Copyright
©The Author(s) 2017.
World J Gastroenterol. Jan 14, 2017; 23(2): 306-317
Published online Jan 14, 2017. doi: 10.3748/wjg.v23.i2.306
Published online Jan 14, 2017. doi: 10.3748/wjg.v23.i2.306
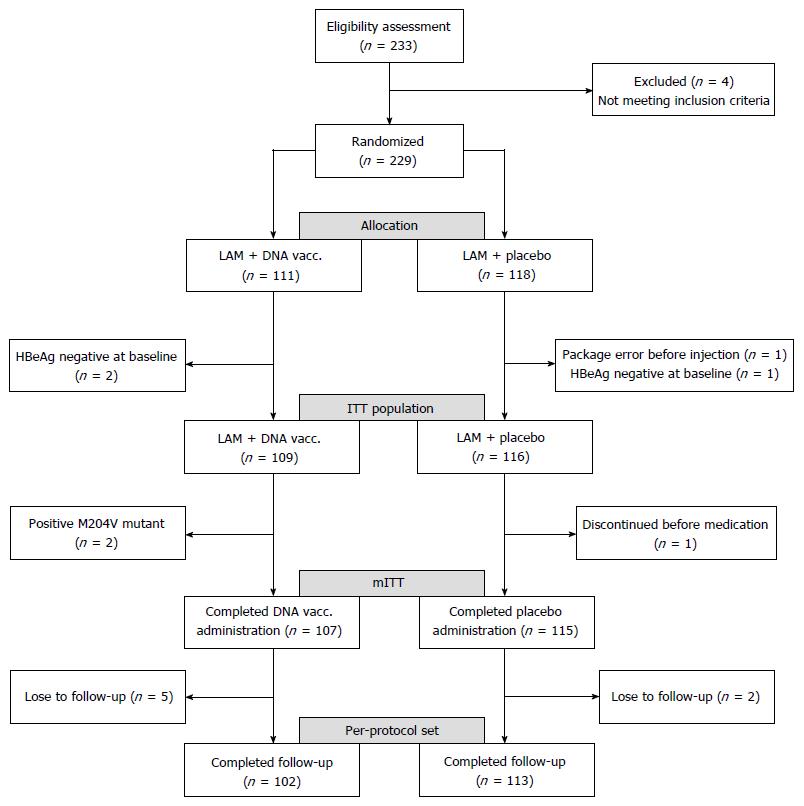
Figure 1 Patient disposition and different analysis populations.
M204V mutant: The lamivudine-resistant amino acid mutation of HBV; mITT: Modified intent-to-treat.
- Citation: Yang FQ, Rao GR, Wang GQ, Li YQ, Xie Y, Zhang ZQ, Deng CL, Mao Q, Li J, Zhao W, Wang MR, Han T, Chen SJ, Pan C, Tan DM, Shang J, Zhang MX, Zhang YX, Yang JM, Chen GM. Phase IIb trial of in vivo electroporation mediated dual-plasmid hepatitis B virus DNA vaccine in chronic hepatitis B patients under lamivudine therapy. World J Gastroenterol 2017; 23(2): 306-317
- URL: https://www.wjgnet.com/1007-9327/full/v23/i2/306.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i2.306