory drug enteropathy - a randomized, double-blind, cross-over, placebo-controlled study. Aliment Pharmacol Ther. 2010;32:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 65. | Montalto M, Gallo A, Gasbarrini A, Landolfi R. NSAID enteropathy: could probiotics prevent it? J Gastroenterol. 2013;48:689-697. [PubMed] |
| 66. | Scheiman JM, Yeomans ND, Talley NJ, Vakil N, Chan FK, Tulassay Z, Rainoldi JL, Szczepanski L, Ung KA, Kleczkowski D. Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors. Am J Gastroenterol. 2006;101:701-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 192] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 67. | Choung RS, Ruff KC, Malhotra A, Herrick L, Locke GR, Harmsen WS, Zinsmeister AR, Talley NJ, Saito YA. Clinical predictors of small intestinal bacterial overgrowth by duodenal aspirate culture. Aliment Pharmacol Ther. 2011;33:1059-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 68. | Lewis SJ, Franco S, Young G, O’Keefe SJ. Altered bowel function and duodenal bacterial overgrowth in patients treated with omeprazole. Aliment Pharmacol Ther. 1996;10:557-561. [PubMed] |
| 69. | Wallace JL, Syer S, Denou E, de Palma G, Vong L, McKnight W, Jury J, Bolla M, Bercik P, Collins SM. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology. 2011;141:1314-122, 1314-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 342] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 70. | Marlicz W, Loniewski I, Grimes DS, Quigley EM. Nonsteroidal anti-inflammatory drugs, proton pump inhibitors, and gastrointestinal injury: contrasting interactions in the stomach and small intestine. Mayo Clin Proc. 2014;89:1699-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 71. | Quigley EM. Gut bacteria in health and disease. Gastroenterol Hepatol (N Y). 2013;9:560-569. [PubMed] |
| 72. | Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146:1513-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 750] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 73. | Sanz Y, Santacruz A, De Palma G. Insights into the roles of gut microbes in obesity. Interdiscip Perspect Infect Dis. 2008;2008:829101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 74. | Jiang W, Wu N, Wang X, Chi Y, Zhang Y, Qiu X, Hu Y, Li J, Liu Y. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci Rep. 2015;5:8096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 473] [Cited by in RCA: 447] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 75. | Farhadi A, Gundlapalli S, Shaikh M, Frantzides C, Harrell L, Kwasny MM, Keshavarzian A. Susceptibility to gut leakiness: a possible mechanism for endotoxaemia in non-alcoholic steatohepatitis. Liver Int. 2008;28:1026-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 76. | Patel V, Sanyal AJ. Drug-induced steatohepatitis. Clin Liver Dis. 2013;17:533-546, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 77. | Benichou C, Danan G, Flahault A. Causality assessment of adverse reactions to drugs--II. An original model for validation of drug causality assessment methods: case reports with positive rechallenge. J Clin Epidemiol. 1993;46:1331-1336. [PubMed] |
| 78. | Danan G, Benichou C. Causality assessment of adverse reactions to drugs--I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323-1330. [PubMed] |
| 79. | Stravitz RT, Sanyal AJ. Drug-induced steatohepatitis. Clin Liver Dis. 2003;7:435-451. [PubMed] |
| 80. | Farrell GC. Drugs and steatohepatitis. Semin Liver Dis. 2002;22:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 149] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 81. | Geneve J, Hayat-Bonan B, Labbe G, Degott C, Letteron P, Freneaux E, Dinh TL, Larrey D, Pessayre D. Inhibition of mitochondrial beta-oxidation of fatty acids by pirprofen. Role in microvesicular steatosis due to this nonsteroidal anti-inflammatory drug. J Pharmacol Exp Ther. 1987;242:1133-1137. [PubMed] |
| 82. | Pessayre D, Fromenty B, Berson A, Robin MA, Lettéron P, Moreau R, Mansouri A. Central role of mitochondria in drug-induced liver injury. Drug Metab Rev. 2012;44:34-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 195] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 83. | Fromenty B, Pessayre D. Inhibition of mitochondrial beta-oxidation as a mechanism of hepatotoxicity. Pharmacol Ther. 1995;67:101-154. [PubMed] |
| 84. | Koek GH, Liedorp PR, Bast A. The role of oxidative stress in non-alcoholic steatohepatitis. Clin Chim Acta. 2011;412:1297-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 244] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 85. | Hagiwara M, Kataoka K, Arimochi H, Kuwahara T, Ohnishi Y. Role of unbalanced growth of gram-negative bacteria in ileal ulcer formation in rats treated with a nonsteroidal anti-inflammatory drug. J Med Invest. 2004;51:43-51. [PubMed] |
| 86. | Park SC, Chun HJ, Kang CD, Sul D. Prevention and management of non-steroidal anti-inflammatory drugs-induced small intestinal injury. World J Gastroenterol. 2011;17:4647-4653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 87. | Blackler R, Syer S, Bolla M, Ongini E, Wallace JL. Gastrointestinal-sparing effects of novel NSAIDs in rats with compromised mucosal defence. PLoS One. 2012;7:e35196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 88. | Rigas B, Kashfi K. Cancer prevention: a new era beyond cyclooxygenase-2. J Pharmacol Exp Ther. 2005;314:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |