Published online Jan 14, 2017. doi: 10.3748/wjg.v23.i2.306
Peer-review started: July 22, 2016
First decision: September 20, 2016
Revised: September 30, 2016
Accepted: October 27, 2016
Article in press: October 27, 2016
Published online: January 14, 2017
Processing time: 174 Days and 20.9 Hours
To assess the efficacy and safety of in vivo electroporation (EP)-mediated dual-plasmid hepatitis B virus (HBV) DNA vaccine vs placebo for sequential combination therapy with lamivudine (LAM) in patients with chronic hepatitis B.
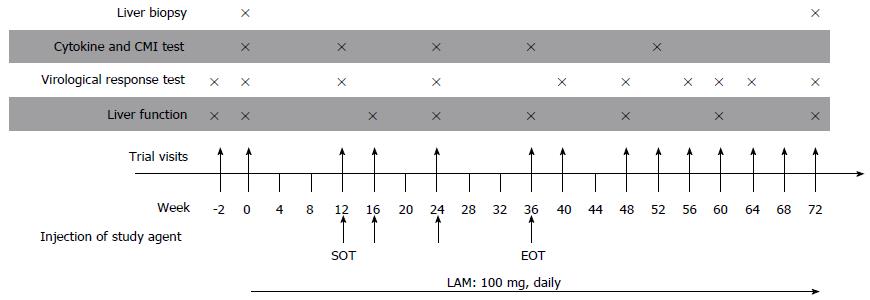
Two hundred and twenty-five patients were randomized to receive either LAM + vaccine (vaccine group, n = 109) or LAM + placebo (control group, n = 116). LAM treatment lasted 72 wk. Patients received the DNA vaccine or placebo by intramuscular injection mediated by EP at weeks 12 (start of treatment with vaccine or placebo, SOT), 16, 24, and 36 (end of treatment with vaccine or placebo, EOT).
In the modified intent-to-treat population, more patients had a decrease in HBV DNA > 2 log10 IU/mL in the vaccine group at week 12 after EOT compared with the control group. A trend toward a difference in the number of patients with undetectable HBV DNA at week 28 after EOT was obtained. Adverse events were similar. In the dynamic per-protocol set, which excluded adefovir (ADV) add-on cases at each time point instantly after ADV administration due to LAM antiviral failure, more patients had a decrease in HBV DNA > 2 log10 IU/mL in the vaccine group at week 12 and 28 after EOT compared with the control group. More patients with undetectable HBV DNA at week 28 after EOT in the vaccine group were also observed. Among patients with a viral load < 1000 copies/mL at week 12, more patients achieved HBeAg seroconversion in the vaccine group than among controls at week 36 after EOT, as well as less virological breakthrough and YMDD mutations.
The primary endpoint was not achieved using the HBV DNA vaccine. The HBV DNA vaccine could only be beneficial in subjects that have achieved initial virological response under LAM chemotherapy.
Core tip: The study aimed to assess the efficacy and safety of in vivo electroporation-mediated dual-plasmid hepatitis B virus (HBV) DNA vaccine vs placebo for sequential combination therapy with lamivudine (LAM) in patients with chronic hepatitis B. The results indicated that the primary endpoint was not achieved using the HBV DNA vaccine. The HBV DNA vaccine might only be beneficial in subjects that have achieved initial virological response under LAM chemotherapy.
- Citation: Yang FQ, Rao GR, Wang GQ, Li YQ, Xie Y, Zhang ZQ, Deng CL, Mao Q, Li J, Zhao W, Wang MR, Han T, Chen SJ, Pan C, Tan DM, Shang J, Zhang MX, Zhang YX, Yang JM, Chen GM. Phase IIb trial of in vivo electroporation mediated dual-plasmid hepatitis B virus DNA vaccine in chronic hepatitis B patients under lamivudine therapy. World J Gastroenterol 2017; 23(2): 306-317
- URL: https://www.wjgnet.com/1007-9327/full/v23/i2/306.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i2.306
Chronic hepatitis B (CHB) is a chronic infection caused by the hepatitis B virus (HBV). There is no specific symptoms and the diagnosis is based on the clinical description accompanied by laboratory findings (IgM anti-HBc-negative and positive results for HBsAg, HBeAg, or HBV DNA)[1]. The CHB burden is global, but more significant in Asia, Pacific Islands, Sub-Saharan Africa, Amazon, and Eastern Europe[2]. HBV infection is endemic in China, with about 110 million HBV carriers and at least 300000 people dying from HBV-related diseases each year[3].
Antiviral therapy aiming at clearing or radically inhibiting virus replication is the most effective treatment for CHB, and consists of interferon and reverse transcriptase inhibitors of oral nucleos(t)ide analogues (NUCs) such as lamivudine (LAM), adefovir (ADV), entecavir (ETV), and tenofovir (TDF). Impaired host immune responses in patients with CHB constitute an obstacle to virus clearance. Therefore, alternative therapeutic strategies using vaccines have been suggested[4,5].
Compared with protein vaccines, DNA vaccines mediate the de novo intracellular viral protein expression and synthesis in host cells, consequently boosting host-specific T cell-mediated response against HBV[6-8]. However, a recent study revealed that DNA vaccines have limited efficacy[9]. The humoral and cellular immune responses to an HBV PreS2/S DNA vaccine were lower in chronic HBV carriers than in healthy volunteers[7,10]. Therefore, alternative gene delivery strategies are being considered, including in vivo electroporation (EP), which has been studied in animal models and approved for a phase I trial among healthy individuals[11].
In order to enhance the cellular immune response to DNA vaccines, we developed a construct encoding an interleukin-2 (IL-2) and interferon-γ (IFN-γ) fusion protein to potentiate local antigen presentation by antigen-presenting cells, and consequently to increase immunogenicity against HBV[12,13].
Therefore, we conducted a phase IIa study in which patients with CHB received LAM with or without EP-mediated dual-plasmid HBV DNA vaccine (ED-DNA). The vaccine has been shown to be safe and immunologically effective, with the HBV-specific T-cell responses induced under LAM chemotherapy showing a correlation with suppression of viral replication[14]. LAM is a well-tolerated suppressor of viral replication with a strong antiviral potential, and its effects are similar in Asian and Western populations[15]. Nevertheless, it was reported that a large number of CHB patients under LAM monotherapy develop drug resistance mutations and virological breakthrough (VBT)[16]. As suggested by a previous study[14], adding vaccine therapy to LAM could be effective to prevent VBT. The aim of the present study was to further assess the efficacy and safety of the dual-plasmid strategy with LAM in patients with CHB compared with placebo plus LAM.
This was a double-blind, randomized, placebo-controlled study, approved by the Chinese State Food and Drug Administration (CFDA) (license number: 2006L03542). Patients with CHB on a 72-wk LAM treatment (100 mg daily; GlaxoSmithKline Pharmaceutical Corporation, Suzhou, Jiangsu Province, China) were enrolled at 18 centers. This trial was registered in ClinicalTrials.org (NCT01487876) and approved by the ethics committee of the Peking University First Hospital, as well as by the committee of each of participating center. Written informed consent was obtained from each participant prior to enrolment. The study was conducted between April 2011 and October 2013 and in compliance with the Declaration of Helsinki. The study was monitored by Beijing Guoxin Ze Ding International Pharmaceutical Co., Ltd. (China), an independent contract research organization.
CHB diagnosis was carried out based on clinical symptoms and liver function tests[17]. Inclusion criteria were: (1) 18-55 years old; (2) HBsAg and HBeAg positivity for at least 6 mo with HBV DNA > 106 copies/mL (1.79 × 105 IU/mL) within 4 wk prior to randomization; and (3) serum alanine aminotransferase (ALT) 2-10 times the upper limit of normal within 4 wk before randomization. Exclusion criteria were: (1) suspected hepatic tumors or alpha-fetoprotein > 100 ng/mL; (2) cirrhosis; (3) co-infection with hepatitis A, C, D, or E virus; (4) co-infection with human immunodeficiency virus; (5) other causes of liver disease; (6) serious medical or psychiatric illness; (7) abnormal serum creatinine, thrombocyte count, hemoglobin, or serum total bilirubin; or (8) pregnancy. Previous treatments for CHB were allowed, but not within 6 mo before study participation.
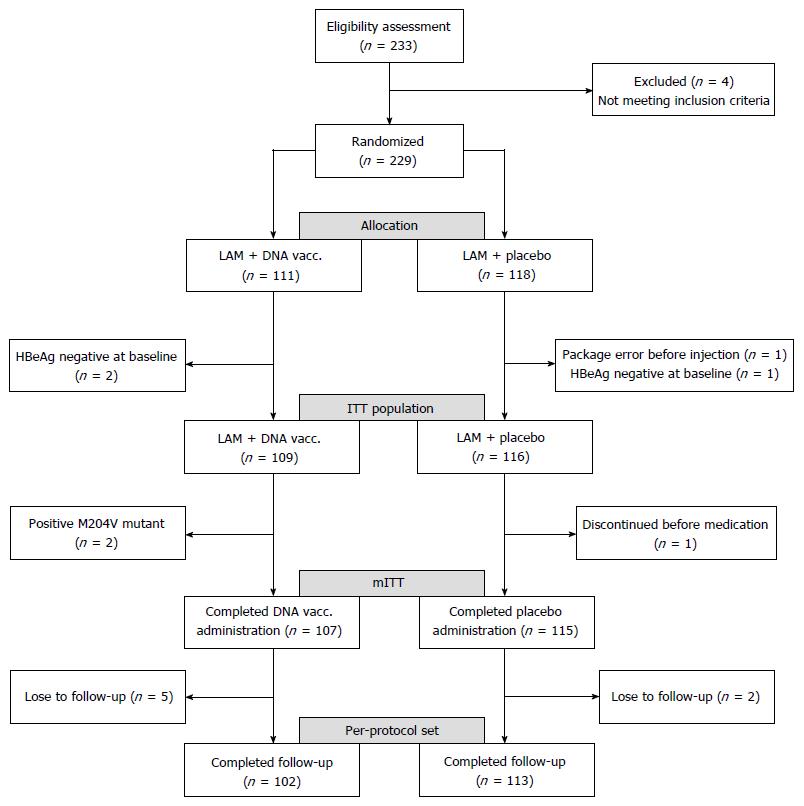
Between April 2011 and October 2013, 229 patients were screened and randomly assigned to LAM + vaccine (vaccine) and LAM + placebo (control) groups (Figure 1); 111 patients were randomized to the vaccine arm and two were excluded due to loss to follow-up or protocol violation; 118 patients were randomized to the control arm and two were excluded (Figure 1). Therefore, the 225 patients that received at least one dose of study medication were designated as the intent-to-treat (ITT) population, including 109 in the vaccine group and 116 in the control group; 222 patients were analyzed as the modified ITT (mITT) population, excluding two patients with HBV M204V mutant in the vaccine arm and one who discontinued the allocated study agent in the control arm. Finally, 102 (91.9%) and 113 (95.7%) patients with good compliance and no additional dosing were included in the per-protocol set (PPS) analysis (Figure 1).
Baseline characteristics were comparable between the two groups (Table 1). At the start of vaccine treatment (SOT), serum HBV DNA, HBeAg, and HBsAg were similar between the two groups (all P > 0.05) (Table 1).
| Characteristic | Vacc. (n = 107) | Ctr. (n = 115) | P value |
| Demographics | |||
| Age, median (IQR) | 28.9 (23.1-37.1) | 29.3 (24.0-35.4) | 0.950 |
| Male sex, n (%) | 86 (78.9) | 86 (74.1) | 0.400 |
| Weight (kg), median (IQR) | 62.5 (56.0-70.0) | 63.0 (56.0-70.0) | 0.907 |
| Genotype B, n (%) | 42/107 (39.3) | 39/115 (33.9) | 0.409 |
| Week 0 laboratory results | |||
| ALT (IU/L), median (IQR) | 119.0 (94.0-167.5) | 136.0 (102.6-195.3) | 0.066 |
| HBV DNA (log10 IU/mL), median (IQR) | 8.1 (7.7-8.3) | 8.1 (7.5-8.4) | 0.9532 |
| HBeAg (COI), median (IQR) | 852.0 (223.5-1093.0) | 926.6 (508.5-1076.0) | 0.1972 |
| HBsAg (IU/mL), median (IQR) | 10261.0 (3953.0-24089.0) | 8637.0 (2748.0-24436.0) | 0.7542 |
| Week 12 laboratory results1 | |||
| HBV DNA (log10 IU/mL), median (IQR) | 3.5 (2.6-4.5) | 3.4 (2.6-4.6) | 0.7542 |
| HBeAg (COI), median (IQR) | 147.4 (15.6-650.0) | 45.9 (11.9-1011.0) | 0.6052 |
| HBsAg (IU/mL) median (IQR) | 4072.0 (1608.0-8514.0) | 3023.5 (1167.0-11194.0) | 0.4602 |
The numbers of patients who needed ADV add-on treatment, as proposed by the protocol, could be determined by the physicians in their respective local clinical centers, and are presented in Table 2. During the 72-wk study period, a similar proportion of patients in the two arms needed ADV add-on therapy.
| Week | Vacc. (n = 102) | Ctr. (n = 113) |
| 24 | 1 (0.98) | 0 (0.00) |
| 36 | 2 (1.96) | 1 (0.88) |
| 40 | 4 (3.92) | 5 (4.42) |
| 48 | 7 (6.86) | 8 (7.08) |
| 52 | 11 (10.78) | 14 (12.39) |
| 56 | 17 (16.67) | 18 (15.93) |
| 60 | 27 (26.47) | 26 (23.01) |
| 64 | 34 (33.33) | 32 (28.32) |
Patients were randomly assigned to either an LAM + vaccine (vaccine) or an LAM + placebo (control) group. Identical labels with computer-generated random unique numeric codes were used to identify all study drug vials. An independent biostatistician was in charge of randomization using the SAS program (SAS Institute Inc., Cary, NC, United States), as well as labeling. The block size and seed numbers were sealed with the randomization list and kept at the CFDA. Participants, investigators, and sponsors were blinded until the end of the trial.
As described previously[18], the dual-plasmid consists of a pS2.S HBV DNA vaccine plasmid (pcDNA3.1+/S2.S) encoding the HBV envelope middle protein and a pFP adjuvant plasmid (pcDNA3.1-/IL2+IFN-γ) containing the fused sequence of human IL-2 (hIL-2) and human IFN-γ (hIFN-γ). The S2.S gene was amplified from the plasmid pHBVα1 with the whole HBV S gene fragment (type ayw) by PCR, and then inserted in the eukaryotic expression vector pcDNA3.1+ after the use of the EcoR I enzyme. Supplementary Figure 1A shows a representative map of the vaccine plasmid pS2.S of the HBV DNA vaccine. Total RNA was extracted from human peripheral blood mononuclear cells (PBMCs), and cDNA was synthesized by reverse transcription. PCR was carried out to amplify the recombined gene fragments of hIL-2 and hIFN-γ. The fusion gene was named hIIF. The hIIF gene was cloned conventionally into the eukaryotic expression vector pcDNA3.1- after digestion with EcoR I. Supplementary Figure 1B shows a representative map of the adjuvant plasmid pFP of the HBV DNA vaccine. The pharmaceutical grade dual-plasmid was re-constituted in phosphate-buffered saline and evaluated for efficacy, toxicity, and safety in animals as an investigative new drug[19,20]. The plasmids were manufactured by Guangzhou Biotech Pharmaceutical Co., Ltd., China.
Patients in the vaccine group received four immunizations with ED-DNA consisting of a primer (week 12/SOT) and three boosting doses (weeks 16, 24, and 36/end of vaccine treatment, EOT) (Figure 2)[14]. Each dose of 4 mL of ED-DNA (2 mg of DNA vaccine plasmid and 2 mg of adjuvant plasmid) was intramuscularly injected into four sites of the deltoid muscle on both sides of the patient, immediately followed by in vivo EP. Placebo (4 mL of vaccine diluent) was administered using the same method.
The custom-designed electro-pulse generator can generate square wave signals with a voltage of 0-300 V and pulse duration of 0.01-100 ms in any specific combination sequences. The device uses a medical-grade sterilized two-needle electrode made of silver Chinese acupuncture needles (diameter: 0.3 mm; length: 12 mm, for single use only). The two needles are 1 cm apart.
Responses were assessed at 12, 28, and 36 wk after EOT. The primary endpoint was the rate of undetectable HBV DNA or HBeAg seroconversion (defined as loss of HBeAg and presence of HBeAb). The secondary endpoints included: (1) HBV DNA load suppression (number of patients who had a decrease of HBV DNA > 2 log10 IU/mL); (2) occurrence of LAM-resistant mutations or VBT; and (3) normalization of ALT levels.
At the end of follow-up, all serum samples were assayed in a central laboratory or at each participating center, as previously described[14]. HBV serological markers at each visit were assayed using the same lots of reagents. Serum HBsAg/HBeAg status and HBV DNA levels were detected at the central laboratory (AxSYM test, dynamic range of HBsAg was 0.05-250 IU/mL, Abbot, Chicago, IL, United States; COBAS AmpliPrep/COBAS TaqMan HBV Test, ranging from 20 to 1.7 × 108 IU/mL, Roche Diagnostics, Basel, Switzerland).
Venous blood samples were obtained and PBMCs were separated for immunologic response test. The number of IFN-γ-secreting T lymphocytes among PBMCs was assessed using an enzyme-linked immunosorbent spot (ELISPOT) assay using the hIFN-γ ELISPOT kit (BD Biosciences, San Diego, CA, United States), as previously described[14]. Peptides synthesized by GL Biochem Ltd (Shanghai, China), including HLA-2 restricted CTL epitope peptides and proteins in HBV (preS2 + S) T cell antigen, were used to stimulate the cells during the ELISPOT assays. The number of IFN-γ spots was counted on an AID ELISPOT reader system (AID, Germany). Data are expressed as mean SFCs/106 PBMCs. On the other hand, PBMCs were cultured under stimulation with HBsAg. After 9 d, supernatants were assessed for HBV antigen-specific IFN-γ secretion by enzyme-linked immunosorbent assay (ELISA).
PBMCs were used for cytotoxicity analysis by flow cytometry (FACS Calibur; BD Biosciences, Franklin Lake, NJ, United States) as follows. PBMCs were first stained with PerCP-conjugated α-CD3 and APC-conjugated α-CD8. Then, fixation and permeabilization were performed, followed by staining with PE-conjugated IFN-γ monoclonal antibodies (BD Biosciences, Franklin Lake, NJ, United States). Results were analyzed using the FlowJo software (version 5.7.2; Tree Star Inc., Ashland, OR, United States).
Among all participants, 45 patients were recruited 50-70 wk after the 72-wk LAM treatment period and before unblinding. Then, IFN-γ levels in PMBC supernatants were detected by ELISA after 9 d of in vitro culture after HBsAg stimulation. In this case, a positive T-cell response was defined as an increase in IFN-γ secretion by two times compared to the control level. Therefore, the threshold of < 100 pg/mL upon 9 d of culture of PBMCs stimulated by HBsAg was selected. A positive response of CD8+ intracellular INF-γ secreting T-cells in PMBCs was defined as no less than 0.1% frequency of CD8 cells showing positive IFN-γ intracellular staining, measured by flow cytometry (FACS), upon stimulation by various HBV envelope antigens.
All health-related events were documented during the 72-wk treatment period. Physical examinations, vital signs, hematology tests, and urinalysis were performed. In addition, anti-nuclear antibodies and anti-DNA antibodies were measured in all subjects. Patients with a failure to LAM antiviral therapy or with VBT occurrence received ADV (10 mg/d) as add-on treatment.
The adverse events were evaluated by clinicians and defined as mild (transient, tolerable, and minor symptoms disappearing by themselves in the intervals of drug administration), moderate (relatively long-lasting symptoms affecting daily life of the patients and requiring reduction of drug dose or administration frequency), or severe (long-lasting symptoms causing disability to the patients who only recovered after drug discontinuation and with appropriate treatments).
We assumed that 40% of patients would have an HBV DNA loss in the DNA vaccine group compared with 20% in the placebo arm at EOT. Therefore, 106 patients per group (a total of 212 patients) were needed to achieve a 90% power with a two-sided significance level of 0.05. Assuming a drop-out rate of 15%, 240 patients were required.
A database was established using Epidata 3.1 (http://www.epidata.dk/). SAS 9.2 (SAS institute Inc., Cary, NC, United States) was used for statistical analyses. Data double entry was performed, including range and consistency checks. Analysis was conducted on all eligible patients according to the ITT principle. A dynamic PPS (dPPS) analysis was performed to take into account the patients who received ADV as add-on treatment. All categorical data were analyzed using the χ2 test, Fisher’s exact test, or Mann-Whitney U test, as appropriate. Continuous data were analyzed using the Student’s t test or Wilcoxon Rank Sum test, as appropriate. P values < 0.05 were considered statistically significant.
The mITT analysis revealed a modest and transient therapeutic effect. At week 12 after EOT, there were more patients with a decrease in HBV DNA > 2 log10 IU/mL in the vaccine group (10.3% vs 3.5%, P = 0.044) compared with the control group. In addition, there was a trend toward differences in the number of patients in the two groups with undetectable HBV DNA at 28 wk after EOT (28.0% vs 17.4%, P = 0.058) (Table 3) and a decrease in HBeAg > 1 log10 IU/mL (34.6% vs 23.5%, P = 0.068) at 36 wk after EOT (Table 3). There was no difference in HBV DNA levels between the two groups at any time point.
| Population | Response | 12 wk after EOT (week 48) | 28 wk after EOT (week 64) | 36 wk after EOT (week 72) | ||||||
| Vacc. | Ctr. | P value | Vacc. | Ctr. | P value | Vacc. | Ctr. | P value | ||
| mITT | No. of patients | 107 | 115 | 107 | 115 | 107 | 115 | |||
| HBV DNA (log10 IU/mL), median (IQR) | 3.06 (0-4.63) | 3.05 (1.43-5.02) | 0.742 | 2.18 (0-5.01) | 2.78 (1.41-4.86) | 0.164 | 1.88 (0-4.36) | 2.37 (0-5.38) | 0.329 | |
| HBV DNA > 2 log10 IU/mL decrease, n (%) | 11 (10.3) | 4 (3.48) | 0.044 | 13 (12.2) | 6 (5.2) | 0.065 | 10 (9.4) | 7 (6.1) | 0.362 | |
| HBV DNA undetectable, n (%) | 27 (25.2) | 23 (20.0) | 0.351 | 30 (28.0) | 20 (17.4) | 0.058 | 24 (22.4) | 26 (22.6) | 0.975 | |
| HBeAg > 1 log10 IU/mL decrease, n (%) | 22 (20.6) | 22 (19.1) | 0.789 | ND | ND | 37 (34.6) | 27 (23.5) | 0.068 | ||
| HBeAg seroconversion, n (%) | 16 (15.0) | 10 (8.7) | 0.147 | ND | ND | 18 (16.8) | 15 (13.0) | 0.429 | ||
| dPPS | No. of patients | 98 | 108 | 75 | 87 | 68 | 81 | |||
| HBV DNA (log10 IU/mL), median (IQR) | 2.99 (0-4.59) | 3.05 (1.43-5.02) | 0.593 | 2.16 (0-4.66) | 2.78 (1.41-4.86) | 0.104 | 1.85 (0-3.78) | 2.37 (0-5.38) | 0.262 | |
| HBV DNA > 2 log10 IU/mL decrease, n (%) | 11 (11.2) | 4 (3.8) | 0.038 | 13 (17.3) | 6 (7.1) | 0.040 | 10 (14.7) | 7 (8.9) | 0.246 | |
| HBV DNA undetectable, n (%) | 27 (27.6) | 23 (21.3) | 0.296 | 29 (38.7) | 20 (23.0) | 0.030 | 24 (35.3) | 26 (32.1) | 0.681 | |
| HBeAg > 1 log10 IU/mL decrease, n (%) | 20 (20.4) | 21 (19.4) | 0.863 | ND | ND | 29 (42.7) | 25 (30.9) | 0.136 | ||
| HBeAg seroconversion, n (%) | 16 (16.3) | 10 (9.3) | 0.127 | ND | ND | 18 (26.5) | 15 (18.5) | 0.244 | ||
| Subgroup1 | No. of patients | 11 | 19 | 11 | 19 | 11 | 19 | |||
| HBV DNA (log10 IU/mL), median (IQR) | 0 (0-1.16) | 1.43 (0-2.64) | 0.268 | 0 (0-0) | 1.73 (0-3.6) | 0.053 | 0 (0-0) | 0 (0-4.32) | 0.172 | |
| HBV DNA undetectable, n (%) | 8 (72.7) | 7 (36.8) | 0.128 | 8 (72.7) | 8 (44.1) | 0.142 | 7 (63.6) | 9 (47.4) | 0.466 | |
| HBeAg seroconversion, n (%) | 5 (45.5) | 2 (10.5) | 0.068 | ND | ND | 6 (54.6) | 3 (15.8) | 0.042 | ||
| dPPS of subgroup2 | No. of patients | 11 | 19 | 9 | 18 | 9 | 17 | |||
| HBV DNA (log10 IU/mL), median (IQR) | 0 (0-1.16) | 1.43 (0-2.64) | 0.268 | 0 (0-0) | 1.73 (0-3.6) | 0.053 | 0 (0-0) | 0 (0-4.32) | 0.172 | |
| HBV DNA undetectable, n (%) | 8 (72.7) | 7 (36.8) | 0.128 | 8 (88.9) | 8 (44.4) | 0.042 | 7 (77.8) | 9 (52.9) | 0.399 | |
| HBeAg seroconversion, n (%) | 5 (45.5) | 2 (10.5) | 0.068 | ND | ND | 6 (66.7) | 3 (17.7) | 0.028 | ||
Detection for drug-resistant mutants, as well as all other HBV serological markers, showed no remarkable differences between the two groups throughout the study.
Taking into account the patients with ADV add-on treatment (Table 2), a dPPS was performed: cases were excluded dynamically from the PPS at each visit when they had received ADV add-on treatment. Compared with the control group, more patients with a decrease in HBV DNA > 2 log10 IU/mL were found in the vaccine group at weeks 12 (11.2% vs 3.8%, P = 0.038) and 28 (17.3% vs 7.1%, P = 0.040) after EOT. In addition, there were more patients with undetectable HBV DNA at week 28 after EOT (38.7% vs 23.0%, P = 0.030) in the vaccine group compared with controls (Table 3).
A stratified analysis was performed, as previously described[21]. At SOT, 30 patients (11 and 19 in the vaccine and control groups, respectively) with serum HBV DNA levels < 1000 copies/mL (1.79 × 102 IU/mL) were selected. More patients achieved HBeAg seroconversion in the vaccine group (6/11, 54.5%) than among controls (3/19, 15.8%) (P = 0.042) at week 36 after EOT (Table 3). At SOT, there were three cases with HBeAg seroconversion in the vaccine group compared to four in the control group. The three patients in the vaccine group remained in seroconversion status throughout the trial until the endpoint, while the seroconversion status among the four control patients was unstable due either to HBeAb loss or reappearance of HBeAg, resulting in only two patients remaining in seroconversion at week 36 after EOT. Two patients in the vaccine group (2/11) had HBsAg levels below 1000 IU/mL at SOT, and showed HBeAg seroconversion at week 36 after EOT. Meanwhile, eight patients in the control group (8/19) had HBsAg levels below 1000 IU/mL at SOT, with two showing HBeAg seroconversion at week 36 after EOT. In addition, less VBT (18.2% vs 47.4%, P = 0.140) and YMDD mutations (9.1% vs 42.1%, P = 0.100) were observed in the vaccine group at week 36 after EOT, but the difference was not statistically significant.
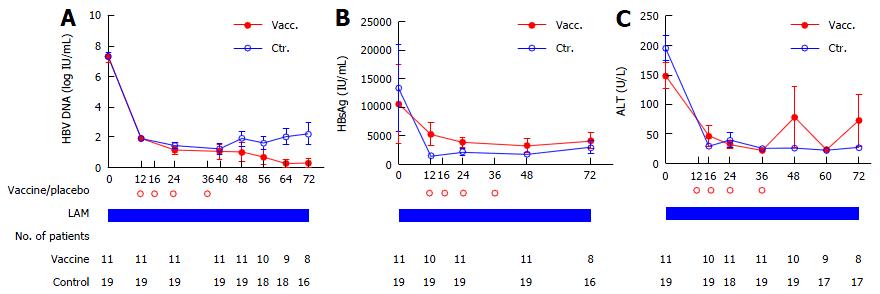
Furthermore, in the dPPS analysis of the 30 patients by excluding the ADV add-on cases at each time point instantly after ADV administration due to LAM antiviral failure, viral load decrease at weeks 28 (P = 0.017) and 36 (P = 0.023) after EOT was more remarkable in the LAM + vaccine group (Figure 3A), in which more patients (8/9 vs 8/18, P = 0.042) had serum HBV DNA maintained at undetectable levels at week 28 after EOT. Similarly, less VBT (0.0% vs 41.2%, P = 0.058) and YMDD mutations (0.0% vs 35.3%, P = 0.063) were observed in the vaccine group at week 36 after EOT.
Baseline serum HBsAg levels at SOT were higher in the vaccine group (mean = 5287 IU/mL) than in controls (1494 IU/mL) (Figure 3B), probably due to lower ALT levels at week 0 (mean of 148 U/L) compared with control values (mean of 195 U/L, Figure 3C). As shown in Figure 3B, throughout the trial, serum HBsAg tended to decrease slightly (4116 IU/mL at week 36 after EOT) in the vaccine group, while remaining at a similar level with some elevation in the control group (2991 IU/mL at week 36 after EOT).
In the present study, normalized ALT levels displayed a substantial decrease during the first 16 wk of treatment and then stabilized (Figure 3C). No significant differences were observed between the two groups. However, two patients in the vaccine group experienced a flare-up with ALT elevation up to 593 U/L in one VBT patient and 424 U/L in another patient with well suppressed viral status at weeks 12 and 36 after EOT.
These analyses were also performed in the group of patients with serum HBV DNA levels > 1000 copies/mL (1.79 × 102 IU/mL), but no difference could be observed in any parameter.
Forty-five patients were recruited 50-70 wk after EOT, including 22 and 23 in the vaccine and control groups, respectively, to assess immuno-memory responses in venous blood samples.
After 9 d of HBsAg stimulation, 19/22 (86.4%) patients showed positive immunological response with IFN-γ secretion in the vaccine group, compared with 13/23 (56.5%) patients in the control group (P = 0.027). In addition, intracellular IFN-γ secreting CD3+/CD8+ T cells among PMBCs were detected using flow cytometry after 6-8 h of stimulation with HBV-related antigens, and 17/22 (77.3%) vs 10/23 (43.5%) patients showed positive results in the vaccine and control groups, respectively (P = 0.021) (Table 4).
In either ex vivo or in vitro ELISPOT assay, the two groups showed similar levels of IFN-γ-secreting T-cells per million PBMCs, after stimulation by HBV specific peptide pools for 48 h or 9 d.
Vaccine injections were well tolerated with limited mild adverse events observed in 14 of 111 (12.6%) DNA vaccine recipients compared with 15 of 118 (12.7%) patients receiving placebo. Adverse effects did not lead to any dropout during the study. Only two (1.8%) patients in the vaccine group and one (0.9%) in the control group reported non-drug-related serious adverse events during treatment (P > 0.05). In the vaccine group, a 38-year-old male patient had a severe CHB relapse due to LAM resistance at week 60. The other patient was a 46-year-old female who underwent surgery for breast fibroma and thyroid adenoma at weeks 54 and 55. In the control group, a 43-year-old female patient was diagnosed with primary liver cancer by ultrasound and computed tomography at week 36 after EOT. All three patients were admitted to the hospital for further treatments. These events were reported as severe adverse events. Four (3.6%) patients receiving the DNA vaccine and five (4.2%) patients receiving the placebo had increased total bilirubin levels at week 36 after EOT (P > 0.05). No clinically significant serological abnormalities were observed. No antinuclear antibodies against double-strand DNA were detected in any patients during and after treatment.
The aim of this study was to assess the efficacy of the dual-plasmid strategy with LAM in patients with CHB compared with placebo + LAM as a phase IIb trial. Our results indicated that the primary endpoint was not achieved using the ED-DNA. The HBV DNA vaccine could only be beneficial in subjects that have achieved initial virological response under LAM chemotherapy.
The efficacy did not reach the pre-set primary endpoint as per the registered protocol, but the mITT analysis showed that serum HBV DNA and HBeAg levels decreased, and the secondary efficacy measures of the treatment were better in the DNA vaccine + LAM group than in the placebo + LAM group. There was a higher number of patients with a decrease in HBV DNA > 2 log10 IU/mL in the vaccine group at week 12 after EOT compared with the control group. There were tendencies toward a difference in the number of patients with undetectable HBV DNA at week 28 after EOT and that with a decrease in HBeAg of > 1 log10 IU/mL at week 36 after EOT. The vaccine was safe, well tolerated, and resulted in a rate of adverse events comparable to that of the control group. Again, the secondary efficacy measures of the vaccine was more obvious in the dPPS analysis, in which there was a higher number of patients with a decrease in HBV DNA > 2 log10 IU/mL in the vaccine group at weeks 12 and 28 after EOT, as well as that with undetectable HBV DNA at week 28 after EOT. The discrepancy between the data sets is that we took into account the patients who needed add-on therapy with ADV in the PPS population and thus suggests that the added value of the DNA vaccine, as a kind of HBV-specific immune modulatory therapy upon LAM antiviral therapy, might be achieved in easy-to-treat patients with CHB but without antiviral failure. Therefore, these results suggest that the effect of the vaccine could be better in highly selected patients, but additional studies are necessary to identify these patients adequately.
In the present study, the frequency of VBT was higher than that in our previous phase IIa study[14] in which the occurrence rate of VBT in patients receiving LAM + vaccine was 50.0% vs 63.5% in the LAM + placebo at week 72, compared with 55.1% vs 56.0% in the present study. In addition, the numbers of patients achieving a decrease in HBV DNA > 2 log10 at week 72, as well as the numbers of patients with undetectable HBV DNA, were comparable between the two groups, which are in disagreement with our previous phase IIa trial[14]. Many reasons could explain these discrepancies. There is a possibility that some patients were not naïve to NUCs and some mutations could already have been present. The patients enrolled in the present study were different from the ones originally recruited in the phase IIa trial, which included only four centers in Beijing, while the present study involved many centers across China. Nevertheless, the results of the stratified analysis are similar to our previous findings[14], and may suggest that the lower HBV DNA load at week 72 is probably due to lower rates of VBT and YMDD mutations, but this needs to be confirmed.
In the stratified analysis of 30 patients with a viral load < 1000 copies/mL (1.79 × 102 IU/mL) at SOT, more patients achieved HBeAg seroconversion in the vaccine group than among controls. In addition, less VBT and YMDD mutations were observed in the vaccine group. If HBeAg seroconversion is selected as trial endpoint for the DNA vaccine, the baseline serum HBsAg at SOT should be considered in this stratified analysis, as it favors HBeAg seroconversion at the end[22]. Interestingly, baseline serum HBsAg levels at SOT were higher in the vaccine group than among controls, probably due to the higher ALT levels at week 0 among controls, which is an indicator of host cellular immune response and would have been favorable for viral clearance among the patients of this group. During the trial course or at the endpoint, serum HBsAg tended to decrease slightly in the vaccine group, while remaining at the relatively same level with some elevation in the control group. ALT flare-ups occurred only in the vaccine arm, but were not associated with VBT in one case. Such host-induced flare-ups have been reported in patients responding to pegylated interferon-alpha 2a (peg-IFN), and are thought to reflect the immune clearance of HBV[21,22].
HBeAg seroconversion has been used as a reliable criterion for evaluating therapeutic efficacy of antiviral treatments and could be the impetus for HBsAg loss and seroconversion[23]. It is considered a therapeutic endpoint and has been associated with immune restoration or control, and with reduced morbidity and mortality of CHB patients[14,23,24]. In the present study, LAM showed good efficacy during the first stage of viral suppression, and the adjusted results provided clinical evidence that DNA vaccine can induce an immune control of HBV infection in CHB patients who had already achieved initial virological responses under LAM antiviral therapy. Indeed, treatment guidelines suggest that therapy may be discontinued after 2-3 years of consolidation therapy in HBeAg-positive non-cirrhotic CHB patients that achieve HBeAg seroconversion during an antiviral therapy[25]. It was reported by recent studies that patients with NUC therapy either switching to or adding peg-IFN achieve higher rates of sustained response than those continuing NUC monotherapy[21,26,27]. Combination therapy of antivirals and immunomodulators, either to improve response to NUCs, IFN, or newly developed therapeutic vaccines, is considered crucial for investigators to achieve “functional” cure of HBV, defined as HBsAg loss or off-therapy immune control[27]. The present study suggests that for patients receiving long-term therapy, it might be possible to enhance the chances of HBeAg seroconversion by adding a finite course of immunomodulatory therapy, especially an HBV-specific one.
Patients with CHB often present immune defects such as weak or undetectable helper and cytotoxic T cell-mediated immune responses[22,28], and immune modulation therapy aims to induce a sustainable HBV-specific multi-epitope and polyclonal T cell response. Therapeutic HBV DNA vaccine, as an HBV-specific immunomodulator, might be superior to interferon, as a non-specific one, in terms of adverse effects and dosing regimens, given that vaccine therapy was well tolerated and only four injections were administered in our present and previous studies. A previous study in macaques revealed that DNA vaccine may induce some immune memory[29]. Results from our phase IIa trial showed that the patients receiving the DNA vaccine had a significant elevation of HBV-specific IFN-γ-secreting T cell count compared with baseline[14], suggesting that the DNA vaccine plays a role in boosting the host cellular immune responses in favor of viral eradication among CHB patients. Because local laboratories were not equipped to assess T cells and because of logistics issues for shipping fresh samples to a central laboratory, we could not assess T cells in the present phase IIb trial. Nevertheless, we attempted to evaluate immune response in this cohort by recalling the patients who completed the follow-up and before unblinding. Compared with the control group, there was a higher proportion of positive responses against HBsAg and HBV T-cell related peptides in terms of IFN-γ secretion and phenotypes of T cells from the PBMCs of patients receiving DNA vaccination. Additional studies are necessary to determine the optimal doses and administration strategies.
This study is not without limitations, as the sample size was small in the stratified analysis, it potentially prevented us from reaching a conclusion on its efficacy. In addition, the follow-up duration was short, which prevented us from evaluating the long-term efficacy and the beneficial outcome of the HBeAg seroconversion achieved with the vaccine. In the present study, only one vaccine administration schedule was tried, based on our previous phase IIa trial[14], but additional studies could explore the best administration schedule. Last but not the least, we failed to assess the real-time HBV-specific T-cell immune responses of the subjects during the trial.
Even though the clinical efficacy as a whole was a minor outcome in this trial, our findings are still of relevance for future studies, in which DNA vaccine will sequentially be combined with more potent NUCs or NUCs less susceptible to drug resistance, like ETV or TDF. Since there were only four doses of the vaccine in the present trial, investigations on more appropriate strategies of vaccination including the number of injections and timing, either in animals or small-scale clinical trials, are crucial before large-scale trials. The results of positive memory T-cell responses in the present study would be implicative for these further studies, aiming to intensify and prolong the HBV-specific T-cell responses induced by the vaccine under NUC chemotherapy among CHB patients.
In conclusion, the primary endpoint was not achieved using the HBV DNA vaccine. The HBV DNA vaccine might only be beneficial in subjects that have achieved initial virological response under LAM chemotherapy. Additional studies are necessary to identify the subset of patients that could benefit the most from the EP-mediated DNA vaccination.
Chronic hepatitis B infection leads to serious morbidity and mortality. Antivirals have some efficacy, but virological breakthrough (VBT) will eventually occur.
DNA vaccine is a novel type of vaccine that induces the production of antigen proteins by the cells.
The primary endpoint was not achieved using the hepatitis B virus (HBV) DNA vaccine. Nevertheless, the HBV DNA vaccine could only be beneficial in subjects that have achieved initial virological response under lamivudine (LAM) chemotherapy.
Patients with a response to LAM could receive the DNA vaccine as an add-on therapy in order to prolong the response.
A DNA vaccine is a vaccine that introduces a DNA sequence in host cells to make them produce the protein that will serve as the antigen for antibody production and T-cell response induction. A VBT occurs when an antiviral therapy fails.
This manuscript is well written and interesting.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Rasti M S- Editor: Yu J L- Editor: Wang TQ E- Editor: Zhang FF
| 1. | Weinbaum CM, Williams I, Mast EE, Wang SA, Finelli L, Wasley A, Neitzel SM, Ward JW. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57:1-20. [PubMed] |
| 2. | Sorrell MF, Belongia EA, Costa J, Gareen IF, Grem JL, Inadomi JM, Kern ER, McHugh JA, Petersen GM, Rein MF. National Institutes of Health Consensus Development Conference Statement: management of hepatitis B. Ann Intern Med. 2009;150:104-110. [PubMed] |
| 3. | Sunbul M. Hepatitis B virus genotypes: global distribution and clinical importance. World J Gastroenterol. 2014;20:5427-5434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 264] [Cited by in RCA: 289] [Article Influence: 26.3] [Reference Citation Analysis (8)] |
| 4. | Gurunathan S, Klinman DM, Seder RA. DNA vaccines: immunology, application, and optimization*. Annu Rev Immunol. 2000;18:927-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 867] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 5. | Shi TD, Wu YZ, Jia ZC, Zou LY, Zhou W. Therapeutic polypeptides based on HBV core 18-27 epitope can induce CD8+ CTL-mediated cytotoxicity in HLA-A2+ human PBMCs. World J Gastroenterol. 2004;10:1902-1906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Kim CY, Kang ES, Kim SB, Kim HE, Choi JH, Lee DS, Im SJ, Yang SH, Sung YC, Kim BM. Increased in vivo immunological potency of HB-110, a novel therapeutic HBV DNA vaccine, by electroporation. Exp Mol Med. 2008;40:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Mancini-Bourgine M, Fontaine H, Scott-Algara D, Pol S, Bréchot C, Michel ML. Induction or expansion of T-cell responses by a hepatitis B DNA vaccine administered to chronic HBV carriers. Hepatology. 2004;40:874-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 146] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Chen M, Jagya N, Bansal R, Frelin L, Sällberg M. Prospects and progress of DNA vaccines for treating hepatitis B. Expert Rev Vaccines. 2016;15:629-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Fontaine H, Kahi S, Chazallon C, Bourgine M, Varaut A, Buffet C, Godon O, Meritet JF, Saïdi Y, Michel ML. Anti-HBV DNA vaccination does not prevent relapse after discontinuation of analogues in the treatment of chronic hepatitis B: a randomised trial--ANRS HB02 VAC-ADN. Gut. 2015;64:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 10. | Roy MJ, Wu MS, Barr LJ, Fuller JT, Tussey LG, Speller S, Culp J, Burkholder JK, Swain WF, Dixon RM. Induction of antigen-specific CD8+ T cells, T helper cells, and protective levels of antibody in humans by particle-mediated administration of a hepatitis B virus DNA vaccine. Vaccine. 2000;19:764-778. [PubMed] |
| 11. | Yang HY, Chen GM, Cui YM, Xia Z, Mei C, Rao GR, Guo-Yu MO, Yang RC. Clinical study on safety and immunogenicity of therapeutic dual-plasmid HBV DNA vaccine mediated by in vivo electroporation. Jiefangjun Yixue Zazhi. 2013;38:204-209. |
| 12. | Yang FQ, Chen GM, Rao GR, Yu-Hong XU, Zhao YG, Xiao-Qiang HE, Sun XH, Hou JL. Interleukin-2 and Interferon-Fusion Protein Expression Plasmid Enhanced Potency of HBV DNA Vaccination in HBV Transgenic Mice. Redai Yixue Zazhi. 2008;. |
| 13. | Yang FQ, Chen GM, Rao GR, Yu-Hong XU, Zhao YG, Xiao-Qiang HE, Sun XH, Hou JL. Adjuvant effect of IL-2 and IFN-γ fusion protein gene plasmid on HBV DNA vaccination in BALB/c mice. Zhongguo Xiandai Yixue Zazhi. 2008;838-842. |
| 14. | Yang FQ, Yu YY, Wang GQ, Chen J, Li JH, Li YQ, Rao GR, Mo GY, Luo XR, Chen GM. A pilot randomized controlled trial of dual-plasmid HBV DNA vaccine mediated by in vivo electroporation in chronic hepatitis B patients under lamivudine chemotherapy. J Viral Hepat. 2012;19:581-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Wu PC, Dent JC, Barber J. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1381] [Cited by in RCA: 1347] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 16. | Schalm SW. Clinical implications of lamivudine resistance by HBV. Lancet. 1997;349:3-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513-1520. [PubMed] |
| 18. | He X, Chen G, Huang Y. Construction and identification of therapeutic double-plasmid hbv DNA vaccine. Jiefangjun Yixue Zazhi. 2003;28:493-496. |
| 19. | Mo G, Chen G, Yang F. Induction of immune responses and safety profiles in rhesus macaques immunized with the therapeutic DNA vaccine against hepatitis B virus. Jiefangjun Yixue Zazhi. 2003;28:508-510. |
| 20. | Mo G, Chen G, Huang Z. A study of distribution in the tissues and long-term toxicity of therapeutic DNA vaccine in mice. Jiefangjun Yixue Zazhi. 2003;28:504-507. |
| 21. | Ning Q, Han M, Sun Y, Jiang J, Tan D, Hou J, Tang H, Sheng J, Zhao M. Switching from entecavir to PegIFN alfa-2a in patients with HBeAg-positive chronic hepatitis B: a randomised open-label trial (OSST trial). J Hepatol. 2014;61:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 199] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 22. | Mukherjee R, Reddy PB, Arava J, Rao P, Mitnala S, Gupta R, Reddy D. Relationship between serum HBsAg level, HBV DNA level, and peripheral immune cells in patients with chronic hepatitis B virus infection. Hepat Med. 2010;2:157-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Xu DZ, Wang XY, Shen XL, Gong GZ, Ren H, Guo LM, Sun AM, Xu M, Li LJ, Guo XH. Results of a phase III clinical trial with an HBsAg-HBIG immunogenic complex therapeutic vaccine for chronic hepatitis B patients: experiences and findings. J Hepatol. 2013;59:450-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 24. | Zhou H, Min J, Zhao Q, Gu Q, Cong H, Li Y, He S. Protective immune response against Toxoplasma gondii elicited by a recombinant DNA vaccine with a novel genetic adjuvant. Vaccine. 2012;30:1800-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | The guideline of prevention and treatment for chronic hepatitis B (2010 version). Zhonghua Liuxingbingxue Zazhi. 2011;32:405-415. [PubMed] |
| 26. | Brouwer WP, Xie Q, Sonneveld MJ, Zhang N, Zhang Q, Tabak F, Streinu-Cercel A, Wang JY, Idilman R, Reesink HW. Adding pegylated interferon to entecavir for hepatitis B e antigen-positive chronic hepatitis B: A multicenter randomized trial (ARES study). Hepatology. 2015;61:1512-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 138] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 27. | Lampertico P. The royal wedding in chronic hepatitis B: The haves and the have-nots for the combination of pegylated interferon and nucleos(t)ide therapy. Hepatology. 2015;61:1459-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Dimitropoulou D, Karakantza M, Tsamandas AC, Mouzaki A, Theodorou G, Gogos CA. T-lymphocyte subsets in peripheral blood and liver tissue of patients with chronic hepatitis B and C. In Vivo. 2011;25:833-840. [PubMed] |
| 29. | Kulkarni V, Rosati M, Jalah R, Ganneru B, Alicea C, Yu L, Guan Y, LaBranche C, Montefiori DC, King AD. DNA vaccination by intradermal electroporation induces long-lasting immune responses in rhesus macaques. J Med Primatol. 2014;43:329-340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |