Published online Jan 14, 2017. doi: 10.3748/wjg.v23.i2.256
Peer-review started: August 28, 2016
First decision: September 21, 2016
Revised: September 29, 2016
Accepted: October 31, 2016
Article in press: October 31, 2016
Published online: January 14, 2017
Processing time: 139 Days and 23.2 Hours
To investigate the dynamic alteration of mitochondrial carnitine palmitoyl transferase II (CPT-II) expression during malignant transformation of rat hepatocytes.
Sprague-Dawley male rats were fed with normal, high fat (HF), and HF containing 2-fluorenylacetamide (2-FAA) diet, respectively. According to the Hematoxylin and Eosin staining of livers, rats were divided into control, fatty liver, degeneration, precancerous, and cancerous groups. Liver lipids were dyed with Oil Red O, CPT-II alterations were analyzed by immunohistochemistry, and compared with CPT-II specific concentration (μg/mg protein). Levels of total cholesterol (Tch), triglyceride (TG), and amino-transferases [alanine aminotransferase (ALT), aspartate aminotransferase (AST)] were determined by the routine methods.
After intake of HF and/or HF+2-FAA diets, the rat livers showed mass lipid accumulation. The lipid level in the control group was significantly lower than that in other groups. The changes of serum TG and Tch levels were abnormally increasing, 2-3 times more than those in the controls (P < 0.05). During the rat liver morphological changes from normal to cancer development process with hepatocyte injury, serum AST and ALT levels were significantly higher (4-8 times, P < 0.05) than those in the control group. The specific concentration of CPT-II in liver tissues progressively decreased during hepatocyte malignant transformation, with the lowest CPT-II levels in the cancer group than in any of the other groups (P < 0.05).
Low CPT-II expression might lead to abnormal hepatic lipid accumulation, which should promote the malignant transformation of hepatocytes.
Core tip: Nonalcoholic fatty liver disease (NAFLD) is one of the main risk factors for hepatocellular carcinoma, except for chronic infection with hepatitis B virus or hepatitis C virus as well as other non-viral liver diseases. However, the pathogenesis of NAFLD formation still need to be elucidated. We have successfully investigated the dynamic alteration of carnitine palmitoyl transferase II (CPT-II) expression located on the mitochondrial inner membrane during malignant transformation of rat hepatocytes under lipid accumulation and first discovered that the progressively decreasing of CPT-II expression in hepatocarcinogenesis might lead to abnormal hepatic lipid accumulation and promote the malignant transformation of hepatocytes.
- Citation: Gu JJ, Yao M, Yang J, Cai Y, Zheng WJ, Wang L, Yao DB, Yao DF. Mitochondrial carnitine palmitoyl transferase-II inactivity aggravates lipid accumulation in rat hepatocarcinogenesis. World J Gastroenterol 2017; 23(2): 256-264
- URL: https://www.wjgnet.com/1007-9327/full/v23/i2/256.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i2.256
Hepatocellular carcinoma (HCC) is one of the most common and rapidly fatal malignancies worldwide[1,2], with a highly chemoresistant cancer with no effective systemic or well-established adjuvant therapies[3,4]. HCC prognosis remains very poor because of its high recurrence. Nonalcoholic fatty liver disease (NAFLD) has been one of the primary causes of cases of chronic liver diseases or HCC in Europe, the United States, and Japan[5,6]. Now, it is also one of the main risk factors for HCC in China, except for chronic persistent infection with hepatitis B viruses or hepatitis C viruses as well as other non-viral liver diseases[7-9].
NAFLD is a disease characterized by diffuse macrovesicular steatosis of hepatocytes without excessive drinking history or other factors of liver damage[10,11]. It mainly includes simple fatty liver, nonalcoholic steatosis hepatitis (NASH), NASH-related cirrhosis, and even ultimate development of HCC[12,13]. However, the pathogenesis of NAFLD formation is very complicated and the relationship between nonalcoholic fatty accumulation and hepatocyte injury or malignant transformation still need to be elucidated[14,15].
Fatty acids are a family of molecules classified within the lipid macronutrient class. One role of fatty acids within metabolism is energy production, captured in the form of adenosine triphosphate (ATP)[16]. When they are completely oxidized to CO2 and water by β-oxidation in mitochondria and the citric acid cycle. The carnitine palmitoyl transferase (CPT) system (EC 2.3.1.21) is a pivotal component of ATP generation through mitochondrial fatty acid oxidation[17]. The CPT complex consists of two enzymes located in the outer (CPT-I) and inner (CPT-II) mitochondrial membranes[18]. The rate-limiting step in the importation of long-chain fatty acids into the mitochondria is the transesterification of acyl-coenzyme A (CoA) to acylcarnitine by CPT-I, while CPT-II changes the imported acylcarnitine back to acyl-CoA[19].
CPT II protein is a homotetramer encoded by a single gene (which spans 20 kb and contains 5 exons ranging from 81 to 1305 bp) located on chromosome 1p32. Its activity is closely associated with energy metabolism and fatty acid oxidation[20]. However, to our knowledge, the relationship between CPT-II activity and lipid accumulation has not been reported, especially in NAFLD or HCC[21,22]. The objectives of this study were to investigate the dynamic alterations of hepatic mitochondrial inner membrane CPT-II activity and hepatic specific concentration during rat hepatocarcinogenesis under the statues of nonalcoholic lipid accumulation.
Ninety-four male Sprague-Dawley rats (4-6-wk-old, body weight 100-120 g) provided by the Experimental Animal Center of Nantong University, China, were divided randomly into control (n = 10), fatty liver (n = 42) and inducing cancer groups (n = 42), and housed under bio-clean conditions at 22 ± 2 °C environment with a 12-h light/dark cycle and 55% humidity according to the previously described method[23]. The starting time for the animal experiment was defined as week 0. The rats were then monitored daily for survival and weight loss and their clinical signs were recorded; the rats were sacrificed at different times. All procedures performed on the animals were conducted in accordance with the guidelines for experimental animals approved by the Animal Care and Use Committee of Nantong University, China.
Rat liver tissues were fixed by 10% (V/V) buffered formalin and then dehydrated, made transparent, dipped in wax, embedded and sliced. The paraffin sections were subjected to histological examination with Hematoxylin and Eosin (HE) staining, with observation and photograph recording by light microscope (BX51; Olympus, Japan); the sections grouped as: control, fatty liver, degeneration, precancerous, and cancerous.
The rats in the fatty liver group were fed with high fat diet (HF; 10% lard, 10% yolk, 4% cholesterol, 1% cholic acid, and 75% normal diet)[24,25]. The rats in the inducing cancer groups were fed with HF diet containing 0.05% 2-fluorenyl-acetamide (2-FAA; Sigma, United States). The rats in the control group were fed with normal diet. The rats with one control, each of the HF and HF-2-FAA group, were sacrificed for blood draw and collection of livers every 2 wk. The morphological changes of rat livers were examined by H&E staining. According to the H&E results, the rats were divided into the control, fatty liver, degeneration, precancerous, and cancerous groups. The hepatic lipid accumulation of different livers was dyed using the Oil Red O method[26], the alterations of CPT-II expression were confirmed by immunohistochemistry, and compared with the CPT-II specific concentration (μg/mg liver protein) among the different groups.
Rat liver tissues stored at -80 °C were made into frozen sections, stained by the Oil Red O solution (0.5%; Nanjing Jiangcheng Bioengineering Institute, China), observed and photographed by light microscopy (IX71-A12FL/PH; Olympus, Japan). The ratio of red area to total tissue area in each microscopic field was determined by Image Pro Plus 6.0. This ratio represents the relative content of fats in each liver tissue.
Liver tissues were rinsed in ice-cold PBS (0.01 moL/L, PH 7.0 approximately 7.2) to remove excess blood thoroughly and weighed before homogenization. After mincing the tissues into small pieces, they were homogenized in 10 mL of PBS on ice with a glass homogenizer. The resulting suspension was subjected to two freeze-thaw cycles to further break the cell membranes. After that, the homogenates were centrifuged for 5 min at 5000 ×g and then the supernatant was removed and either assayed immediately or aliquoted for storage at -20 °C. The protein concentration was determined using a Bicinchoninic acid protein assay kit (Beyotime Institute of Biotechnology, China).
Paraffin tissue slides of the rat liver tissues were dewaxed, rehydrated, and subjected to antigen retrieval. Slides were incubated with 3% H2O2 for 10 min, washed with PBS, and then incubated with rabbit anti-rat monoclonal CPT-II antibody (ab181114, diluted 1:80; Abcam, United Kingdom) at 4 °C overnight. After washing with PBS, the reaction enhancement solution was added, incubated for 20 min and washed. The samples were then incubated with horse radish peroxidase (HRP)-conjugated goat anti-rabbit IgG for 30 min. Finally, the slides were reincubated with diaminobenzidine and counterstained with Hematoxylin solution, dehydrated in ethanol, cleared in xylene, and cover-slipped. The relative quantitative levels of CPT-II expression were calculated using Image Pro Plus 6.0.
The levels of total cholesterol (Tch), triglyceride (TG), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) in sera of rats were quantitatively detected by routine clinical methods and according to the instructions of matched test kits provided by the Nanjing Jiangcheng Bioengineering Institute. Then, the absorbance (A) was determined and the result was calculated as concentration of Tch, TG, AST, and ALT from the respective standard curves.
The levels of hepatic CPT-II expression were detected by using an enzyme-linked immunosorbent assay kit (Cloud-Clone Corp., United States) according to the manufacturer’s instructions, with positive sample as quality control and performed by two researchers working independently. For the study, 100 μL was added of each dilution of the standard, blank and samples into the appropriate wells. After that, the plates were covered by means of a plate sealer and incubated for 2 h at 37 °C, after which the liquid was removed from each well, and 100 μL of biotin-conjugated antibody specific to CPT-II was added to each well and incubated for 1 h at 37 °C. Next, after washing, 100 μL of avidin conjugated to HRP was added and incubated for 30 min at 37 °C. Then, after washing, 90 μL of substrate solution was added to each well and incubated for 15 min at 37 °C. Next, 50 μL of the stop solution was added to each well, and the A value was read at 450 nm using a microplate reader (Synergy HT; BioTek, United States). A standard curve was plotted based on the concentrations of different standards and their corresponding A values. The CPT-II concentration of each sample was calculated according to its A value with the standard curve.
Statistical analysis was carried out by using SPSS software (version 20.0). Image Pro Plus 6.0 image system was used for analysis. Data were calculated as the mean ± SD and compared between two groups by t test. A P value less than 0.05 was set at the significance level for statistical analysis.
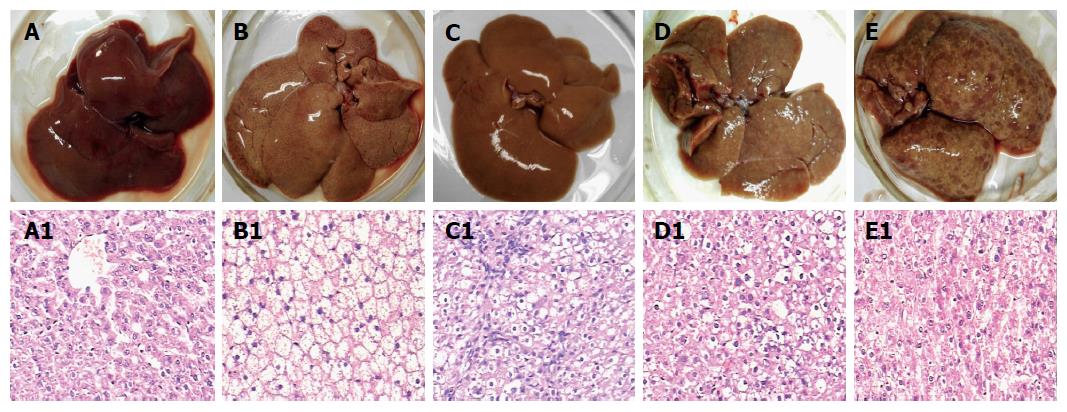
The alterations of morphology and pathology of rat livers are shown in Figure 1. The general specimen of the rat livers in the control group was observed to be reddish brown, soft, and brittle (Figure 1A). The liver cells after the H&E staining showed cords, prominent nucleoli, clear nuclear membrane, sparse nuclear chromatin, and shallow dyeing under microscopy (Figure 1 A1). Of the livers in the fatty liver group, the appearance volumes seem to be increasing slightly with smooth tight membrane, soft texture, yellow color, and greasy feeling (Figure 1B); the liver cells under microscopy showed the cytoplasm appearing in many, ranging from swollen size of the vacuoles (lipid droplets), clear nucleus, and fuzzy intercellular boundary (Figure 1 B1).
The rat liver morphological changes observed with H&E staining after 2-FAA diet were divided into the degeneration, precancerous, and cancerous groups, except for the control or fatty liver groups. At the early stage, the livers in the degeneration group tended to be yellowish and to show, microscopically, a large number of round vacuoles around the nuclei, which could be fused into a large vacuole with mild hepatic fibrosis (Figure 1C and C1). At the medial stage, a small amount of nodules appeared on the liver surface in the precancerous group, and pseudolobuli formation was observed under microscopy, with hyperchromatic nuclei, obvious nucleoli and dysplasia (Figure 1D and D1). At the last stage, the liver surface in the cancerous groups showed diffuse small nodules and pathological observation showed tumor cells that were disordered and nested, neoplastic cells with round, oval or irregular shape (Figure 1E and E1).
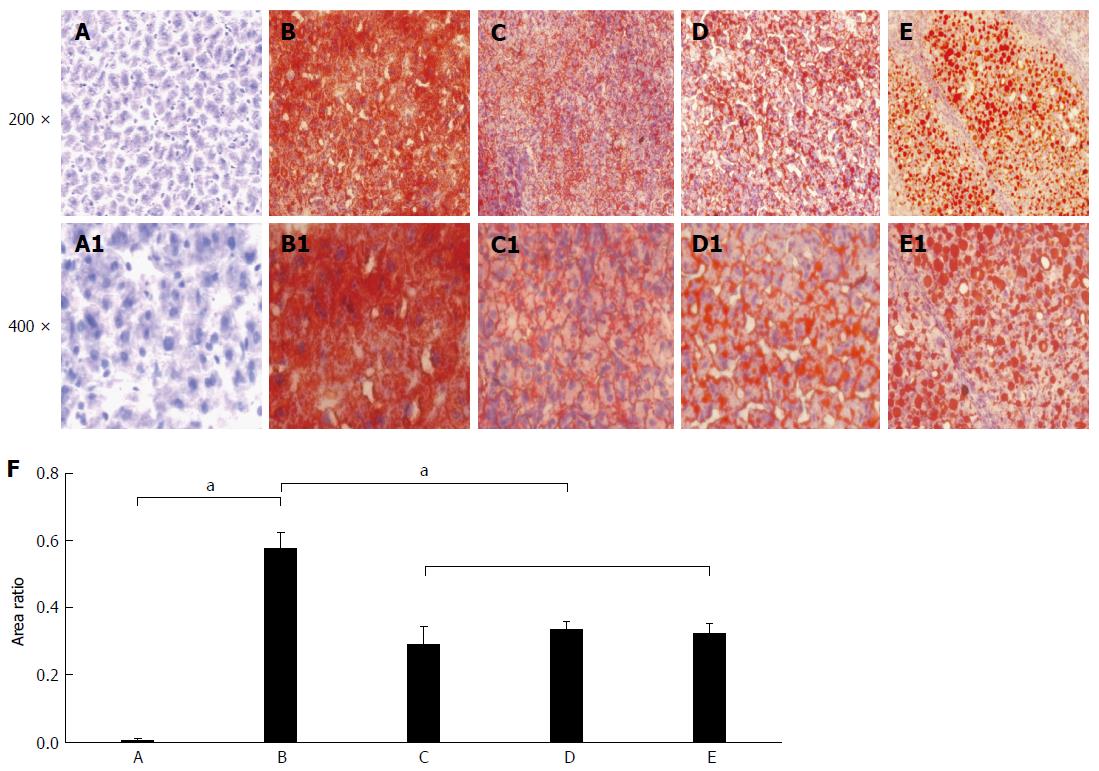
The lipid accumulation after liver sections stained with the Oil Red O are shown in Figure 2. There was no obvious positive lipid staining in the livers of the control group (Figure 2A) and significant positive staining was found in each of the other groups, with large amount of lipid accumulation. The lipid level in the control group was significantly lower than that in the fatty liver group (t = -11.556, P < 0.001; Figure 2B), the denaturation group (t = -4.847, P = 0.04; Figure 2C), the precancerous group (t = -13.652, P = 0.005; Figure 2D), and the cancerous group (t = -10.896, P = 0.008; Figure 2E). The relative fat quantitative analysis in the liver tissues (Figure 2F) indicated that there was no obvious lipid staining in the control group, while the lipid accumulation of livers in the fatty liver group was the highest among different rats with the deepest red by the Oil Red O staining. The lipid content in the inducing cancer groups was about only 50% of the fatty liver group, and the difference was significant (P < 0.05).
During the rat hepatocyte malignant transformation, the changes of circulating lipid levels and hepatic enzyme activities in the Sprague-Dawley rats are shown in Table 1. The changes of serum TG and Tch levels were abnormally increased 2-3 times more than those in the controls (P < 0.05). During the rat liver morphological changes from normal to cancer development process with hepatocyte injury, serum AST and ALT levels were significantly higher (4-8 times, P < 0.05) than those in the control group. All the data showed that liver lipid accumulation was serious with hepatocyte injury in all groups except for the rats in the control group.
| Group | n | Tch (mmol/L) | TG (mmol/L) | ALT (IU) | AST (IU) |
| Control | 10 | 1.88 ± 0.42 | 1.09 ± 0.42 | 6.26 ± 2.76 | 8.15 ± 3.70 |
| Fatty liver | 39 | 3.36 ± 1.43a | 1.69 ± 1.12a | 27.48 ± 21.64a | 22.87 ± 17.51a |
| Degeneration | 17 | 6.26 ± 1.79a | 4.51 ± 3.10a | 54.45 ± 33.82a | 35.09 ± 14.72a |
| Precancerous | 15 | 4.84 ± 1.37a | 4.68 ± 2.20a | 48.83 ± 29.30a | 50.38 ± 22.65a |
| Cancerous | 10 | 5.10 ± 1.18a | 3.10 ± 1.76a | 32.97 ± 21.34a | 31.41 ± 16.13a |
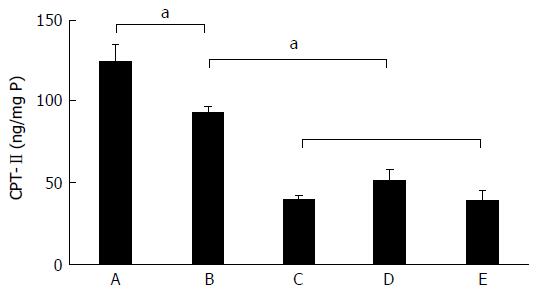
The alterations of CPT-II concentration in liver tissues are shown in Figure 3. The meaning is the concentration of CPT-II/per mg of total protein in the supernatant of liver tissue homogenate (ng/mg P). The specific concentration of liver CPT-II was 124.08 ± 26.73 ng/mg P in the control group, 92.84 ± 11.18 ng/mg P in the fatty liver group, 39.29 ± 6.33 ng/mg P in the denaturation group, 50.49 ± 18.92 ng/mg P in the precancerous group, and 38.73 ± 14.95 ng/mg P in the cancerous group. The hepatic CPT-II level was significantly higher in the control group than in the fatty liver group (t = 2.641, P = 0.035), the denaturation group (t = 7.559, P < 0.001), the precancerous group (t = 5.504, P < 0.001), and the cancerous group (t = 6.825, P < 0.001). Besides, the CPT-II level in the fatty liver group was significantly higher than in the denaturation group (t = 10.210, P < 0.001), the precancerous group (t = 4.721, P = 0.001) and the cancerous group (t = 7.100, P < 0.001). It can be seen that the specific concentration of liver CPT-II expression progressively decreased during hepatocyte malignant transformation.
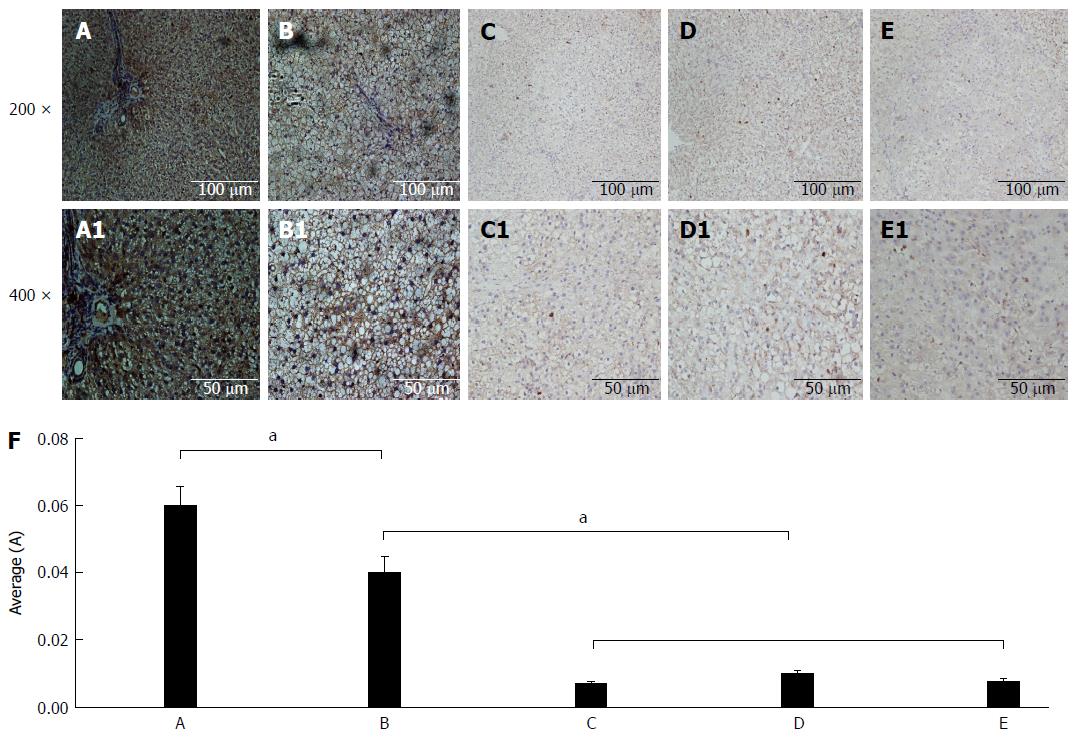
The immunohistochemical analysis results for CPT-II expression in the different rat liver tissues are shown in Figure 4. The average optical density of hepatic CPT-II expression in the control group was significantly higher than in the fatty liver group (t = 2.648, P = 0.025), the denaturation group (t = 9.071, P < 0.001), the precancerous group (t = 8.397, P < 0.001), and the cancerous group (t = 8.836, P < 0.001). Besides, the average optical density in the fatty liver group was significantly higher than in the denaturation group (t = 6.976, P < 0.001), the precancerous group (t = 6.166, P < 0.001), and the cancerous group (t = 6.698, P < 0.001). From these data, the decreasing specific concentration of liver CPT-II expression was revealed during rat hepatocyte malignant transformation.
The formation mechanism of NAFLD is very complex, with many related theories such as insulin resistance, lipid peroxidation, cellular factors’ over-expression, lipid metabolism disorder, genetic, environmental, immune, drugs, iron overload and so on[25,27]. The performance characteristic of NAFLD is metabolic disorder of lipids, and CPT-II located on the mitochondrial inner membrane is the key enzyme of fatty acid metabolism[18,28]. In normal lipid metabolism, the fatty acids in the body are activated into acyl-CoA are carried into the mitochondria by carnitine. In the mitochondrial outer membrane, fatty acids and CoA must be converted to acyltransferases by CPT-I. Once inside the mitochondrial matrix, CPT-II generates acyl-CoAs from acylcarnitines to initiate the β-oxidation of fatty acids to acetyl-CoA[28,29]. Then, carnitine crosses the mitochondrial inner membrane and binds with the endogenous or exogenous acyl-CoA to prevent the accumulation of acyl-CoA and subsequent cell poisoning.
Although the association between NAFLD and HCC has recently attracted a high degree of clinical attention, the pathogenesis of NAFLD occurrence still remains unclear[13-15]. In this study, we successfully made an in vivo model under lipid accumulation conditions for the first time to investigate the dynamic alteration of CPT-II expression during hepatocyte malignant transformation.
Long chain fatty acids are required to be transported by carnitine via the mitochondrial inner membrane to the substrate for oxidation, thus having enough carnitine available is very important for fatty acid oxidation[30]. After virus infection, competitive combination of endogenous metabolites and carnitine inhibits CPT-II expression, which causes a lack of carnitine and accumulation of acyl-CoA and fatty acids, leading to mitochondrial damage and finally resulting in energy dysmetabolism[16,31]. During the process, carnitine deficiency causes a decrease in serum free fatty acids, resulting in visceral fat accumulation and abnormal endogenous metabolites’ (dicarboxylic acid) production, resulting in more severe injury of mitochondria[17,18].
After the Sprague-Dawley rats in-took HF diets, the livers showed mass lipid accumulation. The lipid level in the control group was significantly lower than in the fatty liver the livers of the denaturation, precancerous and cancer groups. The levels of serum TG and Tch abnormally increased 2-3 times more than those in the controls. During the rat liver morphological changes from normal to cancer development process with hepatocyte injury, serum AST and ALT levels were significantly higher (4-8 times) than those in the control group. The results indicated that the fat accumulation in the rat livers with hepatocyte damage could affect the fat oxidation in mitochondria.
Hepatic lipid accumulation is a complicated process with multi-factors, multi-steps and multi-phases, and is closely related to the mitochondrial inner membrane CPT-II activity or function[32,33]. Hepatic CPT-II controls the mitochondria’s lipid oxidation, and its activity is sensitive to temperature, regulated by peroxisome proliferators-activated receptors[17,18], and has multiple variations. Liver derangements in lipid metabolism, importing of free fatty acids and manufacturing, storing and exporting of lipids could lead to NAFLD development.
Although the majority of NAFLD patients presented with steatosis only, about 20% of patients presented with NASH (as defined by microscopic findings and consisting of liver injury, steatosis, parenchymal and portal inflammation, and distinctive fibrosis)[34,35]. Loss of CPT-II catalytic function has direct effect on lipid oxidation. Hepatic CPT-II level in the fatty liver group was significantly lower than that in the control group, indicating that CPT-II reduction could be associated with lipid accumulation or steatosis.
Systemic and genetic mechanisms involved in the malignant trans- formation of liver cells, as well as some biomarkers at early stage HCC, have been investigated[23,36]. Dysregulation of the hormonal axes and cytokines in patients with NAFLD promotes a greater impairment of the cycle between metabolic and inflammatory stimulus that might lead to malignant transformation of hepatocytes[37,38]. However, the exact mechanisms underlying the interrelation of NAFLD and HCC remain largely unknown.
In this study, the dynamic alterations of CPT-II expression were observed by using models of hepatocyte malignant transformation under nonalcoholic lipid accumulation. The rat hepatocyte development from normal to malignant transformation after intake of fatty liver diets with a chemical cancer inducing agent was assessed. The specific contents of hepatic CPT-II in the cancerous groups were significantly lower than those in the fatty liver group, which was negatively correlated with the cell malignancy degree. The present data suggested that decreasing CPT-II accompanying lipid accumulation led to further damage of liver cells and promoted cell malignancy transformation.
In conclusion, to the best of our knowledge, this is the first report to investigate hepatic CPT-II expression in hepatocarcinogenesis and to indicate that it may be a novel important factor for NAFLD[39,14,40]. Here, the findings are promising, and the initial evidence confirmed that CPT-II is one of the key molecules in the β-oxidation of fatty acids. Future studies should clarify the molecular mechanisms of the down-regulation of hepatic CPT-II expression and the relationship between NAFLD and HCC. Although the exact mechanisms underlying the NAFLD tumor-promoting mechanisms triggered by hypernutrition remain to be explored, it is well recognized that NAFLD with excessive fat deposition and loss CPT-II activity represent a greater risk of tumor-promoting inflammation conditions and should be treated in time to avoid liver cell malignant transformation[41,42].
The authors thank Dr. FitzGibbon T for comments on earlier drafts of the manuscript.
Systemic and genetic mechanisms are involved in the malignant transformation of liver cells. Dysregulation of the hormonal axes and cytokines in patients with nonalcoholic fatty liver disease (NAFLD) promotes a greater impairment of the cycle between metabolic and inflammatory stimulus that might lead to malignant transformation of hepatocytes. However, the exact mechanisms underlying the interrelation of NAFLD and hepatocellular carcinoma remain largely unknown.
The formation mechanism of NAFLD is very complex, involving many related theories such as insulin resistance, lipid peroxidation, cellular factors’ over-expression, lipid metabolism disorder, genetic, environmental, immune, drugs, iron overload and so on. The performance characteristic of NAFLD is that of a metabolic disorder of lipids, and carnitine palmitoyl transferase II (CPT-II) located on the mitochondrial inner membrane is the key enzyme of fat metabolism.
This is the first report to investigate hepatic CPT-II expression in hepato- carcinogenesis and to indicate that it may be a novel important factor for NAFLD. The findings are promising, and the initial evidence confirmed that CPT-II is one of the key molecules in the β-oxidation of fatty acids.
Although the exact mechanisms underlying the NAFLD tumor-promoting mechanisms triggered by hypernutrition remain to be explored, it is well recognized that NAFLD with excessive fat deposition and loss CPT-II activity represent a greater risk of tumor-promoting inflammation conditions and should be treated in time to avoid liver cell malignant transformation.
CPT consists of two enzymes located in the outer (CPT-I) and inner (CPT-II) mitochondrial membranes. CPT-II is a homotetramer encoded by a single gene (which spans 20 kb and contains 5 exons, ranging from 81 to 1305 bp) located on chromosome 1p32, and its activity is closely associated with energy metabolism and fatty acid oxidation.
The abnormality of mitochondrial CPT-II expression was progressively decreased in hepatocarcinogenesis, which might lead to abnormal hepatic lipid accumulation that should promote the malignant transformation of hepatocytes, and CPT-II should be an early useful marker for monitoring malignant transformation of rat hepatocytes.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Blaner WS, Ramadan RA, Sarkar D S- Editor: Qi Y L- Editor: Filipodia E- Editor: Zhang FF
| 1. | Singal AG, El-Serag HB. Hepatocellular Carcinoma From Epidemiology to Prevention: Translating Knowledge into Practice. Clin Gastroenterol Hepatol. 2015;13:2140-2151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 408] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 2. | Ashtari S, Pourhoseingholi MA, Sharifian A, Zali MR. Hepatocellular carcinoma in Asia: Prevention strategy and planning. World J Hepatol. 2015;7:1708-1717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (2)] |
| 3. | Yao M, Wang L, Yao Y, Gu HB, Yao DF. Biomarker-based MicroRNA Therapeutic Strategies for Hepatocellular Carcinoma. J Clin Transl Hepatol. 2014;2:253-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Wu W, Yang JL, Wang YL, Wang H, Yao M, Wang L, Gu JJ, Cai Y, Shi Y, Yao DF. Reversal of multidrug resistance of hepatocellular carcinoma cells by metformin through inhibiting NF-κB gene transcription. World J Hepatol. 2016;8:985-993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Okanoue T, Umemura A, Yasui K, Itoh Y. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in Japan. J Gastroenterol Hepatol. 2011;26 Suppl 1:153-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 6. | van Meer S, van Erpecum KJ, Sprengers D, Klümpen HJ, Jansen PL, Ijzermans JN, Siersema PD, de Man RA, Verheij J. Hepatocellular carcinoma in noncirrhotic livers is associated with steatosis rather than steatohepatitis: potential implications for pathogenesis. Eur J Gastroenterol Hepatol. 2016;28:955-962. [PubMed] |
| 7. | de Martel C, Maucort-Boulch D, Plummer M, Franceschi S. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology. 2015;62:1190-1200. [PubMed] |
| 8. | Seyda Seydel G, Kucukoglu O, Altinbasv A, Demir OO, Yilmaz S, Akkiz H, Otan E, Sowa JP, Canbay A. Economic growth leads to increase of obesity and associated hepatocellular carcinoma in developing countries. Ann Hepatol. 2016;15:662-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (1)] |
| 9. | Fan JG. Epidemiology of alcoholic and nonalcoholic fatty liver disease in China. J Gastroenterol Hepatol. 2013;28 Suppl 1:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 213] [Article Influence: 17.8] [Reference Citation Analysis (2)] |
| 10. | Yki-Järvinen H. Diagnosis of non-alcoholic fatty liver disease (NAFLD). Diabetologia. 2016;59:1104-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Ibrahim MA, Kelleni M, Geddawy A. Nonalcoholic fatty liver disease: current and potential therapies. Life Sci. 2013;92:114-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844-855. [PubMed] |
| 13. | Safaei A, Arefi Oskouie A, Mohebbi SR, Rezaei-Tavirani M, Mahboubi M, Peyvandi M, Okhovatian F, Zamanian-Azodi M. Metabolomic analysis of human cirrhosis, hepatocellular carcinoma, non-alcoholic fatty liver disease and non-alcoholic steatohepatitis diseases. Gastroenterol Hepatol Bed Bench. 2016;9:158-173. [PubMed] |
| 14. | Gu J, Yao M, Yao D, Wang L, Yang X, Yao D. Nonalcoholic Lipid Accumulation and Hepatocyte Malignant Transformation. J Clin Transl Hepatol. 2016;4:123-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | De Minicis S, Day C, Svegliati-Baroni G. From NAFLD to NASH and HCC: pathogenetic mechanisms and therapeutic insights. Curr Pharm Des. 2013;19:5239-5249. [PubMed] |
| 16. | Yao M, Cai M, Yao D, Xu X, Yang R, Li Y, Zhang Y, Kido H, Yao D. Abbreviated half-lives and impaired fuel utilization in carnitine palmitoyltransferase II variant fibroblasts. PLoS One. 2015;10:e0119936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Yao M, Yao D, Yamaguchi M, Chida J, Yao D, Kido H. Bezafibrate upregulates carnitine palmitoyltransferase II expression and promotes mitochondrial energy crisis dissipation in fibroblasts of patients with influenza-associated encephalopathy. Mol Genet Metab. 2011;104:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Yao D, Mizuguchi H, Yamaguchi M, Yamada H, Chida J, Shikata K, Kido H. Thermal instability of compound variants of carnitine palmitoyltransferase II and impaired mitochondrial fuel utilization in influenza-associated encephalopathy. Hum Mutat. 2008;29:718-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Yao D, Kuwajima M, Chen Y, Shiota M, Okumura Y, Yamada H, Kido H. Impaired long-chain fatty acid metabolism in mitochondria causes brain vascular invasion by a non-neurotropic epidemic influenza A virus in the newborn/suckling period: implications for influenza-associated encephalopathy. Mol Cell Biochem. 2007;299:85-92. [PubMed] |
| 20. | Yao D, Yao M, Yamaguchi M, Chida J, Kido H. Characterization of compound missense mutation and deletion of carnitine palmitoyltransferase II in a patient with adenovirus-associated encephalopathy. J Med Invest. 2011;58:210-218. [PubMed] |
| 21. | White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012;10:1342-1359.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 586] [Cited by in RCA: 575] [Article Influence: 44.2] [Reference Citation Analysis (2)] |
| 22. | Purohit V, Rapaka R, Shurtleff D. Role of cannabinoids in the development of fatty liver (steatosis). AAPS J. 2010;12:233-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Dong ZZ, Yao DF, Wu W, Yao M, Yu HB, Shen JJ, Qiu LW, Yao NH, Sai WL, Yang JL. Delayed hepatocarcinogenesis through antiangiogenic intervention in the nuclear factor-kappa B activation pathway in rats. Hepatobiliary Pancreat Dis Int. 2010;9:169-174. [PubMed] |
| 24. | Hebbard L, George J. Animal models of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2011;8:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 384] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 25. | Yao P, Qi AL, Zhang Y, Su HJ, Ying M, Zhang J, Huang YF, Yao LB, Wang WQ, Li TY. Non-alcoholic fatty liver in mice experimental models of optimal feed ratio of programs. Med Innovation of China. 2011;8:11-14. |
| 26. | Yao M, Wang XY, Wang L, Yao DF, Yao DB. Antagonistic key molecule of mitochondrial carnitine shuttle system on effects of hepatic fat metabolism. J Nantong Uni (Med Sci). 2014;34:247-250. |
| 27. | Gori M, Simonelli MC, Giannitelli SM, Businaro L, Trombetta M, Rainer A. Investigating Nonalcoholic Fatty Liver Disease in a Liver-on-a-Chip Microfluidic Device. PLoS One. 2016;11:e0159729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 28. | Sagnelli E, Stroffolini T, Sagnelli C, Smedile A, Morisco F, Furlan C, Babudieri S, Brancaccio G, Coppola N, Gaeta GB. Epidemiological and clinical scenario of chronic liver diseases in Italy: Data from a multicenter nationwide survey. Dig Liver Dis. 2016;48:1066-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Kanwar P, Kowdley KV. The Metabolic Syndrome and Its Influence on Nonalcoholic Steatohepatitis. Clin Liver Dis. 2016;20:225-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 30. | Chen Y, Mizuguchi H, Yao D, Ide M, Kuroda Y, Shigematsu Y, Yamaguchi S, Yamaguchi M, Kinoshita M, Kido H. Thermolabile phenotype of carnitine palmitoyltransferase II variations as a predisposing factor for influenza-associated encephalopathy. FEBS Lett. 2005;579:2040-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Knapp AC, Todesco L, Torok M, Beier K, Krähenbühl S. Effect of carnitine deprivation on carnitine homeostasis and energy metabolism in mice with systemic carnitine deficiency. Ann Nutr Metab. 2008;52:136-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Stickel F, Hellerbrand C. Non-alcoholic fatty liver disease as a risk factor for hepatocellular carcinoma: mechanisms and implications. Gut. 2010;59:1303-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 33. | Asgharpour A, Cazanave SC, Pacana T, Seneshaw M, Vincent R, Banini BA, Kumar DP, Daita K, Min HK, Mirshahi F. A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer. J Hepatol. 2016;65:579-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 365] [Cited by in RCA: 392] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 34. | Berlanga A, Guiu-Jurado E, Porras JA, Auguet T. Molecular pathways in non-alcoholic fatty liver disease. Clin Exp Gastroenterol. 2014;7:221-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 159] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 35. | Streba LA, Vere CC, Rogoveanu I, Streba CT. Nonalcoholic fatty liver disease, metabolic risk factors, and hepatocellular carcinoma: an open question. World J Gastroenterol. 2015;21:4103-4110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 125] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 36. | Wu XH, Yao DF, Su XQ, Tai BJ, Huang H, Qiu LW, Wu W, Shao YX. Dynamic expression of rat heat shock protein gp96 and its gene during development of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2007;6:616-621. [PubMed] |
| 37. | Jin J, Valanejad L, Nguyen TP, Lewis K, Wright M, Cast A, Stock L, Timchenko L, Timchenko NA. Activation of CDK4 Triggers Development of Non-alcoholic Fatty Liver Disease. Cell Rep. 2016;16:744-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 38. | Kolly P, Dufour JF. Surveillance for Hepatocellular Carcinoma in Patients with NASH. Diagnostics (Basel). 2016;6. pii:E22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Cotrim HP, Oliveira CP, Coelho HS, Alvares-da-Silva MR, Nabuco L, Parise ER, Ivantes C, Martinelli AL, Galizzi-Filho J, Carrilho FJ. Nonalcoholic steatohepatitis and hepatocellular carcinoma: Brazilian survey. Clinics (Sao Paulo). 2016;71:281-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Raff EJ, Kakati D, Bloomer JR, Shoreibah M, Rasheed K, Singal AK. Diabetes Mellitus Predicts Occurrence of Cirrhosis and Hepatocellular Cancer in Alcoholic Liver and Non-alcoholic Fatty Liver Diseases. J Clin Transl Hepatol. 2015;3:9-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 41. | Font-Burgada J, Sun B, Karin M. Obesity and Cancer: The Oil that Feeds the Flame. Cell Metab. 2016;23:48-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 273] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 42. | Oda K, Uto H, Mawatari S, Ido A. Clinical features of hepatocellular carcinoma associated with nonalcoholic fatty liver disease: a review of human studies. Clin J Gastroenterol. 2015;8:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |