Published online Jun 7, 2017. doi: 10.3748/wjg.v23.i21.3784
Peer-review started: February 6, 2017
First decision: March 16, 2017
Revised: March 24, 2017
Accepted: May 9, 2017
Article in press: May 9, 2017
Published online: June 7, 2017
Processing time: 130 Days and 12.5 Hours
Colonoscopy is the gold standard for colorectal cancer prevention; however, it is still an imperfect modality. Precancerous lesions can be lost during screening examinations, thus increasing the risk of interval cancer. A variety of factors either patient-, or endoscopist dependent or even the procedure itself may contribute to loss of lesions. Sophisticated modalities including advanced technology endoscopes and add-on devices have been developed in an effort to eliminate colonoscopy’s drawbacks and maximize its ability to detect potentially culprit polyps. Novel colonoscopes aim to widen the field of view. They incorporate more than one cameras enabling simultaneous image transmission. In that way the field of view can expand up to 330°. On the other hand a plethora of add-on devices attachable on the standard colonoscope promise to detect lesions in the proximal aspect of colonic folds either by offering a retrograde view of the lumen or by straightening the haustral folds during withdrawal. In this minireview we discuss how these recent advances affect colonoscopy performance by improving its quality indicators (cecal intubation rate, adenoma detection rate) and other metrics (polyp detection rate, adenomas per colonoscopy, polyp/adenoma miss rate) associated with examination’s outcomes.
Core tip: Accomplishing high intra-procedural colonoscopy quality indicators has been associated with better patients’ outcomes. Recently, a number of novel wide-angle view endoscopes as well as different add-on devices have been developed aiming to further improve these metrics. They promise detailed inspection of otherwise difficult to examine parts of the colonic mucosa. Herein, we present the current evidence regarding the efficacy of these scopes and devices.
- Citation: Gkolfakis P, Tziatzios G, Dimitriadis GD, Triantafyllou K. New endoscopes and add-on devices to improve colonoscopy performance. World J Gastroenterol 2017; 23(21): 3784-3796
- URL: https://www.wjgnet.com/1007-9327/full/v23/i21/3784.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i21.3784
Colorectal cancer (CRC) ranks second regarding cancer-related mortality[1]. Colonoscopy interrupts the carcinogenesis by detecting and removing precancerous lesions, namely adenomas, thus providing the opportunity for neoplasia screening[2,3]. Despite its efficacy and widespread use, it is an imperfect examination. Almost a quarter of existing colonic adenomas remain undetected during a screening colonoscopy, while more recent studies raise that percentage up to 40%[4-7]. The so-called missed adenomas are considered independent risk factor for interval CRC[8], defined as CRC rising within the surveillance intervals. Missed adenomas are of particular significance in the right colon (RC), where more than half of the interval CRC incidents occur[9]. Furthermore, the usually flat serrated sessile adenomas (SSA) of the RC represent premalignant lesions of a distinct group of CRCs that also develop predominantly in the proximal colon[10,11].
Missed adenomas are a consequence of multiple factors; poor bowel preparation[12], inability to complete the colonoscopy by visualizing the cecum[13], inadequate withdrawal times[14], lack of expertise[15] and poor inspection of the proximal side of the colonic folds, as well as of the region around the anatomic flexures and the ileocecal valve[16,17].
Recent studies highlighted the importance of accurate adenoma detection during screening colonoscopies. Corley et al[18] evaluated more than 300000 examinations and proved that patients of both genders undergoing screening colonoscopy by an endoscopist with high adenoma detection rate (ADR) are protected against interval CRC both in the proximal and the distal colon in comparison with individuals undergoing colonoscopy by a physician with lower ADR. Similarly, a mathematical model showed that 1% increase of the ADR leads to 3% decrease of colon cancer incidence[8].
Aiming to provide patients the highest level of health services, scientific endoscopy Societies have recommended specific quality indicators to measure colonoscopy outcomes[19]. Similarly, endoscopy industries make continuous efforts to develop novel endoscopes and several devices to improve colonoscopy’s intrinsic technical imperfectness (Table 1). Almost a decade ago, a simple transparent plastic cap was one of the first devices introduced to increase endoscopists’ performance. Since then several studies have been conducted that led to two meta-analyses[20,21]. Their results indicate marginal efficacy of cap-assisted colonoscopy (CAC) to increase detection of patients with polyps. Due to the lack of further remarkable evolvement, cap-assisted colonoscopy will not be discussed in this paper. Marginal improvement of colonoscopy performance was also associated with the advent of high - definition endoscopy[22]. Due to this marginal positive effect and high costs of the investment, this technology is not the standard of care worldwide yet, and its detailed presentation is beyond the scope of this minireview.
| Wide-angle view colonoscopes | Add-on devices | ||
| Brand | Manufacturer | Brand | Manufacturer |
| Full-spectrum endoscopy platform (Fuse) | EndoChoice, GA, United States | Third-Eye Retroscope (TER) | Avantis Medical Systems, Inc, Sunnyvale, CA. United States |
| Extra-wide angle view colonoscope | Olympus Co., Tokyo, Japan | Third-Eye Panoramic | Avantis Medical Systems, Inc, Sunnyvale, CA, United States |
| Self-propelled disposable colonoscopy system (Aer-O-Scope) | GI View Ltd, Ramat Gan, Israel | Endocuff | Arc Medical Design, Leeds, England |
| Endocuff-Vision | Arc Medical Design, Leeds, England | ||
| EndoRings | EndoAid Ltd, Caesarea, Israel | ||
| NaviAid G-EYE | SMART Medical Systems Ltd, Ra’anana, Israel | ||
In this minireview we focus on the intra-procedural quality indicators: cecal intubation rate (CIR), polyp detection rate (PDR), adenoma detection rate (ADR), adenomas per colonoscopy (APC), as well as the polyp- and adenoma miss rates (PMR/AMR) (Table 2) in studies evaluating wide angle view (> 170°) colonoscopes and new add-on colonoscopy accessories. The term ADR -patients with at least one adenoma- will be used to describe not only the adenoma detection rate in screening/surveillance populations, but also in symptomatic individuals. To facilitate readers’ comprehension the exact composition of each study population regarding its indication will be presented, whenever needed.
| Metric | Definition | Suggested target (references) |
| Cecal intubation rate | The frequency of completed colonoscopies (cecum is visualized) | Overall: ≥ 90% |
| Screening: ≥ 95%[19] | ||
| Polyp detection rate | The proportion of patients with at least one polyp | N/A |
| Adenoma detection rate | The proportion of patients with at least one adenoma | Men: ≥ 30% |
| Women: ≥ 20%[19] | ||
| Adenoma per colonoscopy | The mean number of adenomas detected per colonoscopy | N/A |
| Polyp miss rate (PMR) | The proportion of polyps missed during a first pass and detected by a second one. It is used in back-to-back studies. | N/A |
| Adenoma miss rate | The proportion of adenomas missed during a first pass and detected by a second one. It is used in back-to-back studies. | N/A |
We conducted a comprehensive review of English literature published in MEDLINE electronic database from January 2008 until January 2017. The following key words were used: “wide-angle view colonoscopes”, “Third-Eye Retroscope”, “Full-Spectrum Endoscopy”, “balloon assisted colonoscope”, “Endocuff” and “Endorings”. Moreover, data from abstracts presented during the Digestive Diseases Week and the United European Gastroenterology Week from 2010 to 2016 were retrieved and manually searched. First author name, year of publication, study design, number of participants, their age and indications, CIR, PDR, ADR APC, PMR and AMR were extracted either as reported by the authors or after appropriate calculation.
One of the factors potentially accountable for missed lesions during colonoscopy is the relatively narrow field of view (140°-170°) of standard forward viewing (SFV) colonoscopes. In an effort to eliminate this limitation, novel wider field of view endoscopes have been manufactured, allowing meticulous inspection of the proximal aspect of the haustral folds. Table 3 summarizes data from studies regarding wide-angle view colonoscopy platforms.
| Ref. | Study design | Technology | Comparator | N | Indication | Age (yr), range | CIR (%) | PDR (%) | ADR (%) | APC | PMR (%) | AMR (%) |
| Gralnek et al[23], 2013 | Single-center prospective, | FUSE | None | 50 | Mixed | 18-70 | 100% | - | - | N/A | N/A | N/A |
| Gralnek et al[25], 2014 | Multicenter, prospective, randomized, tandem | FUSE | SFV | 101 vs 96 | Mixed | 18-70 | 98.0% vs 98.9% | - | 134.0% vs 28.0% | 10.64 vs 0.33 | 10%vs 43% | 7% vs 41% |
| Papanikolaou et al[26], 2017 | Multicenter, prospective randomized, tandem | FUSE | SFV+R | 107 vs 108 | Mixed | 41-80 | - | - | - | 10.61 vs 0.50 | 13.0% vs 33.5% | 10.9% vs 33.7% |
| Hassan et al[29], 2016 | Multicenter, prospective, randomized parallel | FUSE | SFV | 328vs 330 | Screening after (+) FIT | 50-69 | 92.1% vs 93.3% | - | 43.6% vs 45.5% | 0.81 vs 0.85 | N/A | N/A |
| Song et al[24], 2016 | Singe-center retrospective, | FUSE | None | 262 | Mixed | 22-80 | 100% | 54.20% | 36.3% | 0.66 | N/A | N/A |
| Rath et al[31], 2015 | Multicenter, prospective, parallel | FUSE | SFV | 90 | - | - | - | 36% vs 24.0% | - | - | N/A | N/A |
| Manes et al[27], 2016 Abstract | Single-center prospective, parallel | FUSE | SFV | 264 vs 265 | Mixed | 18-85 | - | 56.6% vs 44.3% | 35.5% vs 29.9% | - | N/A | N/A |
| Roepstorff et al[28], 2016 Abstract | Single-center prospective, parallel | FUSE | SFV | 109 vs 106 | Screening | - | 83.4% vs 93.4% | N/A | 67.0% vs 59.6% | 1.8 vs 1.4 | N/A | N/A |
| Leong et al[30], 2016 Abstract | Single-center, prospective, randomized tandem | FUSE | SFV | 25 vs 27 | IBD | - | - | - | - | - | 225.0% vs 71.4% | - |
| Uraoka et al[33], 2015 | Multicenter, feasibility | EWAVC | None | 47 | Mixed | - | 100% | - | - | 0.64 | N/A | N/A |
| Uraoka et al[34], 2013 Abstract | Multicenter, prospective, randomized parallel | EWAVC | SFV | 316 | Mixed | - | - | - | 50.6% vs 45.6% | 1.1 vs 1.0 | N/A | N/A |
| Gluck et al[35], 2016 | Single-center, prospective, tandem | Aer-O-Scope | SFV | 56 | Screening | 27-72 | 98.2% vs 98.2% | - | 21.4% vs 25.0% | - | 12.5% for Aer-O-Scope | - |
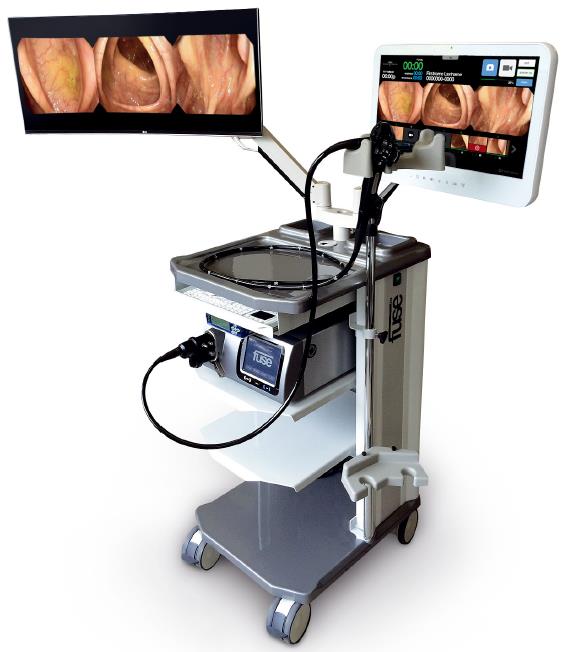
The full-spectrum endoscopy platform (Fuse, EndoChoice, GA, United States) consists of a video colonoscope and a processor. The colonoscope is a normal adult (168 cm working length, 12.8 mm outer diameter) flexible and reusable scope that allows both diagnostic and therapeutic procedures. It provides high-resolution, 330° field of view, achieved by three imagers and LED groups positioned one at the front and two at each side of the scope’s distal tip. The images in the three monitors (Figure 1) reflect transmission from the respective lenses (right image for the right-sided lens, center image for the central positioned lens and left image for the left-sided lens). The endoscopist is allowed to perform all potential maneuvers, such as complete tip deflection (180° up/down direction and 160° left/right direction).
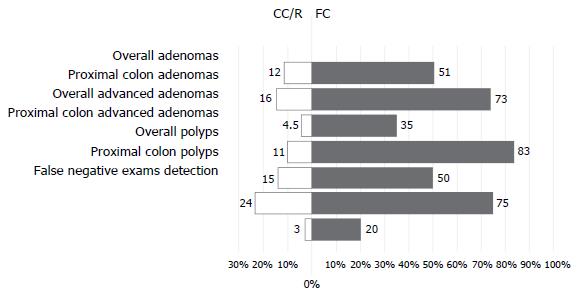
This novel platform has been proven to be safe and feasible with CIR almost 100% in two non-randomized studies[23,24]. Gralnek et al[25] conducted an international, multicenter, randomized back-to-back study to investigate whether Fuse detects more missed adenomas in comparison to SFV colonoscopy. Participants (n = 197, mixed indications) were randomly assigned to undergo same day tandem colonoscopies (either Fuse colonoscopy first followed by SFV colonoscopy or vice versa). The Fuse system had significantly lower miss rates compared to SFV endoscopy for adenomas (7% vs 41%, P < 0.0001) and polyps (10% vs 43%, P < 0.0001). The majority of the 20 adenomas that were missed during SFV examination and detected by the Fuse were sessile (90%), diminutive (70%) and RC sited (70%). In a similar design cross over study, Papanikolaou et al[26] showed that Fuse outperformed SFV complemented by examination of the right colon with scope retroflexion regarding adenoma (10.9% vs 33.7%, P < 0.001) and polyp (3.0% vs 33.5%, P < 0.001) miss rates. The same study showed that the incremental benefit of full-spectrum colonoscopy when performed, as a second examination, was 39% higher compared to that of conventional colonoscopy with retroflexion in the cecum, regarding-adenoma miss-rate overall (Figure 2). Moreover, an even higher incremental benefit was shown in favor of FC in the proximal colon. This benefit might be ameliorated by the fact that the majority of missed lesions measured less than 1 cm, in both study arms.
The ability of Fuse system to improve colonoscopy outcomes has further been evaluated in parallel design non-randomized[27,28] and randomized studies[29-31]. Manes et al[27] conducted a non-randomized study (n = 529) comparing Fuse and standard HD colonoscope. The authors reported increased PDR (56.6% vs 44.3%, P < 0.01) and ADR (35.5% vs 29.9%) in the Fuse arm. In Denmark Roepstorff et al[28] recruited 205 consecutive individuals undergoing screening colonoscopy either with Fuse system or with the conventional endoscope. Completion rate was lower with Fuse (83.4% vs 93.4%, P = 0.04) but Fuse showed numerically higher ADR (67% vs 59.6%, P = 0.36) and APC (1.8 vs 1.4, P = 0.09).
Hassan et al[29] compared the ADR of Fuse and SFV study arms in 658 individuals undergoing colonoscopy after positive FIT test in the context of a population-based massive screening program. Of interest, both ADR and APC were similar (43.6 vs 45.5 and 0.81 vs 0.85, respectively) between Fuse and conventional colonoscopy. Statistical significant difference was neither shown for advanced adenomas, sessile serrated adenomas and proximal adenomas. Authors acknowledged that the high ADR in the control group, potentially related to the disease enriched (FIT+) population of the study might have hindered detection of difference. Apart from that, sample size issues also rise, since randomized control trials of parallel design would normally require significantly more participants in order to achieve sufficient statistical power[32].
Another small (n = 90), randomized, prospective study[31] that assigned patients to undergo either Fuse or conventional colonoscopy showed higher PDR (36% vs 24%) associated with Fuse use.
Finally, the Fuse system has also been evaluated in patients with inflammatory bowel diseases (IBD). In a randomized back-to-back study from Australia[30], 52 IBD patients underwent tandem colonoscopies with Fuse system and conventional colonoscopy in order to evaluate dysplasia miss rate of the first examination (25 patients had Fuse index colonoscopy and 27 started with the conventional examination). Fuse was associated with a significant lower dysplasia miss rate (25% vs 71.4%, P = 0.0001).
This prototype colonoscope introduced by Olympus Co., Tokyo, Japan is composed of two lens’ systems: a standard 140°-angle forward-viewing lens and a 144-232°-angle lateral-backward viewing lens. A video monitor puts together the images of both lens and presents them simultaneously as a single image. Following an initial feasibility study[33] showing CIR of 100%, Uraoka et al[34] compared this prototype scope to SFV in a randomized parallel design study regarding APC and ADR. The sample consisted of 316 individuals undergoing colonoscopy for various indications. The extra-wide angle view colonoscope (EWAVC) had similar to the SFV system APC (1.1 vs 1.0, P = 0.36) and ADR (50.6% vs 45.6%, P = 0.43). However, this novel system may be proven of special importance in angulated and narrow regions of the colon (i.e., sigmoid), as per segment analysis showed a statistically significant higher sigmoid-APC in favor of EAWVC (0.4 vs 0.2, P = 0.04).
The efficacy and safety of this self-propelled disposable colonoscopy (SPDC) system (Aer-O-Scope; GI View Ltd, Ramat Gan, Israel) has been evaluated in one non-randomized prospective study of 56 patients undergoing tandem screening colonoscopies[35]. Its optical system consists of white-light LEDs and a CMOS high-definition digital camera; it allows a simultaneous 57° field of forward view an omni 360° view of a cylindrical band of the colon. Participants underwent colonoscopy with Aer-O-Scope first while SFV colonoscopy followed. SPDC was proven to be safe and effective. CIR were similar between SPDC and SFV endoscopy but SPDC failed to detect 5/40 of the polyps identified by the SFV (PMR 12.5%), leading to a lower ADR (21.4% vs 25%) in comparison to SFV.
With the term “add-on” device we describe all those accessories appended on the distal end of a standard colonoscope to facilitate meticulous inspection of the colonic mucosa. These devices provide either a retrograde view of the lumen (Third-Eye Retroscope, Avantis Medical Systems, Inc, Sunnyvale, CA, United States), or wider field of view (Third-Eye Panoramic, Avantis Medical Systems, Inc, Sunnyvale, CA, United States) or flattening of colonic folds during withdrawal to allow visualization of their proximal side of the colonic folds (Endocuff, Arc Medical Design, Leeds, England; Endocuff-Vision, Arc Medical Design, Leeds, England; EndoRings, EndoAid Ltd., Caesarea, Israel and balloon assisted-colonoscopy using the G-EYE, SMART Medical Systems Ltd, Ra’anana, Israel). Table 4 summarizes data originating from the available studies that evaluated their safety, feasibility and efficacy in improving colonoscopy performance.
| Ref. | Study design | Device | Comparator | N | Indication | Age (yr) | CIR (%) | PDR (%) | ADR (%) | APC | PMR (%) | AMR (%) |
| Triadafilopoulos et al[36], 2008 | Single-center, prospective, pilot | TER | 2SFV | 24 | ScreeningSurveillance | mean: 64 | 310.5 | 311.1 | ||||
| Waye et al[39], 2010 | Multicenter, prospective, open-label | TER | 2SFV | 249 | ScreeningSurveillance | mean: 63 | 0.61 vs 0.55 | 311.7% | 39.9% | |||
| DeMarco et al[37], 2010 | Multicenter, prospective, open-label | TER | 2SFV | 298 | Mixed | mean: 57 | 0.39 vs 0.34 | 312.9% | 313.8% | |||
| Leufkens et al[5], 2011 | Multicenter, prospective, randomized, tandem | TER | SFV | 176 vs 173 | Mixed | range: 23-83 | 15.9 %vs 32.8%(PP) | 18.4% vs 31.4%(PP) | ||||
| Mishkin et al[38], 2012 Abstract | Single-center, prospective | TER | 2SFV | 68 | Mixed | 34.4% | 37.8% | |||||
| Rubin et al[40], 2015 | Single center, Prospective, feasibility | TEP | 2SFV | 33 | Mixed | mean: 60 | 100% | 44% overall | ||||
| Gralnek et al[62], 2014 | Single-center, prospective, cohort | G-EYE | None | 47 | Mixed | mean: 59 | 100% | 53.2 | 44.70% | 0.76 | N/A | N/A |
| Halpern et al[63], 2014 | Multicenter, prospective, randomized, tandem | G-EYE | SFV | 54 vs 52 | Mixed | mean: 55 vs 58 | 100% vs 100% | - | 140.4% vs 25.9% | - | - | 7.5% vs 44.7% |
| Halpern et al[65], 2014 Abstract | Multicenter, prospective, randomized, parallel | G-EYE | SFV | 105 vs 117 | ScreeningSurveillance | ≥ 50 | - | - | 35.4%vs 23.5% | 0.63 vs 0.36 | N/A | N/A |
| Rey et al[64], 2015 Abstract | Multicenter, prospective, randomized, tandem | G-EYE | SFV | 25 vs 24 | Referral for colonoscopy | - | - | - | - | - | 17 vs 41 | - |
| Hendel et al[66], 2015 Abstract | Multicenter, prospective, randomized, parallel | G-EYE HD | SFV | 54 vs 50 | Mixed | ≥ 50 | - | 76% vs 46% | 59% vs 39% | 1.15 vs 0.66 | N/A | N/A |
| Shirin et al[67], 2016 Abstract | Multicenter, prospective, randomized, parallel | G-EYE HD | SFV | 242 vs 238 | Mixed | mean: 65 | - | - | 49.2% vs 33.8% | 0.93 vs 0.57 | N/A | N/A |
| Dik et al[61], 2015 | Multicenter, prospective, randomized, tandem | Endorings | SFV | 57 vs 59 | Mixed | mean: 59 | 100% vs 100% | 168.4% vs 40.7% | 149.% vs 28.8% | 11.05 vs 0.51 | 9.1% vs 52.8% | 10.4% vs 48.3% |
| Lenze et al[41], 2014 | Single-center, retrospective | Endocuff | None | 50 | Mixed | mean: 57 | 98% | - | 34% | 0.72 | N/A | N/A |
| Floer et al[43], 2014 | Multicenter, prospective, randomized, parallel | Endocuff | SFV | 249 vs 243 | Mixed | median: 64 | 96% vs 94% | 55.4% vs 38.4% | 35.4% vs 20.7% | 0.58 vs 0.36 | N/A | N/A |
| Biecker et al[44], 2015 | Two-center, prospective, randomized, parallel | Endocuff | SFV | 245 vs 253 | Mixed | median: 67 | 98% vs 98% | 56% vs 42% | 36% vs 28% | - | N/A | N/A |
| Sawatzki et al[42], 2015 | Multicenter, prospective, feasibility | Endocuff | None | 104 | ScreeningSurveillance | mean: 59 | 99% | 72% | 47% | - | N/A | N/A |
| Van Doorn et al[45], 2015 | Two-center, prospective, randomized, parallel | Endocuff | SFV | 1033(ITT: 504 vs 529 | Mixed | median: 65 vs 65 | ITT: 98% vs 99% | - | ITT: 52% vs 52% | ITT: 1.36 vs 1.17 | N/A | N/A |
| PP: 486 vs 514) | PP: 94% vs 99% | PP: 54% vs 53% | PP: 1.44 vs 1.19 | |||||||||
| De Palma et al[46], 2017 | Single-center, prospective, crossover, tandem | Endocuff | SFV | 137 vs 137 | Mixed | mean: 55 vs 56 | 100% vs 100% | - | 127.7% vs 28.5% | 10.63 vs 0.52 | - | 1.1% vs 29.7% |
| Floer et al[48], 2014 Abstract | Multicenter, prospective, randomized, parallel | Endocuff | SFV | 652 | Screening | mean: 64 | 98.5% vs 99.1% | 55.4% vs 39.9% | - | 0.9 vs 0.54 | N/A | N/A |
| Marsano et al[50], 2014 Abstract | Multicenter, retrospective | Endocuff | SFV | 165 vs 153 | ScreeningSurveillance | - | - | - | 46.6% vs 30% | 0.8vs 0.38 | N/A | N/A |
| Chin et al[53], 2015 Abstract | Single-center, cohort | Endocuff | SFV | 93 vs 143 | Mixed | - | - | 78.5% vs 57.3% | 44.1% vs 27.3% | - | N/A | N/A |
| Patel et al[52], 2016 Abstract | Single-center, cohort | Endocuff | SFV | 452 vs 597 | Mixed | - | - | 79.0% vs 57.4% | 51.8% vs 36.3% | 1.59 vs 0.91 | N/A | N/A |
| Higham-Kessler et al[56], 2016 Abstract | Single-center, cohort | Endocuff | SFV | 77 vs 153 | ScreeningSurveillance | - | - | 67% vs 62.7% | - | N/A | N/A | |
| Garcia et al[51], 2016 Abstract | Single-center, randomized, parallel | Endocuff | SFV | 174 vs 163 | Screening | mean: 61 | - | 29.9% vs 15.9% | 22.4% vs 13.4% | 0.31 vs 0.22 | N/A | N/A |
| Wada et al[49], 2016 Abstract | Two-center, randomized, parallel | Endocuff | SFV | 239 vs 207 | - | - | EAC: 98.8% | 62% vs 50% | 55% vs 40% | - | N/A | N/A |
| Bensuleiman et al[54], 2016 Abstract | Single-center, prospective, randomized, parallel | Endocuff | CAC | 84 vs 75 | Screening | - | 98% vs 99% | - | 53% vs 59% | 1.03 vs 1.00 | N/A | N/A |
| Cavallaro et al[55], 2016 Abstract | Single-center, cohort | Endocuff | SFV | 605 vs 579 | ScreeningSurveillance | mean: 60 vs60 | - | - | 53% vs 48% | 1.1 vs 0.88 | N/A | N/A |
| Triantafyllou et al[47], 2016 Abstract | Multicenter, prospective, randomized, tandem | Endocuff | SFV | 100 vs 100 | Mixed | mean: 61 | - | - | - | 10.93 vs 0.53 | - | 14.7% vs 37.6% |
| Tsiamoulos et al[58], 2015 Abstract | Single-center, cohort | Endocuff-vision | SFV | 133 vs 266 | Screening | - | - | - | 68.9% vs 58.4% | 2.2 vs 1.4 | N/A | N/A |
| Bhattacharyya et al[60], 2016 Abstract | Single-center, prospective, randomized, parallel | Endocuff-vision | SFV | 266 vs 265 | Screening | - | - | 70.3% vs 69.8% | 60.9% vs 63% | 1.26 vs 1.35 | N/A | N/A |
| Ngu et al[59], 2016 Abstract | Multicenter, prospective, randomized, parallel | Endocuff-vision | SFV | 1772 | Mixed | mean: 62 | 96.7% vs 96.4% | - | 40.9% vs 36.2% | 0.95 vs 0.75 | N/A | N/A |
The Third-Eye Retroscope (TER) (Avantis Medical Systems, Inc, Sunnyvale, CA, United States) is one of the first auxiliary imaging devices that tried to extend the field of view of the standard forward viewing colonoscope[36]. The TER is inserted through the working channel, it extends beyond the distal tip of the SFV scope to bend 180° in a J-shape form, looking opposite of the scope main lens; thus, it provides a complement retrograde view of the colonic lumen during scope withdrawal. Three open-label, one-arm prospective studies implementing the device on the SFV colonoscope showed that in the absence of Third Eye the examinations’ polyp and adenoma miss rates would be 4.4%-12.9% and 7.8%-13.8%, respectively[37-39]. In accordance with these findings, Leufkens et al[5] presented the results of a randomized tandem clinical study comparing PMR and AMR of SFV colonoscope with SFV colonoscope plus TER. In the per protocol analysis the TER arm was associated with significantly lower miss rates (PMR: 15.9% vs 32.8% and AMR: 18.4% vs 31.4%). However, the above studies underlined some limitations related to TER use. The device narrows almost 50% the diameter of the working channel making the suction of residues compulsory prior to withdrawal. Moreover, each time a polyp is detected by the retrograde view of TER the device must be removed to allow lesion removal, leading to significant prolongation of the procedure. Finally, the device procurement bears an additional financial burden. For these reasons the device has been abandoned.
A few years later the same manufacturer developed another device called the Third-Eye® Panoramic. This plastic cap can be clipped on to the distal tip of all conventional colonoscopes and contains two side-viewing CMOS chips. The cap is connected to a thin plastic catheter that contains the transmission wires that runs along the scope’s shaft. The catheter ends to an external video processor connected to the conventional colonoscope’s video monitor, resulting in an extended field of view (more than 300°) through three - partially overlapping - images. This novel device has only been evaluated in a feasibility study[40]. In this small study, the device was easy to use and the cecum was intubated in all cases.
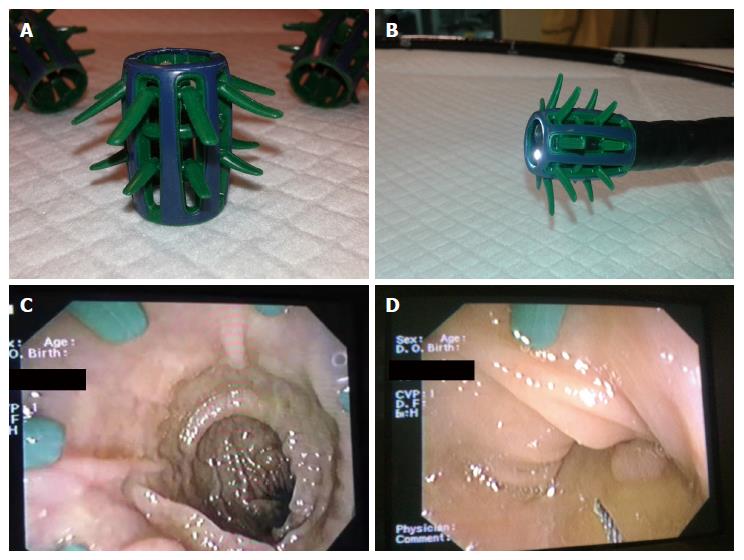
Endocuff (Arc Medical Design, Leeds, United Kingdom) is a plastic, 2 cm long cuff that can be mounted onto the tip of the scope. Endocuff entails two rows of “finger”-like projections, which remain smooth during insertion and bend in the withdrawal phase to flatten the colonic folds and allow assessment of a greater, otherwise unsighted, mucosal area (Figure 3). Endocuff and its “descendant” Endocuff-Vision have been evaluated in numerous studies (Table 4). Feasibility studies[41,42] showed that Endocuff was safe, since only minor insignificant mucosal lacerations were the adverse events related with its use. In these studies the rates of cecal intubation were higher than 98%. Three randomized parallel design studies[43-45] have been published comparing Endocuff-assisted colonoscopy (EAC) to conventional colonoscopy in terms of polyp and adenoma detection. Two studies from Germany[43,44], each recruiting almost 500 patients undergoing colonoscopy for various indications, favored Endocuff use to detect more patients with at least one polyp/adenoma compared to the conventional procedure (PDR: 55.4% vs 38.4% and 56% vs 42% and ADR: 35.4% vs 20.7% and 36% vs 28%, respectively). On the other hand, a similar multicenter Dutch study[45] that randomized more than 1000 patients of various indications failed to reveal any advantage of Endocuff use regarding the proportion of patients with at least one adenoma (ADR: 52% for both arms) and the mean number of adenomas per patient (APC: 1.36 vs 1.17).
Two studies have evaluated Endocuff regarding adenoma miss rate[46,47]. De Palma et al[46] randomized 274 patients to undergo same day back-to-back colonoscopies (either with Endocuff use first and then without it or vice versa). In this study any lesion detected during the first procedure was left in situ in order to be redetected -or not- during the second one. Adenoma miss rate was significantly lower when Endocuff was used (1.1% vs 29.7%, P < 0.001). Similarly, we recently presented the results of a multicenter tandem study[47] showing that Endocuff use outperformed its comparator (standard colonoscopy) in terms of AMR, overall (14.7% vs 37.6%, P = 0.0004) and in the proximal colon (10.4% vs 39%, P = 0.004).
There is also certain amount of data from parallel design studies, reporting increased PDR/ADR[48-53] in the Endocuff arms published in abstract form only. However, three other studies failed to reveal Endocuff superiority[54-56]. A recent meta-analysis tried to sum up these data[57]. Taking into account data from 8 studies (n = 4387) the authors concluded that Endocuff use is associated with higher ADR compared to standard colonoscopy (50.4% vs 43.3%, OR = 1.49, 95%CI: 1.23-1.80, I2 = 50%, P < 0.01).
Endocuff-Vision (Arc Medical Design, Leeds, England) - the evolution of the initial device, with a single row of projections (Figure 4) has also been evaluated in studies measuring colonoscopy outcomes. Tsiamoulos et al[58] reported extremely high ADR for Endocuff-Vision assisted and conventional colonoscopy in a screening population. However, both ADR and APC were even higher in the Endocuff-Vision arms (68.9% vs 58.5 and 2.2 vs 1.4, respectively). In a large study of more than 1700 patients[59], Endocuff-Vision use was associated with a significant higher ADR (40.9% vs 36.2%) in patients of various indications for colonoscopy. Contrariwise, these findings were not confirmed in a single-center prospective parallel design study that involved screening population[60]: similar ADR and APC between Endocuff-Vision-assisted and cap-assisted colonoscopy were noted.
EndoRings (EndoAid Ltd., Caesarea, Israel) is a silicone-rubber add-on device consisting of three circular rings. It fits onto the distal tip of the endoscope and allows not only the mechanical stretching of the haustral folds during withdrawal, but also maintains the lumen in the center of the inspection field. At the time of insertion the view of field is not affected since the device does not project beyond the distal end of the scope, allowing the unimpeded cecal intubation. This device has been evaluated only in one multicenter randomized tandem study[61]. In the per protocol analysis of 116 patients of mixed indications, the use of EndoRings was associated with a statistically significant lower polyp (9.1% vs 52.8%, P < 0.001) and adenoma (10.4% vs 48.3%, P < 0.001) miss rate. The benefit of EndoRings use was higher for the detection of diminutive adenomas (AMR: 13.5% vs 54.2%, P < 0.001) and adenomas found both at the proximal and distal colon (AMR: 10.6% vs 58.1%, P < 0.001 and 10% vs 37%, P < 0.001, respectively)[61].
The NaviAid G-EYE (SMART Medical Systems Ltd, Ra’anana, Israel) is a novel balloon-colonoscope consisting of a standard adult colonoscope combined with an inflatable balloon at the bending part of the scope. The balloon is located 1-2 cm proximally to the distal tip of the colonoscope and it can be inflated up to 60mm diameter with unremarkable alteration in scope’s outer caliber[62]. A special inflation system - the SPARK2C - manipulated by the endoscopist via a foot-pedal, inflates the balloon once cecum intubation achieved and retains a constant pressure within the colon during withdrawal. With the balloon inflated during withdrawal, colonic folds and flexures are mechanically straightened revealing potential suspicious lesions located in their proximal aspect[62]. Two randomized tandem studies[63,64] evaluated G-EYE’s lesions miss rates compared to SFV. Both studies examined individuals undergoing colonoscopy for various reasons. Halpern et al[63] demonstrated a significant lower adenoma miss rate for G-EYE (7.5% vs 44.7%, P = 0.0002), while Rey et al[64] showed a lower polyp miss rate in favor of the G-EYE (7% vs 41%). In terms of ADR and adenomas per colonoscopy this device has been evaluated in three randomized parallel design studies[65-67]. Halpern et al[65] randomized 222 individuals undergoing screening colonoscopy to receive either balloon-assisted assisted colonoscopy or SFV examination. The G-EYE use was related to higher ADR and APC (35.4% vs 23.5% and 0.63 vs 0.36). The last two multicenter randomized trials[66,67] used G-EYE in combination with a HD colonoscope. In both studies the reported rate of adenoma detection was higher in the G-EYE arm (59% vs 39%, and 49.2% vs 33.8%, respectively).
The volume of presented data clearly illustrates the unmet need of optimizing technology to improve colonoscopy performance. The results of the aforementioned studies of novel wide-angle view endoscopes and add-on devices appear promising. Despite some contradictory results the majority of the data are in favor of the new endoscopes/devices regarding polyp and adenoma detection rates, as well as, polyp and adenomas miss rates. However, these data should be interpreted cautiously for a number of reasons:
Firstly, 50% of the reviewed studies have been published as abstracts only. The Extra-wide Angle View Colonoscope and the Third-Eye® Panoramic are still under development, while Aer-O-Scope and Third Eye have been abandoned. Moreover, several new colonoscopy add on devices appear in the endoscopy accessories market without having been adequately evaluated, yet.
Secondly, heterogeneity characterizes the presented studies. Different target populations and lack of a solid integrated design do not allow safe generalization of the results. It should be noted that the plethora of parallel design studies has not enrolled adequate number of participants to detect differences in ADR with sound statistical power. Moreover, the comparator to the examined novelties comprises either standard or high definition endoscopes or both categories, thus adding more confounders to data interpretation. Of note, there are no direct comparisons between new wide angle view colonoscopes and add on devices regarding colonoscopy outcomes, yet and we can hardly expect any to come in the literature soon.
Thirdly, more attention should be paid to studies recruiting asymptomatic subjects at average risk for CRC. This is the particular population in which it is proven that improvement in colonoscopy outcomes (e.g., increased ADR) is correlated to improved patients’ outcomes (reduced risk for interval CRC).
Fourthly, it is still unknown if these novelties are of benefit for the low or the average performing endoscopist only or the benefit is also extended to the high detectors. Whether different levels of endoscopists’ experience and performance or different endoscopic environment (e.g., academic vs community or private practice) could lead to different acceptance of these technologies and to different levels of quality indicators improvement, pends to be answered.
Finally, cost is an important factor that could influence the widespread use of these novelties. It has been shown that in the era of financial recession expensive technologies used for patients’ management are not favored[68]. In this setting, attachable cuffs and rings present a relatively low cost investment.
Summing up, new wide-angle view endoscopes and add-on devices are promising technologies to improve colonoscopy and patients outcomes. More studies are definitely needed in order to provide answers to the aforementioned open questions. Until conclusive data are obtained, endoscopists should use these novelties in a personalized manner taking into account their availability and stuff experience. At the same time, the fundamental principles of colonoscopy like adequate bowel preparation, meticulous inspection independently of endoscope and devices used and suitable withdrawal time should govern our practice.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cao HL, Christodoulou DK S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | SEER Stat Facts Sheets: Colon and Rectum Cancer. Accessed December 2016. Available from: https://seer.cancer.gov/statfacts/html/colorect.html. |
| 2. | Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol. 2009;104:739-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 981] [Cited by in RCA: 1059] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 3. | Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1952] [Cited by in RCA: 2286] [Article Influence: 175.8] [Reference Citation Analysis (2)] |
| 4. | Heresbach D, Barrioz T, Lapalus MG, Coumaros D, Bauret P, Potier P, Sautereau D, Boustière C, Grimaud JC, Barthélémy C. Miss rate for colorectal neoplastic polyps: a prospective multicenter study of back-to-back video colonoscopies. Endoscopy. 2008;40:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 371] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 5. | Leufkens AM, DeMarco DC, Rastogi A, Akerman PA, Azzouzi K, Rothstein RI, Vleggaar FP, Repici A, Rando G, Okolo PI. Effect of a retrograde-viewing device on adenoma detection rate during colonoscopy: the TERRACE study. Gastrointest Endosc. 2011;73:480-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 6. | Rex DK, Cutler CS, Lemmel GT, Rahmani EY, Clark DW, Helper DJ, Lehman GA, Mark DG. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24-28. [PubMed] |
| 7. | van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 878] [Cited by in RCA: 917] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 8. | Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, Zwierko M, Rupinski M, Nowacki MP, Butruk E. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1287] [Cited by in RCA: 1468] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 9. | Brenner H, Chang-Claude J, Seiler CM, Hoffmeister M. Interval cancers after negative colonoscopy: population-based case-control study. Gut. 2012;61:1576-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 10. | Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1445] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 11. | Bressler B, Paszat LF, Vinden C, Li C, He J, Rabeneck L. Colonoscopic miss rates for right-sided colon cancer: a population-based analysis. Gastroenterology. 2004;127:452-456. [PubMed] |
| 12. | Rex DK, Imperiale TF, Latinovich DR, Bratcher LL. Impact of bowel preparation on efficiency and cost of colonoscopy. Am J Gastroenterol. 2002;97:1696-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 471] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 13. | Baxter NN, Sutradhar R, Forbes SS, Paszat LF, Saskin R, Rabeneck L. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology. 2011;140:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 404] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 14. | Lim G, Viney SK, Chapman BA, Frizelle FA, Gearry RB. A prospective study of endoscopist-blinded colonoscopy withdrawal times and polyp detection rates in a tertiary hospital. N Z Med J. 2012;125:52-59. [PubMed] |
| 15. | Baxter NN, Warren JL, Barrett MJ, Stukel TA, Doria-Rose VP. Association between colonoscopy and colorectal cancer mortality in a US cohort according to site of cancer and colonoscopist specialty. J Clin Oncol. 2012;30:2664-2669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 271] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 16. | Leufkens AM, van Oijen MG, Vleggaar FP, Siersema PD. Factors influencing the miss rate of polyps in a back-to-back colonoscopy study. Endoscopy. 2012;44:470-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 202] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 17. | Pickhardt PJ, Nugent PA, Mysliwiec PA, Choi JR, Schindler WR. Location of adenomas missed by optical colonoscopy. Ann Intern Med. 2004;141:352-359. [PubMed] |
| 18. | Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, Zauber AG, de Boer J, Fireman BH, Schottinger JE. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1561] [Article Influence: 141.9] [Reference Citation Analysis (0)] |
| 19. | Rex DK, Schoenfeld PS, Cohen J, Pike IM, Adler DG, Fennerty MB, Lieb JG, Park WG, Rizk MK, Sawhney MS. Quality indicators for colonoscopy. Gastrointest Endosc. 2015;81:31-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 836] [Article Influence: 83.6] [Reference Citation Analysis (0)] |
| 20. | Ng SC, Tsoi KK, Hirai HW, Lee YT, Wu JC, Sung JJ, Chan FK, Lau JY. The efficacy of cap-assisted colonoscopy in polyp detection and cecal intubation: a meta-analysis of randomized controlled trials. Am J Gastroenterol. 2012;107:1165-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 21. | Westwood DA, Alexakis N, Connor SJ. Transparent cap-assisted colonoscopy versus standard adult colonoscopy: a systematic review and meta-analysis. Dis Colon Rectum. 2012;55:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Subramanian V, Mannath J, Hawkey CJ, Ragunath K. High definition colonoscopy vs. standard video endoscopy for the detection of colonic polyps: a meta-analysis. Endoscopy. 2011;43:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 23. | Gralnek IM, Segol O, Suissa A, Siersema PD, Carr-Locke DL, Halpern Z, Santo E, Domanov S. A prospective cohort study evaluating a novel colonoscopy platform featuring full-spectrum endoscopy. Endoscopy. 2013;45:697-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Song JY, Cho YH, Kim MA, Kim JA, Lee CT, Lee MS. Feasibility of full-spectrum endoscopy: Korea’s first full-spectrum endoscopy colonoscopic trial. World J Gastroenterol. 2016;22:2621-2629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Gralnek IM, Siersema PD, Halpern Z, Segol O, Melhem A, Suissa A, Santo E, Sloyer A, Fenster J, Moons LM. Standard forward-viewing colonoscopy versus full-spectrum endoscopy: an international, multicentre, randomised, tandem colonoscopy trial. Lancet Oncol. 2014;15:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 26. | Papanikolaou IS, Apostolopoulos P, Tziatzios G, Vlachou V, Sioulas AD, Polymeros D, Karameris A, Panayiotides I, Alexandrakis G, Dimitriadis GD. Lower adenoma miss rate with FUSE vs. conventional colonoscopy with proximal retroflexion: a randomized back-to-back trial. Endoscopy. 2017;49:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Manes G, Devani M, Saibeni S, Arena I, de Nucci G, Andreozzi P, Omazzi B. Optimizing withdrawal time and use of FUSE endoscope achieve similar results in term of increasing adenoma detection rate. Results of a preliminary observational study. UEG J. 2016;2 Suppl 1. |
| 28. | Roepstorff S, Hadi S, Rasmussen M. Full Spectrum Endoscopy (FUSE) versus standard forward viewing endoscope (SFVE) in a high risk population. UEG J. 2016;2 Suppl 1. |
| 29. | Hassan C, Senore C, Radaelli F, De Pretis G, Sassatelli R, Arrigoni A, Manes G, Amato A, Anderloni A, Armelao F. Full-spectrum (FUSE) versus standard forward-viewing colonoscopy in an organised colorectal cancer screening programme. Gut. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Leong R, Ooi M, Corte C, Yau Y, Kermeen M, Alswaifi A, Katelaris P, McDonald C, Ngu M. Full Spectrum Endoscopy (FUSE) in the detection of inflammatory bowel disease neoplasia (FUSION): A randomized crossover tandem study versus conventional colonoscopy. UEG J. 2016;2 Suppl 1. |
| 31. | Rath T, Gralnek IM, Grauer M, Tontini G, Vieth M, Neurath M, Neumann H. Prospective 1: 1 randomized study to assess the performance characteristics of colorectal full spectrum endoscopy (FUSE). UEG J. 2015;2 Suppl 1. |
| 32. | van den Broek FJ, Kuiper T, Dekker E, Zwinderman AH, Fockens P, Reitsma JB. Study designs to compare new colonoscopic techniques: clinical considerations, data analysis, and sample size calculations. Endoscopy. 2013;45:922-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Uraoka T, Tanaka S, Oka S, Matsuda T, Saito Y, Moriyama T, Higashi R, Matsumoto T. Feasibility of a novel colonoscope with extra-wide angle of view: a clinical study. Endoscopy. 2015;47:444-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Uraoka T, Tanaka S, Matsuda T, Matsumoto T, Oka S, Nakadoi K, Moriyama T, Ogata H, Yahagi N, Saito Y. Impact of Prototype Extra-Wide-Angle-View Colonoscope in the Adenoma Detection Rate: A Multicenter Randomized Controlled Trial. Gastrointest Endosc. 2013;77:AB440. |
| 35. | Gluck N, Melhem A, Halpern Z, Mergener K, Santo E. A novel self-propelled disposable colonoscope is effective for colonoscopy in humans (with video). Gastrointest Endosc. 2016;83:998-1004.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Triadafilopoulos G, Li J. A pilot study to assess the safety and efficacy of the Third Eye retrograde auxiliary imaging system during colonoscopy. Endoscopy. 2008;40:478-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | DeMarco DC, Odstrcil E, Lara LF, Bass D, Herdman C, Kinney T, Gupta K, Wolf L, Dewar T, Deas TM. Impact of experience with a retrograde-viewing device on adenoma detection rates and withdrawal times during colonoscopy: the Third Eye Retroscope study group. Gastrointest Endosc. 2010;71:542-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 38. | Mishkin D. The Effect f the Third Eye® Retroscope® (TER) on Additional Adenoma Detection Rates (DR) During Colonoscopy in Above- Average Risk Patients for Colorectal Cancer in a Community Setting. Gastrointest Endosc. 2012;75:AB480-AB481. |
| 39. | Waye JD, Heigh RI, Fleischer DE, Leighton JA, Gurudu S, Aldrich LB, Li J, Ramrakhiani S, Edmundowicz SA, Early DS. A retrograde-viewing device improves detection of adenomas in the colon: a prospective efficacy evaluation (with videos). Gastrointest Endosc. 2010;71:551-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 40. | Rubin M, Lurie L, Bose K, Kim SH. Expanding the view of a standard colonoscope with the Third Eye Panoramic cap. World J Gastroenterol. 2015;21:10683-10687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Lenze F, Beyna T, Lenz P, Heinzow HS, Hengst K, Ullerich H. Endocuff-assisted colonoscopy: a new accessory to improve adenoma detection rate? Technical aspects and first clinical experiences. Endoscopy. 2014;46:610-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 42. | Sawatzki M, Meyenberger C, Marbet UA, Haarer J, Frei R. Prospective Swiss pilot study of Endocuff-assisted colonoscopy in a screening population. Endosc Int Open. 2015;3:E236-E239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 43. | Floer M, Biecker E, Fitzlaff R, Röming H, Ameis D, Heinecke A, Kunsch S, Ellenrieder V, Ströbel P, Schepke M. Higher adenoma detection rates with endocuff-assisted colonoscopy - a randomized controlled multicenter trial. PLoS One. 2014;9:e114267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 44. | Biecker E, Floer M, Heinecke A, Ströbel P, Böhme R, Schepke M, Meister T. Novel endocuff-assisted colonoscopy significantly increases the polyp detection rate: a randomized controlled trial. J Clin Gastroenterol. 2015;49:413-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 45. | van Doorn SC, van der Vlugt M, Depla A, Wientjes CA, Mallant-Hent RC, Siersema PD, Tytgat K, Tuynman H, Kuiken SD, Houben G. Adenoma detection with Endocuff colonoscopy versus conventional colonoscopy: a multicentre randomised controlled trial. Gut. 2017;66:438-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 46. | De Palma GD, Giglio MC, Bruzzese D, Gennarelli N, Maione F, Siciliano S, Manzo B, Cassese G, Luglio G. Cap cuff-assisted colonoscopy versus standard colonoscopy for adenoma detection: a randomized back-to-back study. Gastrointest Endosc. 2017; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 47. | Triantafyllou K, Polymeros D, Apostolopoulos P, Brandão C, Gkolfakis P, Repici A, Papanikolaou IS, Dinis-Ribeiro M, Alexandrakis G, Hassan C. Endocuff-assisted colonoscopy outperforms conventional colonoscopy to detect missed-adenomas: european multicenter, randomized, back-to-back study. UEG J. 2016;2 Suppl 1. |
| 48. | Floer M, Biecker E, Ameis D, Heinecke A, Ströbel P, Domagk D, Schepke M, Meister T. Endocuff-assisted colonoscopy significantly increases the adenoma detection rate: a randomized multicenter trial with 652 patients. UEG J. 2014;2 Suppl 1. |
| 49. | Wada Y, Wada Y, Wada M, Fukuma Y, Fukuda M, Ohtsuka K. Efficacy of Endocuff-assisted colonoscopy in the detection of colorectal polyps. UEG J. 2016;2 Suppl 1. |
| 50. | Marsano J, Tzimas D, Mckinley M, Robbins D, Mammen A, Sun E, Chugh P, Razavi F, Hasan N, Buscaglia J. P, Gross S. Endocuff Assisted Colonoscopy Increases Adenoma Detection Rates: a Multi-Center Study. Gastrointest Endosc. 2014;79:AB550. |
| 51. | García D, Gonzalez-Fernandez C, Barreto-Zuñiga R, Aguilar-Olivos N, Romano A, Grajales-Figueroa G, Tellez-Avila F. Higher Adenoma Detection Rate With Endocuff: A Randomized Controlled Trial. Gastrointest Endosc. 2016;83:AB193. |
| 52. | Patel A, Grewal J, Karnes W. Endocuff Improves GI Fellows Colonoscopy Performance (PDR, ADR and number of polyps/colonoscopy) Without Affecting Time of Procedure. Gastrointest Endosc. 2016;83:AB535. |
| 53. | Chin M, Chen CL, Karnes W. Improved Polyp Detection Among High Risk Patients With Endocuff. Gastrointest Endosc. 2015;81:AB283. |
| 54. | Bensuleiman Y, Ikezawa N, Sasaki K, Yoshida S, Myoujou S, Nakajima T, Yoshida S. Adenoma detection with Endocuff-assisted colonoscopy versus cap-assisted colonoscopy. UEG J. 2016;2 Suppl 1. |
| 55. | Cavallaro L, Pierenrico L, Galliani E, Dal Pont E, Giacomin A, Iuzzolino P, E . M, Roldo C, Soppelsa F, Di Camillo S, Mel R, Germanà B. Higher number of small (< 10mm) adenomas detected with Endocuff-assisted colonoscopy in a screeening popultion. UEG J. 2016;2 Suppl 1. |
| 56. | Higham-Kessler J, Austin G. Endocuff-Assisted Colonoscopy is Associated with an Increase in the Mean Number of Polyps but a Similar Adenoma Detection Rate, Surveillance Interval Recommendation, and Amount of Sedation Medications. Gastrointest Endosc. 2016;83:AB538. |
| 57. | Chin M, Karnes W, Jamal MM, Lee JG, Lee R, Samarasena J, Bechtold ML, Nguyen DL. Use of the Endocuff during routine colonoscopy examination improves adenoma detection: A meta-analysis. World J Gastroenterol. 2016;22:9642-9649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 58. | Tsiamoulos Z, Misra R, Bourikas L, Rajaratnam R, Patel K, Thomas-Gibson S, Haycock A, Suzuki N, Beintaris I, Saunders B. Endocuff-Vision: Impact on Colonoscopist Performance During Screening. Gastrointest Endosc. 2015;81:AB209. |
| 59. | Ngu WS, Bevan R, Tsiamoulos Z, Bassett P, Hoare Z, Rutter M, Totton N, Lee T, Ramadas A, Silcock J. Rees C. Improved adenoma detection with Endocuff-VisionTM - a multicentre randomisedcontrolled trial. UEG J. 2016;2 Suppl 1. |
| 60. | Bhattacharyya R, Chedgy F, Kandiah K, Fogg C, Higgins B, Haysom-Newport B, Gadeke L, Thursby-Pelham F, Ellis R, Goggin P. The first randomized controlled trial of Endocuff Vision® assisted colonoscopy versus standard colonoscopy for polyp detection in bowel cancer screening (E-cap study). UEG J. 2016;2 Suppl 1. |
| 61. | Dik VK, Gralnek IM, Segol O, Suissa A, Belderbos TD, Moons LM, Segev M, Domanov S, Rex DK, Siersema PD. Multicenter, randomized, tandem evaluation of EndoRings colonoscopy--results of the CLEVER study. Endoscopy. 2015;47:1151-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 62. | Gralnek IM, Suissa A, Domanov S. Safety and efficacy of a novel balloon colonoscope: a prospective cohort study. Endoscopy. 2014;46:883-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 63. | Halpern Z, Gross SA, Gralnek IM, Shpak B, Pochapin M, Hoffman A, Mizrahi M, Rochberger YS, Moshkowitz M, Santo E. Comparison of adenoma detection and miss rates between a novel balloon colonoscope and standard colonoscopy: a randomized tandem study. Endoscopy. 2015;47:301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 64. | Rey JW, Haschemi J, Tresch A, DüMcke S, Borger D, Kirchner A, Kiesslich R, Hoffman A. G-EYE Advanced Colonoscopy for Increased Polyp Detection Rate- Randomized Tandem Study With Different Endoscopist. Gastrointest Endosc. 2015;81:AB215. |
| 65. | Halpern Z, Ishaq S, Neumann H, Dobosz M, Viale E, Hoffman A, Hendel J, Senturk H, Jacob H, Kiesslich R. G-EYE colonoscopysignificantly improves adenoma detection rates - initila resuts of a multicenter prospective cohort study. UEG J. 2014;2 Suppl 1. |
| 66. | Hendel J, Mizrahi M, Hoffman A, Epshtein J, Ishaq S, Jacob H, Israeli E, Vilmann P, Hershcovici T, Rey JW. Prospective Randomized Multicenter Trial to Compare Adenoma Detection Rate of HD Colonoscopy With Standard HD Colonoscopy - Intermediate Results. Gastrointest Endosc. 2015;81:AB145-AB146. |
| 67. | Shirin H, Shpak B, Epshtein J, Vilmann P, Hoffman A, Ishaq S, Testoni P, Sanduleanu S, Neumann H, Goetz M. Comparison of Adenoma Detection Rate by a High Definition Colonoscopy versus Standard High Definition Colonoscopy- A Prospective Randomized Multicenter Trial. Gastrointest Endosc. 2016;83:AB192. |
| 68. | Triantafyllou K, Gkolfakis P, Viazis N, Tsibouris P, Tsigaridas A, Apostolopoulos P, Anastasiou J, Hounda E, Skianis I, Katopodi K. A 13-year time trend analysis of 3724 small bowel video capsule endoscopies and a forecast model during the financial crisis in Greece. Eur J Gastroenterol Hepatol. 2017;29:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |