Published online Jun 7, 2017. doi: 10.3748/wjg.v23.i21.3825
Peer-review started: November 25, 2016
First decision: December 19, 2016
Revised: January 18, 2017
Accepted: April 13, 2017
Article in press: April 13, 2017
Published online: June 7, 2017
Processing time: 195 Days and 10.6 Hours
To measure exogenous corticotropin-releasing factor (CRF)-induced motility of the isolated rat colon and to demonstrate the effect of pharmacologic inhibition on CRF-induced motility.
The isolated vascularly-perfused rat colon was used. Luminal pressure was monitored via microtip catheter pressure transducers in the proximal and distal colon. At first, exogenous CRF was administered in a stepwise manner and the concentration of CRF yielding maximal colonic motility was selected. After recording basal colonic motility, hexamethonium, phentolamine, propranolol, atropine and tetrodotoxin were infused into the isolated colon. Initially, only the test drug was infused; then, CRF was added. The motility index was expressed as percentage change over basal level.
Administration of 1.4, 14.4, 144 and 288 pmol/L CRF progressively increased colonic motility in the proximal and distal colon. Infusion of atropine or tetrodotoxin reduced CRF-induced motility of both the proximal and distal colon, whereas hexamethonium, phentolamine and propranolol had no effect.
CRF-induced colonic motility appears to be mediated by local cholinergic signaling via muscarinic receptors. Muscarinic receptors are potential targets for counteracting CRF-induced colonic hypermotility.
Core tip: Corticotropin-releasing factor (CRF) has emerged as a key mediator of functional bowel disorders and the effects of stress and inflammation on the gastrointestinal tract. CRF-induced colonic motility is mediated by local cholinergic signaling via muscarinic receptors, and blocking muscarinic receptors is a potential way to prevent CRF-induced hypermotility of the colon.
- Citation: Kim KJ, Kim KB, Yoon SM, Han JH, Chae HB, Park SM, Youn SJ. Corticotropin-releasing factor stimulates colonic motility via muscarinic receptors in the rat. World J Gastroenterol 2017; 23(21): 3825-3831
- URL: https://www.wjgnet.com/1007-9327/full/v23/i21/3825.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i21.3825
Corticotropin-releasing factor (CRF), a 41-amino acid peptide of hypothalamic origin[1], was initially identified as the main activator of the pituitary adrenal axis in stress[2]. It also plays a key role in endocrine, behavioral (anxiety/depression), autonomic (sympathetic activation) and immune responses to stress in the brain[3,4].
In addition to the established role of CRF in the brain, both peripheral and central administration of CRF cause stress-like gastrointestinal (GI) motor responses, including stress-induced inhibition of gastric emptying and stimulation of colonic motor function[5]. Accordingly, CRF has emerged as a key mediator of functional bowel disorders and of the effects of stress and inflammation on the GI tract[6,7].
Maillot et al[5] reported that peripheral administration of CRF stimulated colonic motility via peripheral CRF receptors, and this effect was antagonized by peripheral injection of CRF antagonists. There was also earlier evidence that CRF activated the colonic motility of isolated rat colons when it was perfused directly into the bath[8]. In contrast, Tsukamoto et al[9] suggest that peripheral administration of CRF activates the dorsal nucleus of vagi via central CRF receptors, resulting in stimulation of the vagal efferent and cholinergic transmission to the proximal colon.
We conducted the present study to measure the concentration dependence of CRF-induced motility and to assess the influence of pharmacologic inhibitors on CRF-induced motility using the isolated vascularly perfused rat colon model.
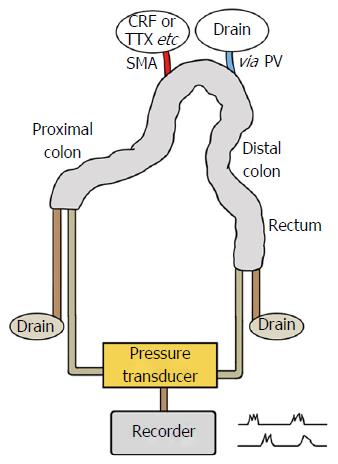
All experiments were approved by the Animal Care Committee of Chungbuk National University. Fifty male Sprague-Dawley rats, weighing between 250 g and 300 g, were starved and given free access to tap water for 48 h to clean out their bowels before surgery. The surgical procedures were similar to those described by Cuber et al[10,11] for isolation and vascular perfusion of the rat duodenojejunum[10] and ileum[11]. Anesthesia was introduced by intraperitoneal injection of xylazine 10 mg/kg and zolazepam 50 mg/kg, and the abdomen was opened via midline incision. The stomach, spleen and small intestine were removed after ligating the blood vessels supplying the area. The whole colon together with the superior mesenteric artery (SMA) and portal vein (PV) was freed of its visceral and retroperitoneal fixations and dissected. The rectum was excised at the level of the pelvic brim and the point at which the retroperitoneum reflects. The resected colon along with SMA and PV was transferred to wet guaze on a Petri dish at 37 °C. The cecum was removed. Two cm on the anal side of the proximal resected margin was defined as the proximal colon, and 2 cm on the oral side of the distal resected margin as the distal colon. A polyethylene cannula [0.58 mm in inner diameter (ID), 0.96 mm in outer diameter (OD)] was inserted into the SMA and another (0.58 mm in ID, 0.96 mm in OD) into the PV, and secured. Krebs solution containing 0.1% bovine serum albumin and 3% dextran was given immediately at a rate of 1.2 mL/min through the SMA. The solution was continuously gassed with 95% O2 and 5% CO2 and warmed at 37 °C.
A rubber cannula (5 mm ID, 6.5 mm OD) was then inserted and secured at both ends of the colon to drain the luminal secretion. The loop was gently flushed out once or twice with 10 mL prewarmed 0.15 mol/L NaCl. Figure 1 shows a schematic view of a rat colon comprising the proximal and the distal colon after removing the cecum and rectum.
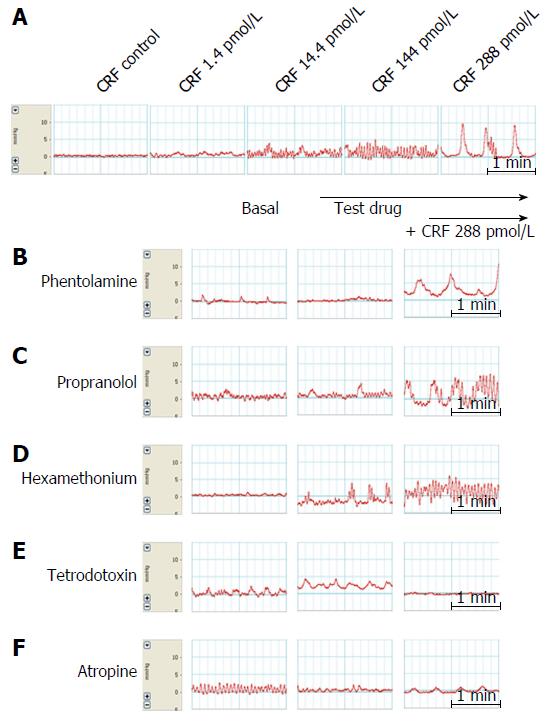
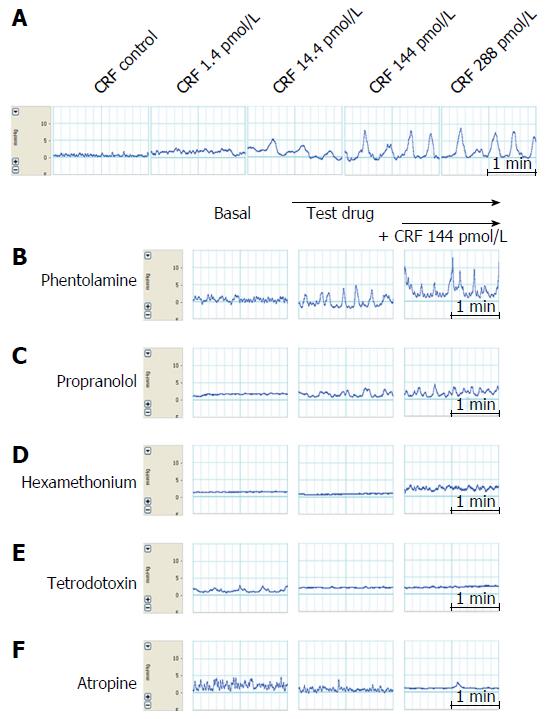
Effects of exogenous CRF infusion on colonic motility: Following a 30 min basal period, CRF (Sigma Chemical Co., St. Louis, MO, United States) was administered at doses of 1.4, 14.4, 144 and 288 pmol/L every 15 min to 6 male Sprague-Dawley rats.
Effects of drugs on CRF-induced colonic motility: To analyze the effects of CRF (used at a concentration of 144 pmol/L in the distal colon and 288 pmol/L in the proximal colon) on colonic motility, 10-5 mol/L phentolamine mesylate (Ciba, Basel, Switzerland), 10-5 mol/L propranolol HCl (AstraZeneca, Cambridge, United Kingdom), hexamethonium bromide (Sigma Chemical Co.), 10-6 mol/L tetrodotoxin (Tocris Cookson Inc., Ballwin, MO, United States) and 10-5 mol/L atropine (atropine sulfate; Jeil Pharmacy, Suncheon, South Korea) were each given to 5 or 6 male Sprague-Dawley rats.
Arterial perfusion was commenced at a rate of 1.2 mL/min (72 mL/h) and each test drug was infused at a rate of 2 mL/h through a side channel, as described previously by Plaisancié et al[12] and Mancinelli et al[8] with some modifications. The effect of each test drug was examined as follows. First, baseline colonic motility was measured for 30 min; then, each test drug was infused separately for 15 min at a rate of 2 mL/h followed by 144 pmol/L CRF for the distal colon or 288 pmol/L CRF for the proximal colon, these being the concentrations of CRF with maximal effects on colonic motility.
Microtip catheter pressure transducers of 2 mm diameter (Millar Inc., Houston, TX, United States) were calibrated in accordance with the manufacturer’s instructions. Briefly, to calibrate a pressure transducer in a channel, recording was started and two known pressures were applied to the transducer to obtain two voltages. Then, recording was stopped and the regions of the two readings were selected and Unit Conversions were chosen from the Channel Function pop-up menu for the channel. Using the 2 Point Calibration mode, the area in the waveform corresponding to one pressure was selected and a value button was used to enter its value, with the known pressure entered into the box beside it on the right. This process was repeated for the other pressure, with the data entered in the unused row.
Microtip catheter pressure transducers (2 mm diameter; Millar Inc.) were placed separately 2.0 cm apart through the proximal and distal ends of the colon. Intraluminal pressure was continuously monitored with a microtip catheter pressure transducer and recorded with a data acquisition system (ML846 Powerlab 4/25; AD Instruments Inc., Victoria, Australia).
Motility indices (MIs) were automatically calculated with the Medical Measurement Systems computer program (ML846 Powerlab 4/25) by multiplying the amplitude of each contractile wave by its recorded duration for the last 5 min of each 15 min interval, and were expressed as percentage changes over basal level. All records were analyzed by the same observer.
Statistical analysis was performed using SPSS for Windows, Version 18 (SPSS Inc., Chicago, IL, United States). Data are presented as mean ± SE. Kendall’s rank correlation coefficient was used to measure the association between drug concentration and motility response. Because of the small sample size, continuous variables, such as “CRF” and “CRF and atropine”, were compared by the Mann-Whitney U test. A P value of < 0.05 was considered statistically significant.
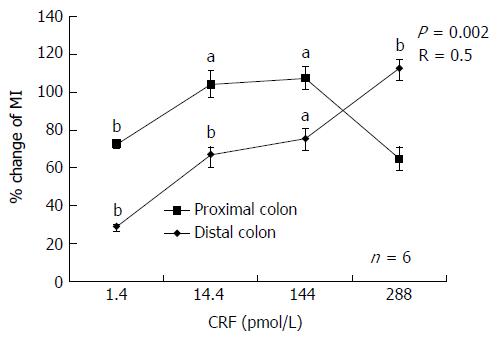
Exogenous infusion of CRF increased colonic motility significantly (P < 0.05) at concentrations of 1.4, 14.4, 144 and 288 pmol/L in the proximal colon (28.3% ± 5.5%, 66.9% ± 18.2%, 75.4% ± 27.1% and 112.5% ± 22.0%, respectively) and in the distal colon (72.6% ± 10.6%, 104.1% ± 34.7%, 107.4% ± 31.4% and 64.7% ± 31.1%, respectively) The mechanical responses induced by infusion of CRF tended to be stronger in the distal colon than in the proximal colon, although the difference was not statistically significant. A maximal response (a 112.5% increase in MI) was observed at 288 pmol/L CRF in the proximal colon, whereas a maximal response (107.4% increase) was observed in the distal colon at 144 pmol/L.
A concentration-response curve for the contractile effects of CRF was observed in the proximal colon only (Figure 2). The correlation coefficient was 0.5 (P = 0.002).
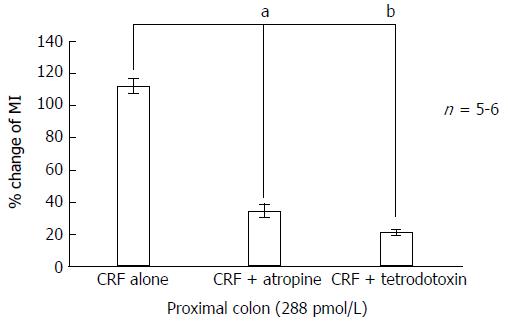
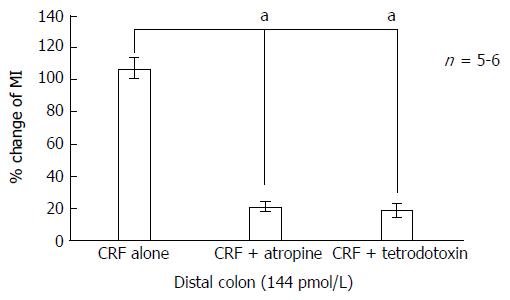
The administration of tetrodotoxin (10-6 mol/L) markedly inhibited CRF-induced colonic motility. MIs decreased from 112.5% ± 22.0% in the proximal colon and from 107.3% ± 31.4% in the distal colon to 21.08% ± 9.8% (P < 0.05) and 18.62% ± 18.6% (P < 0.01), respectively (Figures 3, 4, 5E and 6E).
Infusion of atropine (10-5 mol/L) on its own did not alter basal MI but it markedly inhibited CRF-induced colonic motility. MIs decreased from 112.5% ± 22.0% in the proximal colon and 107.3% ± 31.4% in the distal colon to 34.3% ± 17.8% (P < 0.05) and 20.5% ± 15.2% (P < 0.01), respectively (Figures 3, 4, 5F and 6F).
Since maximal responses were observed in the proximal colon at 288 pmol/L CRF and in the distal colon at 144 pmol/L, we selected these concentrations for assessing the effects of phentolamine, propranolol or hexamethonium on CRF-induced motility.
As shown in Figures 5B and 6B, phentolamine (10-5 mol/L), a reversible nonselective alpha-adrenergic antagonist, had no significant effect on colonic motility when 288 pmol/L CRF was applied to the proximal colon. Infusion of propranolol (10-5 mol/L), a nonselective beta-adrenergic antagonist, tended to reduce CRF-stimulated colonic motility (from a MI value of 112.5% ± 22.0% to 65.9% ± 24.9% in the proximal colon, and from 107.3% ± 31.4% to 34.9% ± 15.2% in the distal colon), although this effect was not statistically significant (Figures 5C and 6C). Furthermore, infusion of hexamethonium (10-3 mol/L), a nicotinic receptor antagonist, had no effect on CRF-induced colonic motility (Figures 5D and 6D).
In the present study, administration of CRF via the superior mesenteric artery increased colonic motility in both the proximal and distal colon, and we obtained evidence that this effect of CRF was mediated by local cholinergic signaling via muscarinic receptors.
CRF stimulates the dorsal motor nucleus of the vagus nerve both in vivo and in vitro[13], and may have a direct effect on the enteric nervous system by binding cholinergic and nitrenergic myenteric and submucosal neurons expressing CRF1 receptors[3,14,15]. Maillot et al[5] have reported that vascularly-perfused CRF stimulated colonic myoelectric activity and peristalsis in a similar isolated rat colon model[8].
For our experiments, we used the isolated rat colon, which may be an optimum model for investigating colonic motility[12,16]. Although the isolated bowel is free from control by the autonomic nervous system, the enteric nervous system and regulatory systems (endocrine, paracrine) remain functional.
The primary outcome of this study was the MI at each concentration of CRF. CRF increased the MI and tended to exert a more potent influence on the distal colon than the proximal colon, although the difference was not statistically significant. It is possible that the number of contractions on the tracings affected the values of MI obtained. However, MI was automatically measured with the Medical Measurement Systems computer program and expressed as percentage change over basal levels.
Although the mechanical responses induced by infusion of CRF tended to be more potent in the distal colon in this study, a concentration-dependent MI increase was only observed in the proximal colon, and the correlation coefficient R was quite low. Since it is known that the CRF gene is more highly expressed in the distal rat colon[17], a more vigorous response might have been expected in the distal colon. However, in our experiments, the difference in mechanical response to CRF between the distal and proximal colon was not significant, perhaps because of the small number of mice. Another explanation for the absence of a difference and the modest correlation between dose and response may have been a decline in CRF action at the highest doses in the distal colon due to an inhibitory effect of the CRF-R2 receptor[18].
Fast rhythmic activities at around 15-20 per min in our experiments were recorded in tracings of both the proximal and distal colon before administration of the test drugs. These activities seemed not to be influenced by phenotolamine, propranolol, hexamethonium, atropine or tetrodotoxin (Figures 5 and 6). Therefore, we speculate that they may originate in the stellate interstitial cells of Cajal near the myenteric border[19].
Hexamethonium, a nicotinic receptor antagonist[20], had no effect on either basal colonic motility or CRF-induced motility. Atropine, a non-selective muscarinic receptor antagonist, is known to suppress colonic contractions. A specific antagonistic effect of atropine on CRF-induced motility in our experiments is shown by the fact that it suppressed CRF-induced hypermotility but did not alter basal motility. Taken together, our findings indicate that CRF does not act on nicotinic receptors in the myenteric ganglia but on muscarinic receptors.
Tetrodotoxin, a voltage-gated sodium channel blocker, abolished CRF-induced colonic hypermotility in both the proximal and distal colon[21]. Overall, we found that CRF action was inhibited by atropine and tetrodotoxin, but not by hexamethonium, phentolamine or propranolol. We conclude that CRF action on the colon requires local cholinergic input via the muscarinic receptors of intrinsic cholinergic neurons in the enteric nervous system.
Although the role of CRF in colonic motility is well-established, in this study we used a pharmacological approach to assess the mechanisms of peripheral CRF-induced activation of colonic motility in rats using isolated colonic segments. Our data may help to further elucidate the role of CRF in gastrointestinal physiology and promote a novel approach to developing therapeutics for stress-related disorders of the gut.
However, our experiments have some noteworthy limitations and there are issues that remain to be addressed. Thus, we did not determine which of the CRF receptor subtypes was involved in the effects of CRF on the isolated colon. In addition, we did not show whether the increased MI translates into propulsive motility or not, and whether the contractile effects of CRF were propagative or not.
In conclusion, CRF stimulates colonic motility via peripheral CRF receptors, and CRF effects on the colon require local cholinergic signaling via muscarinic receptors. Although intrinsic cholinergic neurons in the enteric nervous system may contribute to this cholinergic input, further study is required to establish whether the cholinergic input is from the parasympathetic nerves innervating the colon or from intrinsic cholinergic neurons in the enteric nervous system.
All procedures performed in the studies involving animals were carried out in accordance with the ethical standards of the institution or practice at which the studies were conducted. This study was presented as an oral presentation at the 18th United European Gastroenterology Week, October 2010, in Barcelona, Spain.
Both peripheral and central administration of corticotropin-releasing factor (CRF) cause stress-like gastrointestinal motor responses, including stress-induced inhibition of gastric emptying and stimulation of colonic motor function.
There is no pharmacologic intervention study to reduce the action of CRF.
The authors conducted the present study to measure exogenous CRF-induced motility according to the concentration and to demonstrate the influence of the pharmacologic inhibition on CRF-induced motility using an isolated vascularly perfused rat colon model.
CRF stimulates colonic motility via peripheral CRF receptors, and CRF effects on the colon require local cholinergic signaling via muscarinic receptors.
A motility index (MI) was calculated with the Medical Measurement Systems computer program (ML846 Powerlab 4/25; AD Instruments Inc., Victoria, Australia) automatically by multiplying the amplitude of each contractile wave by the recorded duration for the last 5 min of each 15 min interval, and was expressed as percentage change over basal levels.
The theme of this paper is interesting and this is a very well-written manuscript and well-done basic research study. Please clarify and explore differences between the proximal and the distal colon. Some differences between distal and proximal colon may not have been significant because of number. Please clarify that. The R, although significant, is low, indicating only a moderate correlation.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Americo MF, Capasso R, Ukleja A S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Zhang FF
| 1. | Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394-1397. [PubMed] |
| 2. | Kyrou I, Tsigos C. Stress hormones: physiological stress and regulation of metabolism. Curr Opin Pharmacol. 2009;9:787-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 240] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 3. | Chatzaki E, Crowe PD, Wang L, Million M, Taché Y, Grigoriadis DE. CRF receptor type 1 and 2 expression and anatomical distribution in the rat colon. J Neurochem. 2004;90:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Rivier JE, Rivier CL. Corticotropin-releasing factor peptide antagonists: design, characterization and potential clinical relevance. Front Neuroendocrinol. 2014;35:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Maillot C, Million M, Wei JY, Gauthier A, Taché Y. Peripheral corticotropin-releasing factor and stress-stimulated colonic motor activity involve type 1 receptor in rats. Gastroenterology. 2000;119:1569-1579. [PubMed] |
| 6. | Larauche M, Kiank C, Tache Y. Corticotropin releasing factor signaling in colon and ileum: regulation by stress and pathophysiological implications. J Physiol Pharmacol. 2009;60 Suppl 7:33-46. [PubMed] |
| 7. | Kiank C, Taché Y, Larauche M. Stress-related modulation of inflammation in experimental models of bowel disease and post-infectious irritable bowel syndrome: role of corticotropin-releasing factor receptors. Brain Behav Immun. 2010;24:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Mancinelli R, Azzena GB, Diana M, Forgione A, Fratta W. In vitro excitatory actions of corticotropin-releasing factor on rat colonic motility. J Auton Pharmacol. 1998;18:319-324. [PubMed] |
| 9. | Tsukamoto K, Nakade Y, Mantyh C, Ludwig K, Pappas TN, Takahashi T. Peripherally administered CRF stimulates colonic motility via central CRF receptors and vagal pathways in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1537-R1541. [PubMed] |
| 10. | Cuber JC, Vilas F, Charles N, Bernard C, Chayvialle JA. Bombesin and nutrients stimulate release of CCK through distinct pathways in the rat. Am J Physiol. 1989;256:G989-G996. [PubMed] |
| 11. | Cuber JC, Herrmann C, Kitabgi P, Bosshard A, Bernard C, De Nadai F, Chayvialle JA. Neuromedin-N is not released with neurotensin from rat ileum. Endocrinology. 1990;126:1584-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Plaisancié P, Bernard C, Chayvialle JA, Cuber JC. Release of peptide YY by neurotransmitters and gut hormones in the isolated, vascularly perfused rat colon. Scand J Gastroenterol. 1995;30:568-574. [PubMed] |
| 13. | Lewis MW, Hermann GE, Rogers RC, Travagli RA. In vitro and in vivo analysis of the effects of corticotropin releasing factor on rat dorsal vagal complex. J Physiol. 2002;543:135-146. [PubMed] |
| 14. | Taché Y, Martinez V, Million M, Wang L. Stress and the gastrointestinal tract III. Stress-related alterations of gut motor function: role of brain corticotropin-releasing factor receptors. Am J Physiol Gastrointest Liver Physiol. 2001;280:G173-G177. [PubMed] |
| 15. | Gue M, Junien JL, Bueno L. Conditioned emotional response in rats enhances colonic motility through the central release of corticotropin-releasing factor. Gastroenterology. 1991;100:964-970. [PubMed] |
| 16. | Brechet S, Plaisancié P, Dumoulin V, Chayvialle JA, Cuber JC, Claustre J. Involvement of beta1- and beta2- but not beta3-adrenoceptor activation in adrenergic PYY secretion from the isolated colon. J Endocrinol. 2001;168:177-183. [PubMed] |
| 17. | Yuan PQ, Wu SV, Wang L, Taché Y. Corticotropin releasing factor in the rat colon: expression, localization and upregulation by endotoxin. Peptides. 2010;31:322-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Gourcerol G, Wu SV, Yuan PQ, Pham H, Miampamba M, Larauche M, Sanders P, Amano T, Mulak A, Im E. Activation of corticotropin-releasing factor receptor 2 mediates the colonic motor coping response to acute stress in rodents. Gastroenterology. 2011;140:1586-1596.e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Sarna SK. Physiology and pathophysiology of colonic motor activity (1). Dig Dis Sci. 1991;36:827-862. [PubMed] |
| 20. | Ruckebusch Y, Malbert CH, Crichlow EC. Hexamethonium: a probe to assess autonomic nervous system involvement in upper gastrointestinal functions in conscious sheep. Vet Res Commun. 1987;11:293-303. [PubMed] |
| 21. | Kimura T, Amano T, Uehara H, Ariga H, Ishida T, Torii A, Tajiri H, Matsueda K, Yamato S. Urocortin I is present in the enteric nervous system and exerts an excitatory effect via cholinergic and serotonergic pathways in the rat colon. Am J Physiol Gastrointest Liver Physiol. 2007;293:G903-G910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |