Published online Jun 7, 2017. doi: 10.3748/wjg.v23.i21.3839
Peer-review started: December 6, 2016
First decision: January 19, 2017
Revised: February 9, 2017
Accepted: March 2, 2017
Article in press: March 2, 2017
Published online: June 7, 2017
Processing time: 187 Days and 8.2 Hours
To evaluate the effect of oxymatrine (OMT) on hepatocyte apoptosis in rats with lipopolysaccharide (LPS)/D-galactosamine (D-GalN)-induced acute liver failure (ALF).
LPS/D-GalN was used to establish a model of ALF in rats. To evaluate the effect of OMT, we assessed apoptosis by transmission electron microscopy, and the pathological changes in the liver by light microscopy with hematoxylin and eosin staining. An automated biochemical analyzer was used to measure serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Enzyme-linked immunosorbent assay was used to determine the levels of tumor necrosis factor (TNF)-α and interleukin (IL)-1β. Western blotting was used to detect protein levels in liver tissues. Streptavidin peroxidase immunohistochemistry was used to observe expression of Toll-like receptor (TLR)4, active caspase-3, Bax and Bcl-2.
All rats in the normal control and OMT-pretreated groups survived. The mortality rate in the model group was 30%. OMT preconditioning down-regulated apoptosis of hepatocytes and ameliorated pathological changes in liver tissue. The levels of AST, ALT, TNF-α and IL-1β in the model group increased significantly, and were significantly reduced by OMT pretreatment. OMT pretreatment down-regulated expression of TLR4 and active caspase-3 and the Bax/Bcl-2 ratio, and up-regulated expression of P-AktSer473 (Akt phosphorylated at serine 473) and P-GSK3βSer9 (glycogen synthase kinase 3β phosphorylated at serine 9) induced by LPS/D-GalN.
OMT inhibits hepatocyte apoptosis by suppressing the TLR4/PI3K/Akt/GSK-3β signaling pathway, which suggests that OMT is an effective candidate for ameliorating acute liver failure.
Core tip: The role of oxymatrine (OMT) in inhibiting apoptosis in acute liver failure (ALF) was investigated. OMT pretreatment protected liver cells by improving the liver pathological change and reducing serum aminotransferase in lipopolysaccharide/D-galactosamine-induced ALF in rats. OMT preconditioning down-regulated apoptosis of hepatocytes and ameliorated pathological changes in liver tissue. The levels of alanine aminotransferase, aspartate aminotransferase, tumor necrosis factor-α and interleukin-1β in the model group increased significantly, and were significantly reduced by OMT pretreatment. OMT pretreatment down-regulated expression of Toll-like receptor (TLR)4 and active caspase-3 and the Bax/Bcl-2 ratio, and up-regulated expression of P-AKTSer473 and P-GSK3βSer9. OMT could inhibit hepatocyte apoptosis through the TLR4/PI3K/Akt/GSK-3β signaling pathway.
- Citation: Zhang X, Jiang W, Zhou AL, Zhao M, Jiang DR. Inhibitory effect of oxymatrine on hepatocyte apoptosis via TLR4/PI3K/Akt/GSK-3β signaling pathway. World J Gastroenterol 2017; 23(21): 3839-3849
- URL: https://www.wjgnet.com/1007-9327/full/v23/i21/3839.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i21.3839
Acute liver failure (ALF) is a destructive clinical syndrome with high mortality, which is caused by the acute loss of function and viability of a majority of hepatocytes[1,2]. ALF occurs when the extent of hepatocyte death exceeds the regenerative capacity of the liver. Hepatocyte death can occur via distinct biochemical pathways and morphological alterations, including apoptosis, autophagic cell death and necrosis. Apoptosis is the first cellular response of the liver to viruses, drugs, alcohol, toxins and ischemic injury, and is followed by necrosis[3]. Massive hepatic apoptosis and necrosis is a key mechanism underlying ALF[4,5].
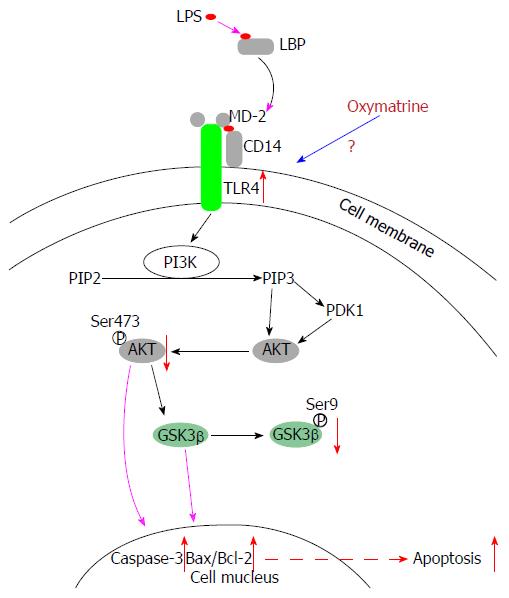
Toll-like receptor (TLR) 4 mediates multiple signaling pathways, and its expression is increased in liver injury and ALF[6]. The phosphatidylinositol-3-kinase (PI3K)/Akt signaling pathway leads to reduced apoptosis, stimulates cell growth, regulates glucose metabolism and promotes cell proliferation[7]. Under normal conditions, PI3K/Akt activation is tightly controlled and dependent on both extracellular growth signals and the availability of amino acids and glucose. Four principal types of sensors control PI3K/Akt pathway activation. Through appropriate binding, these sensors activate downstream kinases in the PI3K family, including Akt. Phosphorylated Akt (P-Akt) activates a multitude of downstream targets. Through its numerous substrates, Akt mediates signals leading to cell growth, cell differentiation and angiogenesis, and prevents apoptosis[8]. We have previously confirmed that the TLR4/PI3K/Akt/GSK-3β pathway participates in the regulation of apoptosis in BRL-3A cells[9] (Figure 1).
Oxymatrine (OMT) is a quinolizidine alkaloid extracted from the Chinese herb Sophora flavescens Ait, which possesses antitumor, antioxidant, anti-inflammatory, anti-allergic, antiviral, antifibrotic and anti-apoptotic activities. It has been used for the treatment of some inflammatory diseases. However, it has not been reported that OMT inhibits the apoptosis of liver cells through the TLR4/PI3K/Akt/GSK-3β signaling pathway.
In this study, we evaluated the therapeutic effect of OMT on lipopolysaccharide (LPS)/D-galactosamine (D-GalN)-induced ALF in rats, thus exploring whether OMT can inhibit hepatocyte apoptosis via the TLR4/PI3K/Akt/GSK-3β signaling pathway.
OMT (98% purity) was purchased from Shaanxi Huike Botanical Development Co. Ltd. (Shaanxi, China). LPS and D-GalN were obtained from Sigma-Aldrich (St. Louis, MO, United States). Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide were obtained from Biouniquer Technology Co., Ltd. (Nanjing, China). Antibodies against TLR4, Akt, P-Akt, glycogen synthase kinase (GSK)-3β, phosphorylated GSK-3β (P-GSK-3β), active caspase-3, Bax and Bcl-2 were obtained from Cell Signaling Technology (Beverly, MA, United States).
Animals were maintained in a temperature-controlled (18-22 °C) and humidity-controlled (50%-70%) environment with a 12 h light/dark cycle. They were adapted to the environment for 1 wk. One hundred male Sprague-Dawley rats weighing 220-250 g were randomly assigned to five groups: normal control group, model group, OMT low-dose group, OMT middle-dose group, and OMT high-dose group. Rats in the OMT low-dose group, OMT middle-dose group and OMT high-dose group were injected intraperitoneally with OMT at 30, 60 and 120 mg/kg once daily, respectively, for 3 consecutive days before creating the ALF model. The normal control and model groups received an equivalent volume of saline. The rats were fasted for 24 h and injected (except for controls) intraperitoneally with D-GalN (700 mg/kg) and LPS (10 μg/kg) dissolved in saline. Rats were anesthetized with ketamine and killed by decapitation at 24 h after LPS/GalN injection. Blood and liver samples were collected for further assessment.
Liver tissues were fixed in paraformaldehyde for 24-48 h, dehydrated in ethanol, treated in xylene, embedded in paraffin, and cut into 4-μm sections, followed by hematoxylin and eosin (HE) staining. After mounting, sections were observed under a light microscope and pathological examination was conducted by two experienced pathologists. Consensus was obtained between two pathologists.
Liver tissues were fixed in 2.5% glutaraldehyde solution for 2 h, washed in 0.1 mol/L phosphate buffer, fixed in 1% osmium tetroxide for 2-3 h, dehydrated in ethanol, embedded and cut into 50-60-nm sections. The sections were stained with 3% uranyl acetate and lead citrate double staining. Sections were observed under a transmission electron microscope.
Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were determined by biochemical method using an automatic biochemical analyzer (Olympus, Tokyo, Japan). This quantitative test was conducted in the Department of Biochemistry at the Affiliated Hospital of Nantong University.
We detected expression of tumor necrosis factor (TNF)-α and interleukin (IL)-1β using enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer’s instructions (RapidBio, Palo Alto, CA, United States), drew the standard curve, and calculated the concentration of unknown samples.
Liver tissue was made into a single cell suspension. Cells were suspended in annexin V binding buffer and stained with annexin V-FITC and propidium iodide. Ten thousand cells were collected for each sample. The stained cells were analyzed within 1 h using flow cytometry (BD Biosciences, San Jose, CA, United States).
Equal amounts of protein were extracted from each group and separated by 8%-12% SDS-PAGE, then transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA, United States). The membranes were blocked with 5% skimmed milk in 1 × Tris-buffered saline/Tween 20 (TBST) for 2 h at room temperature and incubated overnight at 4 °C with corresponding primary antibodies against TLR4 (1:1000), AKT (1:1000), P-AKT (1:1000), GSK-3β (1:1000), P-GSK-3β (1:1000), active caspase-3 (1:1000), Bax (1:1000) and Bcl-2 (1:1000). The membranes were washed three times with 1 × TBST, followed by incubation with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (1:10000; Bioworld, Minneapolis, MN, United States) for 2 h and visualized with the Enhanced Chemiluminescence (ECL) Detection Kit (Millipore, New York, NY, United States). The optical densities (ODs) of the protein bands were analyzed with a ChemiScope (Bio-Rad, Hercules, CA, United States) analysis program.
Immunohistochemistry was performed to detect TLR4, active caspase-3, Bax and Bcl-2 in the liver. Liver sections were heated for 2 d, deparaffinized and dehydrated. After antigen retrieval, sections were incubated with 3% H2O2 for 10 min and blocked in goat serum, then incubated with antibodies to TLR4 (1:100), active caspase-3 (1:300), Bax (1:100) and Bcl-2 (1:100) antibody at 4 °C overnight. After incubation with HRP-conjugated goat anti-rabbit IgG (1:200) at room temperature for 30 min, anti-protein-peroxidase solution was added, and visualized with diaminobenzidine. Sections were observed under a light microscope. Ten fields were randomly selected from each section. TLR4-positive cells showed tan cytoplasm. Active-caspase-3-positive cells showed tan nucleus and cytoplasm. Bax- and Bcl-2-positive cells showed brown granules in the cytoplasm and cell membrane. Image Pro-Plus 6.0 was used for the detection of OD, as a marker of TLR4, active-caspase-3, Bax and Bcl-2 expression.
The results were analyzed with SPSS version 18.0 (SPSS Inc., Chicago, IL, United States). Data are presented as mean ± SEM. Statistical significance of differences between groups was evaluated by one-way analysis of variance with Tukey’s multiple comparison test. P < 0.05 was considered statistically significant.
All rats in the normal control and OMT-pretreated groups survived. The mortality rate in the model group was 30%. Gross observation of the liver in the model group showed marked swelling of liver tissue, diffuse hemorrhage and ecchymosis on the dark red liver surface. OMT preconditioning reduced the severity of liver injury.
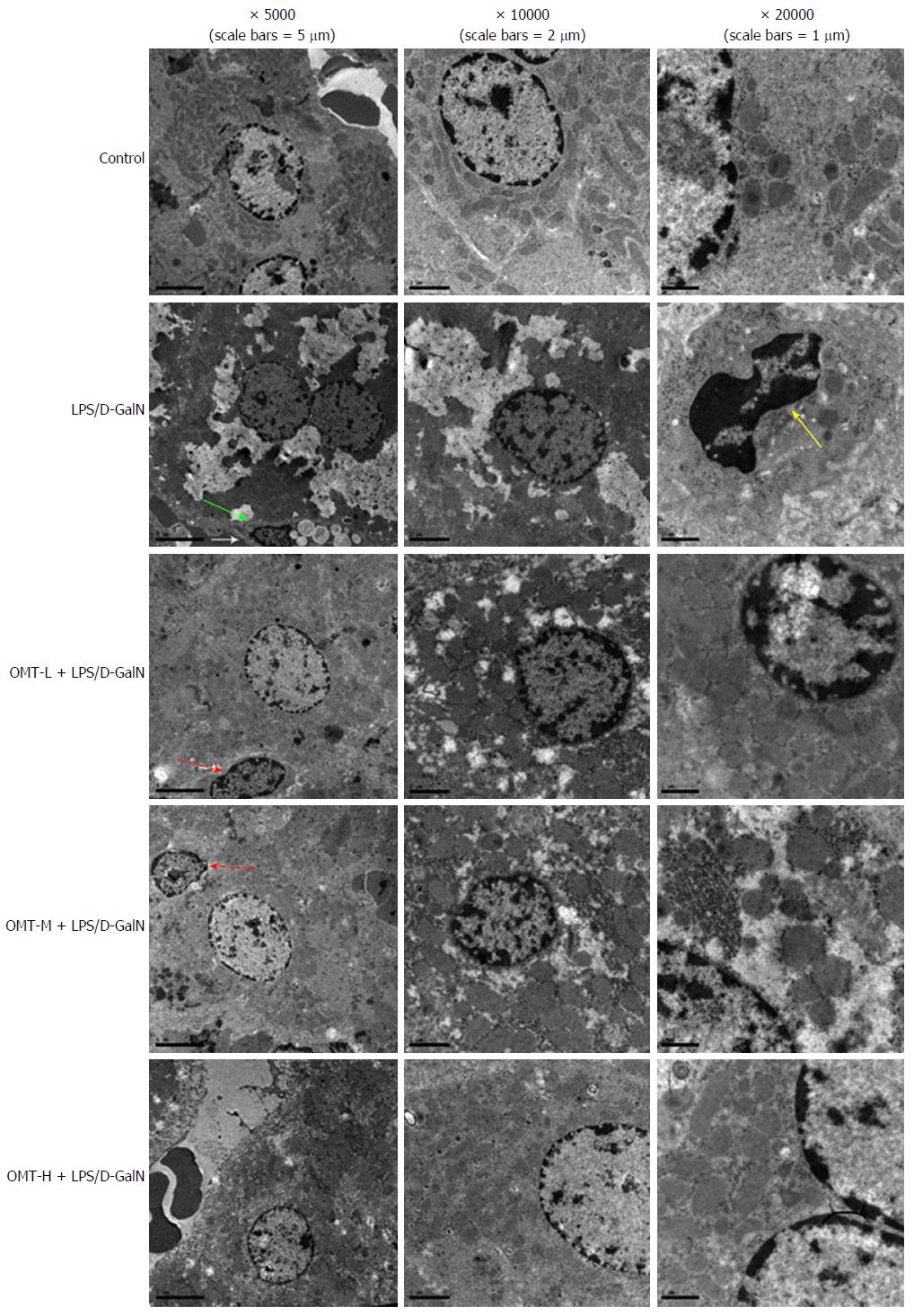
In the model group, transmission electron microscopy (TEM) showed significant necrosis of liver cells, irregular shape of liver cells, visible pyknosis and apoptotic bodies, hepatocyte mitochondrial damage, and no obvious cell structure. OMT preconditioning reduced the pathological changes in the liver tissue (Figure 2).
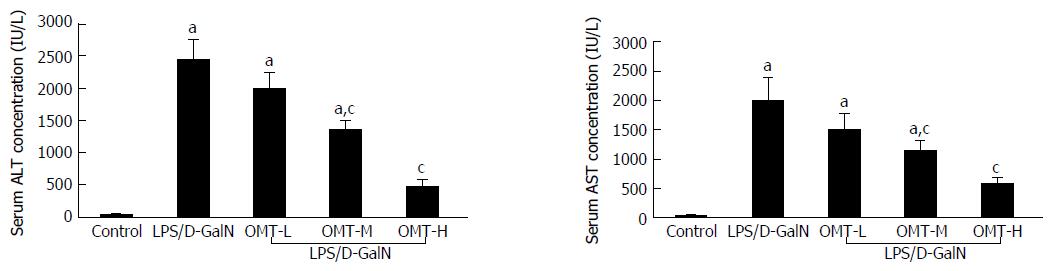
Compared with the control group, the levels of ALT and AST in the model group increased significantly (P < 0.01). OMT pretreatment significantly reduced the levels of AST and ALT (P < 0.05) (Figure 3).
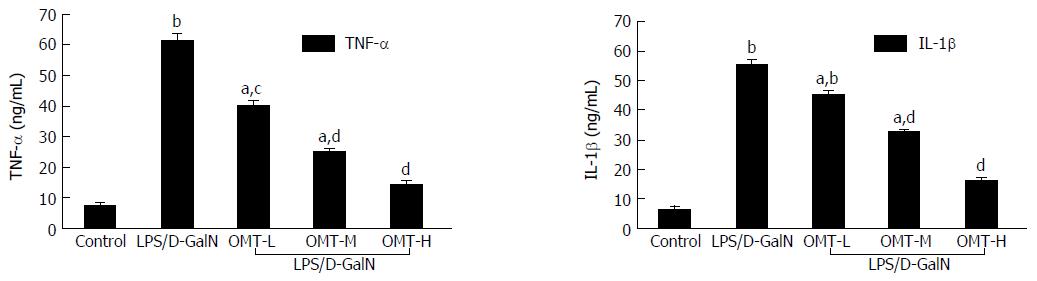
The results of ELISA showed that expression of TNF-α and IL-1β in the LPS/D-GalN group increased significantly (P < 0.01). Compared with the LPS/D-GalN group, OMT pretreatment significantly decreased expression of TNF-α and IL-1β (P < 0.05 or P < 0.01) (Figure 4).
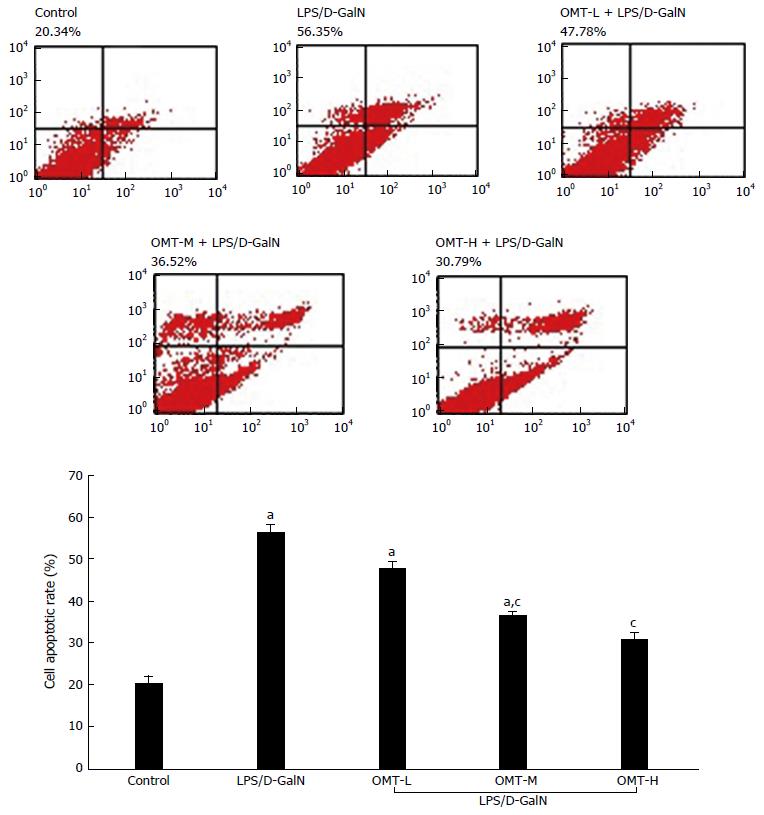
Flow cytometry showed that the rate of hepatocyte apoptosis in the model group was significantly higher than that in the control group (P < 0.05). The middle and high doses of OMT significantly down-regulated apoptosis of hepatocytes (P < 0.05) (Figure 5).
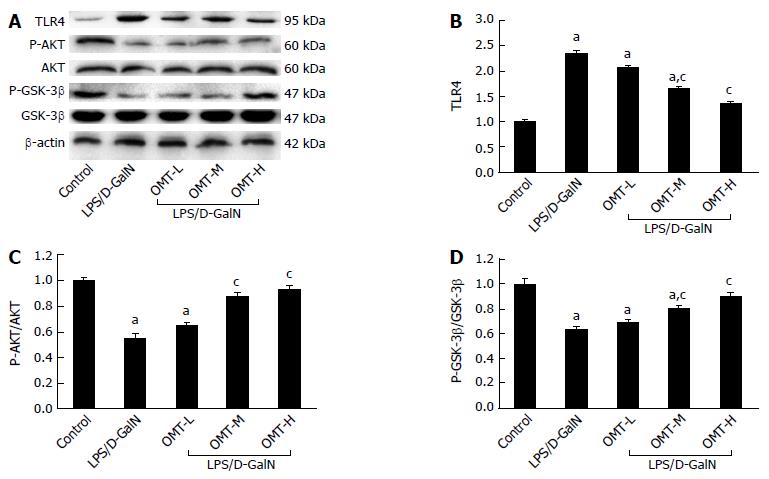
Western blotting showed that expression of TLR4 in the model group significantly increased (P < 0.05), and protein expression of Akt phosphorylated at serine 473 (P-AktSer473) and GSK-3β phosphorylated at serine 9 (P-GSK-3βSer9) significantly decreased (P < 0.05). Compared with the model group, the middle and high doses of OMT significantly down-regulated TLR4 expression (P < 0.05) and up-regulated expression of P-AktSer473 and P-GSK-3βSer9 (P < 0.05) (Figure 6).
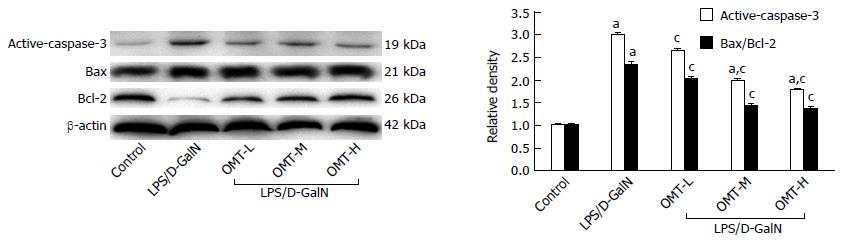
Expression of active caspase-3 protein and Bax/Bcl-2 ratio in the model group was significantly increased (P < 0.05). Compared with the model group, OMT pretreatment significantly down-regulated expression of active caspase-3 and Bax/Bcl-2 ratio (P < 0.05) (Figure 7).
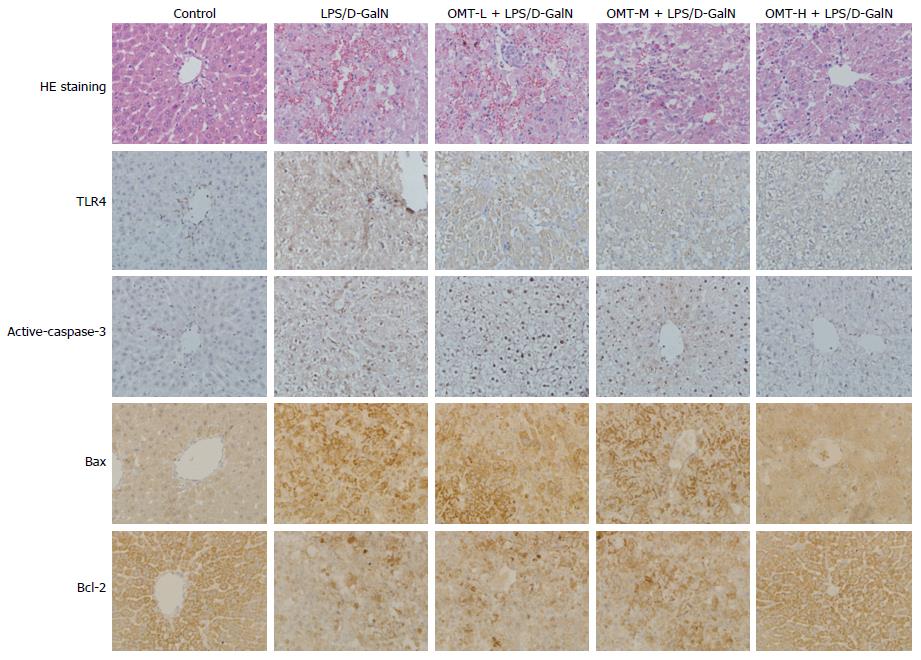
In the model group, HE staining showed obvious necrosis of liver cells, large patchy hemorrhage and necrosis. Only a small number of liver cells survived. There was no normal structure of hepatic lobules, and fibrous mesh scaffolds collapsed, accompanied by periportal infiltration of many inflammatory cells. OMT preconditioning reduced the pathological changes in liver tissue. Immunohistochemistry showed that expression of TLR4, Bax and active caspase-3 in the model group increased significantly and Bcl-2 expression decreased significantly compared with the normal control group (P < 0.05). Compared with the model group, OMT pretreatment significantly down-regulated expression of TLR4, Bax and active caspase-3, and increased expression of Bcl-2 (P < 0.05) (Figure 8).
Apoptosis is a major pathological feature of ALF[10-14]. Apoptosis is a form of programmed cell death that is required for the maintenance of tissue homeostasis by counter-balancing cell proliferation and eliminating damaged, infected or transformed cells. The process of liver cell apoptosis plays a vital role in the formation of subsequent necrosis[11,15]. Excessive apoptosis, resulting in too much cell death, has potentially devastating effects and may lead to tissue destruction and ALF[16,17].
OMT has a tetracyclic quinolizine structure. Its molecular formula is C15H24N2O. It is an alkaloid extracted from Radix Sophorae Flavescentis, Sophora alopecuroide and Radix Sophorae Subprostrata (traditional Chinese medicine belonging to the legumes). The anti-inflammatory, anti-oxidative and antiviral effects of OMT, as well as its role in immunological regulation, have been reported[18-21]. OMT has a variety of pharmacological actions. It has a protective effect on ischemic and reperfusion injury in liver, and the intestines and heart via its anti-apoptotic and anti-inflammatory activity[21-23]. We are the first to investigate the role of OMT in the inhibition of apoptosis in ALF and its potential mechanisms.
LPS is the major structural component of the cell wall of Gram-negative bacteria. LPS can initiate intracellular signals, express and release many types of inflammatory factors and cellular toxic substances, and thus induce apoptosis and necrosis of liver cells[24,25]. Through binding with LPS, TLR4 is initiated, which activates nuclear transcription factor (NF)-κB with the assistance of CD14 and myeloid differentiation protein 2[26,27]. NF-κB then translocates to the nucleus, where it activates and regulates the transcription of genes related to inflammatory responses, such as TNF-α, IL-1β, IL-6, nitric oxide and superoxide, which further induces apoptosis and necrosis of liver cells or more intricate biological responses[28,29]. Previous studies have replicated apoptosis in the hepatic cell line, BRL-3A, and in animals with liver failure using LPS[30,31].
D-GalN is a specific hepatic transcription inhibitor, which consumes UTP in the liver, inhibits RNA synthesis and damages liver biosynthetic function. Lesions in the D-GalN-induced model of ALF are restricted to the liver, without affecting other organs. The biochemical and morphological changes in the model are similar to those in human ALF[32]. LPS causes hepatic sinus injury and liver fibrin deposition, thus leading to severe liver dysfunction[33]. D-GalN can amplify and enhance the toxic effect of LPS within a few hours, thus aggravating liver failure. In the animal model of LPS/D-GalN-induced ALF, D-GalN blocks transcription of hepatic genes, and LPS induces cytokine-dependent liver inflammation, accompanied by cell apoptosis and death[34].
In the present study, LPS and D-GalN were used to establish the model of ALF in rats. HE staining showed obvious pathological changes in the model group, including multiple areas of periportal inflammation, bleeding, necrosis and inflammatory cell infiltration. TEM showed irregular liver cells with apoptotic bodies and mitochondrial damage. Serum aminotransferases were elevated, and expression of TNF-α and IL-1β in the LPS/D-GalN group increased significantly. Apoptotic rate and mortality of rats were significantly increased. The changes in liver function and pathology were all consistent with ALF.
Our results indicate that D-GalN specifically enhances the hepatotoxicity of LPS and promotes the development of ALF. We constructed a model of LPS/D-GalN-induced ALF in rats. OMT pretreatment significantly improved the mental state of the rats, reduced the serum levels of ALT and AST, down-regulated expression of TNF-α and IL-1β, ameliorated liver tissue pathology, decreased apoptotic rate, and improved the survival rate of the rats. The results showed that OMT pretreatment had an obvious protective effect on LPS/D-GalN-induced ALF in rats, and has the potential for clinical treatment of ALF.
Studies have confirmed that the expression of TLR4 is increased in liver injury and ALF[6]. We have previously confirmed that the TLR4/PI3K/Akt/GSK-3β pathway participates in the regulation of apoptosis in BRL-3A cells[9]. In the present study, we further evaluated the effect of OMT on hepatocyte apoptosis and explored its underlying mechanism. TLR4 is one of the first members of the TLR family and has been well characterized as a pattern-recognition receptor[35]. Unlike other TLRs, TLR4 can activate the MyD88-dependent and TRIF signaling pathways[36].
The PI3K/Akt signaling pathway is of importance in regulating cell survival and apoptosis[37]. Activated PI3K can promote formation of the second messenger, phosphatidylinositol (3,4,5)-trisphosphate, which activates Akt through phosphorylation. Akt is a serine/threonine kinase that can be fully activated by phosphorylation at T308 and S473. Activated Akt then activates or inhibits the phosphorylation of its downstream substrates including GSK3 and FOXOs (forkhead family of transcription factors) to regulate cell proliferation, differentiation, apoptosis, migration and other important processes[38-40]. Akt also prevents cytochrome c release and inhibits apoptosis by phosphorylating B-cell-lymphoma-associated death protein at Ser136 to release its inhibition of Bcl-xL. Additional Akt substrates include transcription factors p53, FOXO1 and NF-κB.
To explore further the underlying mechanism of the anti-apoptotic effect of OMT, we detected expression of TLR4, P-AktSer473, Akt, P-GSK-3βSer9 and GSK-3β. Our results indicated that LPS/D-GalN increased protein levels of TLR4 and decreased levels of P-AktSer473, and P-GSK-3βSer9 were dramatically blocked by OMT pretreatment. OMT markedly decreased the protein levels of TLR4. LPS treatment dramatically increased the TLR4 expression on hepatocyte surfaces and PI3K/Akt/GSK-3β activation, while OMT inhibited the LPS-induced invasion and the phosphorylation of GSK3 and Akt. The above results suggest that OMT exerts its anti-apoptotic effect through the TLR4/PI3K/Akt/GSK-3β signaling pathway.
Apoptosis is the result of a series of highly regulated caspase cascades, which are actively executed by the caspase family, including caspase-3. Activated caspase-3 induces apoptosis by inactivating the related protease in repairing DNA, which is the final execution phase of apoptosis[41-43]. The PI3K/Akt signaling pathway inhibits apoptosis and protects cell survival through decreasing the expression of p53 and its downstream Bax (proapoptotic gene), enhancing the mitochondrial membrane potential, inhibiting the production of reactive oxygen species, up-regulating the proportion of Bcl-2 and Bcl-xL (anti-apoptotic genes), inhibiting the release of cytochrome c, and reducing the expression of caspase-3 and caspase-9[44-46].
The balance between anti-apoptotic protein Bcl-2 and proapoptotic protein Bax plays a key role in regulating cell death[47]. Therefore, anti-apoptotic therapy via inhibition of caspase-3 expression and regulation of the balance of Bcl-2/Bax has been proposed to be useful for attenuating ALF. Our results showed that the level of caspase-3 and the ratio of Bax/Bcl-2 in liver tissue stimulated by LPS/D-GalN significantly increased. The effect was obviously attenuated by pretreatment with OMT.
We conclude that OMT inhibits hepatocyte apoptosis through the TLR4/PI3K/Akt/GSK-3β signaling pathway. OMT could be an effective candidate for ameliorating ALF.
Lipopolysaccharide/D-galactosamine (LPS/D-GalN) was used to establish a model of acute liver failure (ALF) in rats. In this study, the author demonstrated the role of oxymatrine (OMT) in inhibiting apoptosis in ALF.
OMT pretreatment protected liver cells by improving the liver pathological change and reducing serum aminotransferase in lipopolysaccharide/D-galactosamine-induced ALF in rats. OMT preconditioning down-regulated apoptosis of hepatocytes and ameliorated pathological changes in liver tissue.
The levels of alanine aminotransferase, aspartate aminotransferase, tumor necrosis factor-α and interleukin-1β in the model group increased significantly, and were significantly reduced by OMT pretreatment. OMT pretreatment down-regulated expression of Toll-like receptor (TLR)4 and active caspase-3 and the Bax/Bcl-2 ratio, and up-regulated expression of P-AKTSer473 and P-GSK3βSer9. OMT could inhibit hepatocyte apoptosis through the TLR4/PI3K/Akt/GSK-3β signaling pathway. OMT inhibits hepatocyte apoptosis by suppressing the TLR4/PI3K/Akt/GSK-3β signaling pathway, which suggests that OMT is an effective candidate for ameliorating ALF.
The authors have come to the conclusion, that oxymatrine can inhibit hepatocyte apoptosis by suppressing the TLR4/PI3K/AKT/GSK-3β signaling pathway, which suggest that OMT could be an effective candidate to ameliorate the course of ALF. The study is generally well designed, well presented and the results are potentially of a certain interest. The main idea of the manuscript gives a new approach to the treatment of extremely serious clinical problem - the acute liver failure and investigation of precise pathomechanism of disease is the only way to implement an effective therapy in clinic.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Saracyn M S- Editor: Yu J L- Editor: Filipodia E- Editor: Zhang FF
| 1. | Lee WM. Acute liver failure in the United States. Semin Liver Dis. 2003;23:217-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 241] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 2. | Kramer L. Acute liver failure. Wien Klin Wochenschr. 2004;116:67-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Doggrell SA. Suramin: potential in acute liver failure. Expert Opin Investig Drugs. 2004;13:1361-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Weng HL, Cai X, Yuan X, Liebe R, Dooley S, Li H, Wang TL. Two sides of one coin: massive hepatic necrosis and progenitor cell-mediated regeneration in acute liver failure. Front Physiol. 2015;6:178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Sun Z, Klein AS, Radaeva S, Hong F, El-Assal O, Pan HN, Jaruga B, Batkai S, Hoshino S, Tian Z. In vitro interleukin-6 treatment prevents mortality associated with fatty liver transplants in rats. Gastroenterology. 2003;125:202-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 108] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Cook DN, Pisetsky DS, Schwartz DA. Toll-like receptors in the pathogenesis of human disease. Nat Immunol. 2004;5:975-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 628] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 7. | Liu X, Cohen JI. The role of PI3K/Akt in human herpesvirus infection: From the bench to the bedside. Virology. 2015;479-480:568-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Danielsen SA, Eide PW, Nesbakken A, Guren T, Leithe E, Lothe RA. Portrait of the PI3K/AKT pathway in colorectal cancer. Biochim Biophys Acta. 2015;1855:104-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 185] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 9. | Zhang S, Wang S, Wan Z, Li R, Yu J. The diagnosis of invasive and noninvasive pulmonary aspergillosis by serum and bronchoalveolar lavage fluid galactomannan assay. Biomed Res Int. 2015;2015:943691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Guicciardi ME, Gores GJ. Apoptosis as a mechanism for liver disease progression. Semin Liver Dis. 2010;30:402-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 11. | Wang K. Molecular mechanisms of liver injury: apoptosis or necrosis. Exp Toxicol Pathol. 2014;66:351-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Tagami A, Ohnishi H, Hughes RD. Increased serum soluble Fas in patients with acute liver failure due to paracetamol overdose. Hepatogastroenterology. 2003;50:742-745. [PubMed] |
| 13. | Kim SJ, Kim KM, Park J, Kwak JH, Kim YS, Lee SM. Geniposidic acid protects against D-galactosamine and lipopolysaccharide-induced hepatic failure in mice. J Ethnopharmacol. 2013;146:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Riordan SM, Williams R. Mechanisms of hepatocyte injury, multiorgan failure, and prognostic criteria in acute liver failure. Semin Liver Dis. 2003;23:203-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Kobayashi M, Tsujitani S, Kurisu Y, Kaibara N. Bcl-2 and Bax expression for hepatocellular apoptosis in a murine endotoxin shock model. Hepatogastroenterology. 2002;49:1602-1606. [PubMed] |
| 16. | Bantel H, Schulze-Osthoff K. Mechanisms of cell death in acute liver failure. Front Physiol. 2012;3:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Rutherford A, Chung RT. Acute liver failure: mechanisms of hepatocyte injury and regeneration. Semin Liver Dis. 2008;28:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Cao CX, Yang QW, Lv FL, Cui J, Fu HB, Wang JZ. Reduced cerebral ischemia-reperfusion injury in Toll-like receptor 4 deficient mice. Biochem Biophys Res Commun. 2007;353:509-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 193] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 19. | Lu LG, Zeng MD, Mao YM, Li JQ, Wan MB, Li CZ, Chen CW, Fu QC, Wang JY, She WM. Oxymatrine therapy for chronic hepatitis B: a randomized double-blind and placebo-controlled multi-center trial. World J Gastroenterol. 2003;9:2480-2483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Liu Y, Zhang XJ, Yang CH, Fan HG. Oxymatrine protects rat brains against permanent focal ischemia and downregulates NF-kappaB expression. Brain Res. 2009;1268:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Hong-Li S, Lei L, Lei S, Dan Z, De-Li D, Guo-Fen Q, Yan L, Wen-Feng C, Bao-Feng Y. Cardioprotective effects and underlying mechanisms of oxymatrine against Ischemic myocardial injuries of rats. Phytother Res. 2008;22:985-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Zhao J, Yu S, Tong L, Zhang F, Jiang X, Pan S, Jiang H, Sun X. Oxymatrine attenuates intestinal ischemia/reperfusion injury in rats. Surg Today. 2008;38:931-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Xiang X, Wang G, Cai X, Li Y. Effect of oxymatrine on murine fulminant hepatitis and hepatocyte apoptosis. Chin Med J (Engl). 2002;115:593-596. [PubMed] |
| 24. | Wang YM, Feng GH, Huang F, Li Y, Zhao GZ. [Tumor necrosis factor-alpha, caspase-3 expression and hepatocyte apoptosis in fulminanting hepatic failure]. Zhonghua Neike Zazhi. 2003;42:566-570. [PubMed] |
| 25. | Herzum I, Renz H. Inflammatory markers in SIRS, sepsis and septic shock. Curr Med Chem. 2008;15:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Possamai LA, McPhail MJ, Quaglia A, Zingarelli V, Abeles RD, Tidswell R, Puthucheary Z, Rawal J, Karvellas CJ, Leslie EM. Character and temporal evolution of apoptosis in acetaminophen-induced acute liver failure*. Crit Care Med. 2013;41:2543-2550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Byun EB, Sung NY, Park JN, Yang MS, Park SH, Byun EH. Gamma-irradiated resveratrol negatively regulates LPS-induced MAPK and NF-κB signaling through TLR4 in macrophages. Int Immunopharmacol. 2015;25:249-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3474] [Cited by in RCA: 3511] [Article Influence: 146.3] [Reference Citation Analysis (0)] |
| 29. | Anderson KV. Toll signaling pathways in the innate immune response. Curr Opin Immunol. 2000;12:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 457] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 30. | Kim SJ, Cho HI, Kim SJ, Park JH, Kim JS, Kim YH, Lee SK, Kwak JH, Lee SM. Protective effect of linarin against D-galactosamine and lipopolysaccharide-induced fulminant hepatic failure. Eur J Pharmacol. 2014;738:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Su GL. Lipopolysaccharides in liver injury: molecular mechanisms of Kupffer cell activation. Am J Physiol Gastrointest Liver Physiol. 2002;283:G256-G265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Wullaert A, van Loo G, Heyninck K, Beyaert R. Hepatic tumor necrosis factor signaling and nuclear factor-kappaB: effects on liver homeostasis and beyond. Endocr Rev. 2007;28:365-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 180] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 33. | Nakao A, Taki S, Yasui M, Kimura Y, Nonami T, Harada A, Takagi H. The fate of intravenously injected endotoxin in normal rats and in rats with liver failure. Hepatology. 1994;19:1251-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Liu LM, Zhang JX, Luo J, Guo HX, Deng H, Chen JY, Sun SL. A role of cell apoptosis in lipopolysaccharide (LPS)-induced nonlethal liver injury in D-galactosamine (D-GalN)-sensitized rats. Dig Dis Sci. 2008;53:1316-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 35. | Medvedev AE. Toll-like receptor polymorphisms, inflammatory and infectious diseases, allergies, and cancer. J Interferon Cytokine Res. 2013;33:467-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 36. | Thada S, Valluri VL, Gaddam SL. Influence of Toll-like receptor gene polymorphisms to tuberculosis susceptibility in humans. Scand J Immunol. 2013;78:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB/AKT--a major therapeutic target. Biochim Biophys Acta. 2004;1697:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 552] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 38. | LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat. 2009;11:32-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 617] [Cited by in RCA: 621] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 39. | Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2188] [Cited by in RCA: 2157] [Article Influence: 134.8] [Reference Citation Analysis (0)] |
| 40. | Atkins RJ, Dimou J, Paradiso L, Morokoff AP, Kaye AH, Drummond KJ, Hovens CM. Regulation of glycogen synthase kinase-3 beta (GSK-3β) by the Akt pathway in gliomas. J Clin Neurosci. 2012;19:1558-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 41. | Han W, Sun Y, Wang X, Zhu C, Blomgren K. Delayed, long-term administration of the caspase inhibitor Q-VD-OPh reduced brain injury induced by neonatal hypoxia-ischemia. Dev Neurosci. 2014;36:64-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Algeciras-Schimnich A, Barnhart BC, Peter ME. Apoptosis-independent functions of killer caspases. Curr Opin Cell Biol. 2002;14:721-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | D’Amelio M, Sheng M, Cecconi F. Caspase-3 in the central nervous system: beyond apoptosis. Trends Neurosci. 2012;35:700-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 190] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 44. | Lee WS, Yi SM, Yun JW, Jung JH, Kim DH, Kim HJ, Chang SH, Kim G, Ryu CH, Shin SC. Polyphenols Isolated from Allium cepa L. Induces Apoptosis by Induction of p53 and Suppression of Bcl-2 through Inhibiting PI3K/Akt Signaling Pathway in AGS Human Cancer Cells. J Cancer Prev. 2014;19:14-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Yuan L, Wei S, Wang J, Liu X. Isoorientin induces apoptosis and autophagy simultaneously by reactive oxygen species (ROS)-related p53, PI3K/Akt, JNK, and p38 signaling pathways in HepG2 cancer cells. J Agric Food Chem. 2014;62:5390-5400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 46. | Seo BR, Min KJ, Cho IJ, Kim SC, Kwon TK. Curcumin significantly enhances dual PI3K/Akt and mTOR inhibitor NVP-BEZ235-induced apoptosis in human renal carcinoma Caki cells through down-regulation of p53-dependent Bcl-2 expression and inhibition of Mcl-1 protein stability. PLoS One. 2014;9:e95588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 47. | Tsujimoto Y. Bcl-2 family of proteins: life-or-death switch in mitochondria. Biosci Rep. 2002;22:47-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |