Published online Jun 7, 2017. doi: 10.3748/wjg.v23.i21.3876
Peer-review started: February 8, 2017
First decision: March 3, 2017
Revised: March 13, 2017
Accepted: April 12, 2017
Article in press: April 12, 2017
Published online: June 7, 2017
Processing time: 121 Days and 11.4 Hours
To evaluate the relationship between serum adenosine deaminase (ADA) levels and histological features in patients with autoimmune hepatitis (AIH).
A total of 80 subjects (52 AIH cases and 28 healthy controls) were included in the study. Patients were diagnosed according to the simplified criteria suggested by the International Autoimmune Hepatitis Group. All of the cases had been diagnosed with AIH between 2010-2015 at Hacettepe University, Department of Gastroenterology. Serum blood samples were collected and stored at -80 °C until the biochemical estimation of ADA activity. The diagnosis of patients was confirmed by liver biopsy. Serum ADA > 20 U/L was considered to be high level.
Mean serum ADA levels were significantly higher in AIH patients than those in healthy controls (25.4 ± 9.6 U/L vs 12.8 ± 2.2 U/L, P < 0.001). Serum ADA levels > 20 U/L were found in 63.5% AIH patients and in 0% healthy controls (P < 0.001). Mean serum ADA levels were significantly increased in each stage of histological activity: 15.2 ± 3.5 U/L for patients with mild interface hepatitis, 23.1 ± 10.0 U/L for patients with moderate interface hepatitis and 30.9 ± 7.0 U/L for patients with severe interface hepatitis (P < 0.001). Correlation analysis showed that there was a positive association between serum ADA levels and histological activity (r = 0.71, P < 0.001). Receiver operating characteristic analysis suggested that 24.5 U/L was the optimum cut-off point of ADA level for severe interface hepatitis (sensitivity 88%, specificity 85.2%, area under the curve: 0.88).
Because of the positive correlation with inflammatory activity, serum ADA level may be a potential biomarker for predicting or monitoring histological activity in patients with AIH.
Core tip: Autoimmune hepatitis (AIH) is a chronic liver disease which can cause cirrhosis. Liver biopsy is still used as the gold standard in determining grade of fibrosis and disease activity in AIH. However, it is an invasive and difficult-to-repeat method, with some minor and major complications. In this study, we aimed to compare AIH patients’ serum adenosine deaminase (ADA) level with histopathological features of liver biopsy. To our knowledge, this is the first study investigating serum ADA activity in AIH. We showed that ADA level was a useful laboratory parameter that reflected histological activity in AIH.
- Citation: Torgutalp M, Efe C, Babaoglu H, Kav T. Relationship between serum adenosine deaminase levels and liver histology in autoimmune hepatitis. World J Gastroenterol 2017; 23(21): 3876-3882
- URL: https://www.wjgnet.com/1007-9327/full/v23/i21/3876.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i21.3876
Autoimmune hepatitis (AIH) is a chronic inflammatory liver disease that can lead to end-stage liver disease, cirrhosis and death[1]. The International Autoimmune Hepatitis Group (IAIHG) suggested simplified criteria - hyperglobulinemia, presence of circulating autoantibodies, interface hepatitis on histological examination and absence of viral hepatitis - for diagnosis of AIH[2,3].
The etiology and pathophysiology of AIH is not well understood. It is believed that genetic factors, environmental factors and immune dysregulation act together by inducing autoreactive B and T cell responses against autoantigens, causing hepatocyte destruction and inflammation[4,5].
Adenosine deaminase (ADA), an enzyme involved in the catabolism of purine bases, catalyzes the irreversible conversion of (deoxy) adenosine to (deoxy) inosine[6]. ADA may be present in various tissues and body fluids but its major function is in lymphoid tissues, where it is essential for lymphocytic proliferation and differentiation[7]. Adenosine is an endogenous anti-inflammatory molecule which is decreased in inflammatory conditions with increased ADA activity[8]. Increased ADA activity has been shown in various autoimmune diseases, such as systemic lupus erythematosus, rheumatoid arthritis and Crohn’s disease[9-11]. Some studies have suggested that ADA levels are associated with disease activation in viral hepatitis[12,13].
In this study, we aimed to compare the AIH patients’ serum ADA level with histopathological features of liver biopsy. To our knowledge, this is the first study investigating serum ADA activity in AIH and its results will provide a useful tool for monitoring of clinical status of AIH patients.
The study was carried out between 2010-2015 and involved 52 newly diagnosed AIH patients who were admitted to the Hacettepe University, Department of Gastroenterology. Patients were excluded if they had concomitant hepatitis B virus (HBV), hepatitis C virus (HCV) or human immunodeficiency virus infections, primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC), excessive alcohol consumption, decompensated cirrhosis, hemochromatosis, Wilson’s disease, α1-anti trypsin deficiency, hepatic or extra-hepatic malignancies, inflammatory bowel diseases and other autoimmune diseases. Patients who had infections that alter serum ADA levels, such as tuberculosis and brucellosis, were also excluded. Clinical characteristics of patients were recorded at the time of AIH diagnosis. AIH was diagnosed according to the simplified criteria suggested by the IAIHG[3]. The diagnosis of patients was confirmed by liver biopsy. After collection of the serum samples, the patients were treated with prednisone (30-60 mg/d) alone or in combination with azathioprine (50-100 mg/d). Biochemical remission was defined as the normalization of aminotransferase and IgG levels while receiving treatment, relapse was defined as a rise in aminotransferases of more than 3-fold the upper limit of normal after a response or remission.
We included age- and sex-matched healthy subjects, who did not have evidence of liver diseases or other chronic disorders. Neither patients nor controls had the diagnosis or sign of tuberculosis.
Antinuclear antibodies (ANA), smooth muscle antibody (SMA) and liver-kidney microsomal antibodies (LKM-1) were detected according to standard autoantibody testing procedures and laboratory standards. An indirect immunofluorescence titer of 1:40 or higher for ANA, SMA and LKM-1 was considered to be positive.
Fasting serum blood samples were collected from peripheral vein and were transferred into anticoagulant-free tubes. Tubes were centrifuged at 3500 rpm for 10 min and stored at -80 °C until the biochemical estimation of ADA activity. Serum ADA levels were measured with commercially available ADA Quantitative UV Assay Kit (Ben S.r.l. Biochemical Enterprise, Milano, Italy) on the COBAS MIRA (Roche Diagnostics, Basel, Switzerland) spectrophotometric chemistry analyzer. Serum ADA values between 5-20 U/L were considered to be within the normal range according to the manufacturer’s instructions.
The METAVİR score was used for staging liver fibrosis on a five (0-4)-point scale, where F0 = no fibrosis; F1 = portal fibrosis without septa; F2 = few septa; F3 = numerous septa without cirrhosis; F4 = cirrhosis[14]. Presence of lymphocytic piecemeal necrosis was graded as mild (necrosis around 1-2 portal tracts), moderate (necrosis at the periphery of about half of the portal tracts) and severe (necrosis surrounding more than half of the circumference of almost all portal tracts). The typical and compatible histology for AIH was defined according to the IAIHG criteria[3].
Statistical analyses were performed using the SPSS software version 21 (SPSS, Chicago, IL, United States). The variables were investigated using visual (histograms, probability plots) and analytical methods (Kolmogorov-Smirnov/Shapiro Wilk’s test) to determine whether or not they are normally distributed. Descriptive analyses were presented using means and standard deviations for normally distributed variables and using medians and interquartile range (IQR) for the non-normally distributed and ordinal variables. The χ2 test or Fisher’s exact test, where appropriate, was used to compare proportions in different groups. Since the age and ADA levels were normally distributed, Student’s t-test was used to compare between patients/control groups and in the interface hepatitis levels. The Mann-Whitney U test was used to compare non-normally distributed variables. One-way ANOVA was used to compare ADA levels among the interface hepatitis (mild, moderate, severe) groups. Levene’s test was used to assess the homogeneity of variances.
Spearman’s rank correlation coefficients were used to evaluate the correlations between serum ADA levels and the inflammatory level of AIH. The capacity ADA level in predicting presence of inflammation was analyzed using receiver operating characteristic (ROC) curve analysis. When a significant cut-off value was observed, the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were presented. A P value of less than 0.05 was considered to show a statistically significant result.
The baseline features of AIH patients are presented in Table 1. A total of 52 patients (37 women, 15 men), with a mean age of 40.1 ± 12.8, and 28 healthy controls, with a mean age of 45.1 ± 10.7, were included in this study. Nine patients (17.3%) had mild interface hepatitis, 18 (34.6%) had moderate interface hepatitis and 25 (48.1%) had severe interface hepatitis. The fibrosis stages were F0 in 6 (11.5%) patients, F1 in 9 (17.3%), F2 in 11 (21.2%), F3 in 16 (30.8%) and F4 in 10 (19.2%).
| Parameter | AIH (n = 52) |
| Mean age in yr | 40.1 ± 12.8 |
| Sex as female/male | 37 (71.2)/15 (28.8) |
| Laboratory findings | |
| ALT as × ULN | 7.0 (IQR = 9.3) |
| AST as × ULN | 6.2 (IQR = 9.1) |
| ALP as × ULN | 1.3 (IQR = 1.7) |
| Total bilirubin as × ULN | 0.9 (IQR = 1.6) |
| Albumin in gr/dL | 3.8 (IQR = 0.8) |
| Serum IgG in gr/dL | 2035 (IQR = 840) |
| Platelet counts as × 103/mm3 | 219.0 (IQR = 66.8) |
| INR | 1.1 (IQR = 0.2) |
| Autoimmune serology | |
| ANA | 38 (73.1) |
| SMA | 20 (38.5) |
| Anti LKM-1 | 1 (2) |
| Histological features | |
| İnterface hepatitis | |
| Mild | 9 (17.3) |
| Moderate | 18 (34.6) |
| Severe | 25 (48.1) |
| Fibrosis score | |
| 0 | 6 (11.5) |
| 1 | 9 (17.3) |
| 2 | 11 (21.2) |
| 3 | 16 (30.8) |
| 4 | 10 (19.2) |
Table 2 shows the comparative characteristics of AIH patients and the healthy controls. Age and sex were similar in both groups. The mean serum ADA level was significantly higher in AIH patients than in controls (25.4 ± 9.6 U/L vs 12.8 ± 2.2 U/L, P < 0.001). A high serum ADA level (> 20 U/L) was found in 33 (63.5%) patients with AIH; however, there was no value greater than 20 U/L detected in any of the healthy controls.
| AIH (n = 52) | Controls (n = 28) | P value | |
| Age in years | 40.1 ± 12.8 | 45.1 ± 10.7 | 0.080 |
| Sex, F/M as n (%) | 37 (71.2)/15 (28.8) | 24 (85.7)/4 (14.3) | 0.144 |
| ADA level in U/L | 25.4 ± 9.6 | 12.8 ± 2.2 | < 0.001 |
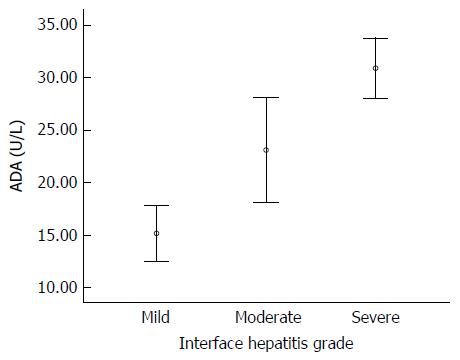
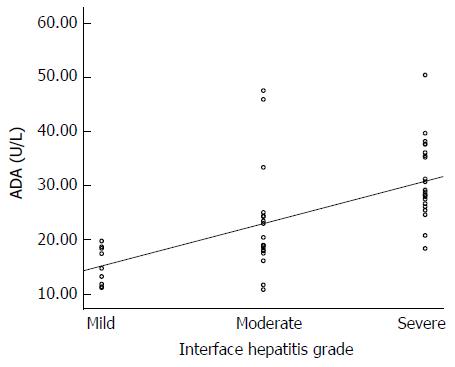
A positive correlation was observed between serum ADA levels and inflammatory activity. The mean serum ADA levels increased for each step of inflammatory activity. ADA levels for different inflammatory activity grades are shown in Figure 1. In patients with mild interface hepatitis, the mean serum ADA was 15.2 ± 3.5 U/L; in patients with moderate interface hepatitis, the mean serum ADA was 23.1 ± 10.0 and in patients with severe interface hepatitis, the mean serum ADA was 30.9 ± 7.0. Age and sex were not related to interface hepatitis score but ALT and IgG were associated with interface hepatitis score like serum ADA level (Table 3). The differences were statistically significant between interface hepatitis grades: mild vs moderate (P = 0.014), moderate vs severe (P < 0.001). Spearman’s correlation analysis showed that significant association between serum ADA levels and inflammatory activity (r = 0.71, P < 0.001) (Figure 2).
| Interface hepatitis | P value | |||
| Mild (n = 9) | Moderate (n = 18) | Severe (n = 25) | ||
| Age in years | 39.3 ± 14.5 | 41.1 ± 11.0 | 39.7 ± 13.9 | 0.927 |
| Sex, F/M | 6-3 | 12-6 | 6-19 | 0.759 |
| ALT as × ULN | 3.9 (IQR = 1.6) | 7.3 (IQR = 11.3) | 10.9 (IQR = 12) | 0.0321 |
| Serum IgG in gr/dL | 1810 (IQR = 745) | 1955 (IQR = 570) | 2390 (IQR = 1205) | 0.0291 |
| ADA in U/L | 15.2 ± 3.5 | 23.1 ± 10.0 | 30.9 ± 7.0 | < 0.0011 |
| ADA > 20 U/L, n (%) | 0 | 9 (50) | 24 (96) | < 0.0011 |
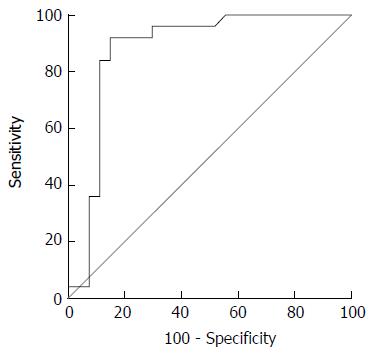
Optimal ADA cut-off level for interface hepatitis is shown in Table 4. A 24.55 U/L cut-off point had 88% sensitivity, 85.2% specificity, 84.6% NPV and 88.5% PPV. A cut-off point of 25.25 U/L had 84% sensitivity, 88.9% specificity, 87.5% NPV, 85.7% PPV and area under the curve (AUC) of 0.88 (95%CI: 0.77-0.99) (Figure 3).
The mean serum ADA level was 17.4 ± 5 U/L in patients with fibrosis score 0 (F0), 21.8 ± 8.7 U/L in patients with F1, 28.5 ± 9.5 U/L in patients with F2, 27.3 ± 9 U/L in patients with F3 and 25.5±9.6 U/L with patients with F4. There was no statistically significant difference between these groups (P = 0.102). Spearman’s correlation analysis also did not show an association between serum ADA levels and liver fibrosis (r = 0.22, P = 0.123).
Liver inflammation is clinically relevant, as it is an important factor that predicts therapy response and long-term outcome of patients with chronic liver diseases. AIH is a rare disease and its estimated prevalence is lower than for many other chronic liver diseases. However, recent studies reported that the incidence of AIH is increasing and that AIH is associated with a high morbidity and mortality[15,16]. These results suggest that better treatment options and new biomarkers or radiological methods capable of predicting disease progression are necessary in AIH.
Liver biopsy is still used as the gold standard in determining grade of fibrosis and disease activity in liver diseases. However, it is an invasive and difficult-to-repeat method, with some minor and major complications. Several non-invasive laboratory and radiological methods have been developed to assess the stage of fibrosis in liver diseases. The performance of these non-invasive methods has been studied in HCV, HBV, non-alcoholic steatohepatitis, PBC, PSC and other liver diseases. Nevertheless, efficacy of these methods in AIH was confined to a few small-sized studies.
Normalization of serum aminotransferase and IgG levels are currently described as the biochemical remission. A recent study reported that AIH patients with long-term biochemical remission had signs of inflammation on liver biopsy, and it was shown to be associated with increased liver disease-related mortality and morbidity[17]. The results of this study strongly suggest the need for further laboratory parameters that reveal liver inflammation in AIH. In the study conducted by Gutkowski et al[18], authors proposed a new laboratory parameter-based model that showed degree of inflammation in AIH, having a high sensitivity and specificity. Similarly, we showed that ADA can predict severe inflammation with considerably high sensitivity and specificity.
Several studies reported that AIH pathogenesis might involve excessive proliferation of cytotoxic T cells by defective T cell activation or impaired number and function of regulatory T cells (commonly known as Tregs)[19-21]. ADA, an enzyme involved in purine metabolism, plays an important role in growth and differentiation of lymphocytes and macrophages[22]. Since the activity of ADA is elevated during T cell response to antigenic stimulus, this enzyme is considered as a non-specific marker of T lymphocyte-mediated cellular immunity[23]. This association appears to imply the fact that serum ADA level may determine T cell activity and severity of inflammation in AIH.
ADA level was previously shown to predict severity of disease in hepatitis B and C[12,13]. In our study, we demonstrated that serum ADA level was markedly elevated at every grade of hepatic inflammation. Furthermore, ADA levels had an 85% sensitivity and 88% specificity for severe inflammation in AIH.
Association between ADA levels and disease activity was also investigated in patients with inflammatory bowel diseases. While some studies reported an association between serum ADA levels and disease activation both in ulcerative colitis and Crohn’s disease[11,24], some others found no relation of serum ADA levels and disease activity in Crohn’s disease[25]. Similarly, it has been shown that ADA levels are associated with disease activation in patients with other autoimmune diseases, such as systemic lupus erythematosus, juvenile idiopathic arthritis, rheumatoid arthritis and Still’s disease[9,10,26,27]. Multiple and complex mechanisms are involved in the pathogenesis of immune-mediated disorders, and this process does not seem to be disease specific. Therefore, performance of ADA levels in predicting disease activation may vary among patients with organ or non-organ specific immune-mediated disease.
Our study has several limitations. First, it is not a prospective study and the value of sequential ADA measurements for predicting subsequent complications of AIH needs to be evaluated in prospective studies with long-term follow up. Secondly, small sample size precludes detecting the best optimal cut-off values of ADA in determining interface hepatitis. Considering the low prevalence of AIH, we believe our study to constitute a base for future large-sized studies.
In conclusion, we showed that ADA level was a laboratory parameter that reflected histological activity in AIH. Owing to its easy-to-repeat nature and cheaper cost, it may be used as a non-invasive marker for monitoring liver inflammation in AIH. Larger-sized prospective trials are warranted to show the extent of prognostic importance of ADA in AIH.
Autoimmune hepatitis (AIH) is a chronic inflammatory liver disease that can lead to end-stage liver disease, cirrhosis and death. Adenosine deaminase (ADA) is an enzyme involved in the catabolism of purine bases. Adenosine is an endogenous anti-inflammatory molecule that is decreased in inflammatory conditions with increased ADA activity. Liver biopsy is still used as the gold standard in determining grade of fibrosis and disease activity in AIH. However, it is an invasive and difficult-to-repeat method with some minor and major complications. In this study, the authors aimed to compare the AIH patients’ serum ADA level with histopathological features of liver biopsy.
The result of this study contributes to using serum ADA level as a non-invasive marker for monitoring liver inflammation in AIH.
In this study, ADA was a useful tool for reflecting histological activity in AIH. The concordance rate between ADA and inflammation on liver biopsy was 71%. To our knowledge, this is the first study investigating serum ADA activity in AIH. Owing to its easy-to-repeat nature and cheaper cost, ADA may be used as a non-invasive marker for monitoring liver inflammation in AIH.
This study suggests that serum ADA level is a useful parameter for predicting inflammation in AIH. During the follow-up period, ADA can be used instead of liver biopsy for determining inflammation in AIH patients.
ADA is a laboratory parameter that is measured from serum. Because of its easy-to-repeat nature and cheaper cost, it may be used as a non-invasive marker for monitoring liver inflammation in AIH.
The author of this paper evaluated the efficacy of serum ADA level for predicting inflammation in AIH. A promising correlation between serum ADA levels and liver biopsy was found. Larger-sized prospective trials are warranted to show the extent of prognostic importance of ADA in AIH.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Rodrigues AT, Uyanik M S- Editor: Yu J L- Editor: Filipodia E- Editor: Zhang FF
| 1. | Krawitt EL. Autoimmune hepatitis. N Engl J Med. 2006;354:54-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 604] [Article Influence: 31.8] [Reference Citation Analysis (1)] |
| 2. | Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, Chapman RW, Cooksley WG, Czaja AJ, Desmet VJ. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929-938. [PubMed] |
| 3. | Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, Bittencourt PL, Porta G, Boberg KM, Hofer H. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1205] [Cited by in RCA: 1252] [Article Influence: 73.6] [Reference Citation Analysis (0)] |
| 4. | Liberal R, Longhi MS, Mieli-Vergani G, Vergani D. Pathogenesis of autoimmune hepatitis. Best Pract Res Clin Gastroenterol. 2011;25:653-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Efe C, Kav T, Aydin C, Cengiz M, Imga NN, Purnak T, Smyk DS, Torgutalp M, Turhan T, Ozenirler S. Low serum vitamin D levels are associated with severe histological features and poor response to therapy in patients with autoimmune hepatitis. Dig Dis Sci. 2014;59:3035-3042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Sauer AV, Brigida I, Carriglio N, Aiuti A. Autoimmune dysregulation and purine metabolism in adenosine deaminase deficiency. Front Immunol. 2012;3:265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Antonioli L, Colucci R, La Motta C, Tuccori M, Awwad O, Da Settimo F, Blandizzi C, Fornai M. Adenosine deaminase in the modulation of immune system and its potential as a novel target for treatment of inflammatory disorders. Curr Drug Targets. 2012;13:842-862. [PubMed] |
| 8. | Cronstein BN. Adenosine, an endogenous anti-inflammatory agent. J Appl Physiol (1985). 1994;76:5-13. [PubMed] |
| 9. | Saghiri R, Ghashghai N, Movaseghi S, Poursharifi P, Jalilfar S, Bidhendi MA, Ghazizadeh L, Ebrahimi-Rad M. Serum adenosine deaminase activity in patients with systemic lupus erythematosus: a study based on ADA1 and ADA2 isoenzymes pattern. Rheumatol Int. 2012;32:1633-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Hitoglou S, Hatzistilianou M, Gougoustamou D, Athanassiadou F, Kotsis A, Catriu D. Adenosine deaminase activity and its isoenzyme pattern in patients with juvenile rheumatoid arthritis and systemic lupus erythematosus. Clin Rheumatol. 2001;20:411-416. [PubMed] |
| 11. | Maor I, Rainis T, Lanir A, Lavy A. Adenosine deaminase activity in patients with Crohn’s disease: distinction between active and nonactive disease. Eur J Gastroenterol Hepatol. 2011;23:598-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Kaya S, Cetin ES, Aridogan BC, Arikan S, Demirci M. Adenosine deaminase activity in serum of patients with hepatitis -- a useful tool in monitoring clinical status. J Microbiol Immunol Infect. 2007;40:288-292. [PubMed] |
| 13. | Umaramani G, Sameera K, Suryanarayana D. Evaluation of Serum Adenosine Deaminase Activity in Viral Hepatitis. Int J Biol Med Res. 2012;3:2017-2019. |
| 14. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 3081] [Article Influence: 106.2] [Reference Citation Analysis (0)] |
| 15. | Hoeroldt B, McFarlane E, Dube A, Basumani P, Karajeh M, Campbell MJ, Gleeson D. Long-term outcomes of patients with autoimmune hepatitis managed at a nontransplant center. Gastroenterology. 2011;140:1980-1989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 159] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 16. | Grønbæk L, Vilstrup H, Jepsen P. Autoimmune hepatitis in Denmark: incidence, prevalence, prognosis, and causes of death. A nationwide registry-based cohort study. J Hepatol. 2014;60:612-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 246] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 17. | Dhaliwal HK, Hoeroldt BS, Dube AK, McFarlane E, Underwood JC, Karajeh MA, Gleeson D. Long-Term Prognostic Significance of Persisting Histological Activity Despite Biochemical Remission in Autoimmune Hepatitis. Am J Gastroenterol. 2015;110:993-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 18. | Gutkowski K, Hartleb M, Kacperek-Hartleb T, Kajor M, Mazur W, Zych W, Walewska-Zielecka B, Habior A, Sobolewski M. Laboratory-based scoring system for prediction of hepatic inflammatory activity in patients with autoimmune hepatitis. Liver Int. 2013;33:1370-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Longhi MS, Hussain MJ, Mitry RR, Arora SK, Mieli-Vergani G, Vergani D, Ma Y. Functional study of CD4+CD25+ regulatory T cells in health and autoimmune hepatitis. J Immunol. 2006;176:4484-4491. [PubMed] |
| 20. | Peiseler M, Sebode M, Franke B, Wortmann F, Schwinge D, Quaas A, Baron U, Olek S, Wiegard C, Lohse AW. FOXP3+ regulatory T cells in autoimmune hepatitis are fully functional and not reduced in frequency. J Hepatol. 2012;57:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 21. | Czaja AJ. Autoantibodies in autoimmune liver disease. Adv Clin Chem. 2005;40:127-164. [PubMed] |
| 22. | Fröde TS, Medeiros YS. Myeloperoxidase and adenosine-deaminase levels in the pleural fluid leakage induced by carrageenan in the mouse model of pleurisy. Mediators Inflamm. 2001;10:223-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Cristalli G, Costanzi S, Lambertucci C, Lupidi G, Vittori S, Volpini R, Camaioni E. Adenosine deaminase: functional implications and different classes of inhibitors. Med Res Rev. 2001;21:105-128. [PubMed] |
| 24. | Beyazit Y, Koklu S, Tas A, Purnak T, Sayilir A, Kurt M, Turhan T, Celik T, Suvak B, Torun S. Serum adenosine deaminase activity as a predictor of disease severity in ulcerative colitis. J Crohns Colitis. 2012;6:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Sajjadi M, Gholamrezaei A, Daryani NE. No association between serum adenosine deaminase activity and disease activity in Crohn’s disease. Dig Dis Sci. 2015;60:1755-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Sari RA, Taysi S, Yilmaz O, Bakan N. Correlation of serum levels of adenosine deaminase activity and its isoenzymes with disease activity in rheumatoid arthritis. Clin Exp Rheumatol. 2003;21:87-90. [PubMed] |
| 27. | Xun C, Zhao Y, Hu ZJ. Potential role of adenosine deaminase in the diagnosis of adult-onset Still’s disease. Rheumatol Int. 2012;33:1255-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |