Published online May 21, 2017. doi: 10.3748/wjg.v23.i19.3418
Peer-review started: January 28, 2017
First decision: March 3, 2017
Revised: March 13, 2017
Accepted: May 4, 2017
Article in press: May 4, 2017
Published online: May 21, 2017
Processing time: 116 Days and 17.8 Hours
Pruritus is a symptom found in patients with chronic liver diseases, especially cholestatic liver diseases such as primary biliary cholangitis. This symptom impairs patient quality of life by disturbing sleep and may lead to consideration of liver transplantation. Mechanisms implicated in pruritus have been associated with the peripheral and central nervous systems, leading to the development of various therapeutic options. Little evidence for the efficacy of most of these treatments is currently available, indicating a need for further investigations.
Core tip: Pruritus is a symptom influencing the quality of life in patients with chronic liver diseases especially with cholestatic liver diseases. Complex underlying mechanisms have been identified and various therapeutic options developed. More evidence is needed for these treatments, as well as improvements in their tolerability.
- Citation: Tajiri K, Shimizu Y. Recent advances in the management of pruritus in chronic liver diseases. World J Gastroenterol 2017; 23(19): 3418-3426
- URL: https://www.wjgnet.com/1007-9327/full/v23/i19/3418.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i19.3418
Pruritus is one of the symptoms encountered in patients with chronic liver diseases, especially in those with cholestatic liver diseases such as primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC)[1-3]. Pruritus in patients with cholestasis is characterized by a circadian rhythm, with the highest intensity during the evening and early at night[4]. Chronic pruritus generally tends to increase with warmth and at night[4,5]. Women with cholestasis frequently show worsening of pruritus during the progesterone phase of the menstrual cycle, in late pregnancy, and during hormone replacement treatment[4-6]. Although pruritus may not be directly associated with the prognosis or outcome of liver diseases, a recent systematic review showed that pruritus has an impact on health-related quality of life in patients with cholestatic liver diseases[7]. Pruritus may be an indication for liver transplantation even in the absence of liver failure[8,9]. Recently, several mechanisms underlying pruritus, as well as treatment advances have been identified. This review describes recent advances in the management of pruritus in chronic liver diseases.
Several lines of evidence have suggested mechanisms by which pruritus is induced in cholestatic conditions. First, accumulated bile salts are thought to act as pruritogens[10]. Bile salts have been reported to induce degranulation of mast cells in vitro, which may contribute to pruritus in cholestatic patients. However the relationship between bile salts and pruritus has not been clarified[11], although some metabolites of bile salts may contribute to pruritus[12]. Second, endogenous opioid levels have been reported increased in cholestatic patients[12,13]. Activation of μ-opioid receptors may cause pruritus by reducing pain signaling, with μ-opioid receptor antagonists showing antipruritic effects in patients with chronic cholestasis[14,15]. However the correlation between those increased opioid levels and pruritus remains unclear[12,13]. Thus, these mechanisms could not fully explain the pathogenesis of pruritus. Furthermore, conditions that may cause skin itching are often found in cirrhotic patients. These include hyperhemodynamic conditions and skin dryness caused by administration of diuretics for hepatic edema[16], complicating the mechanism by which pruritus is induced in cirrhotic patients.
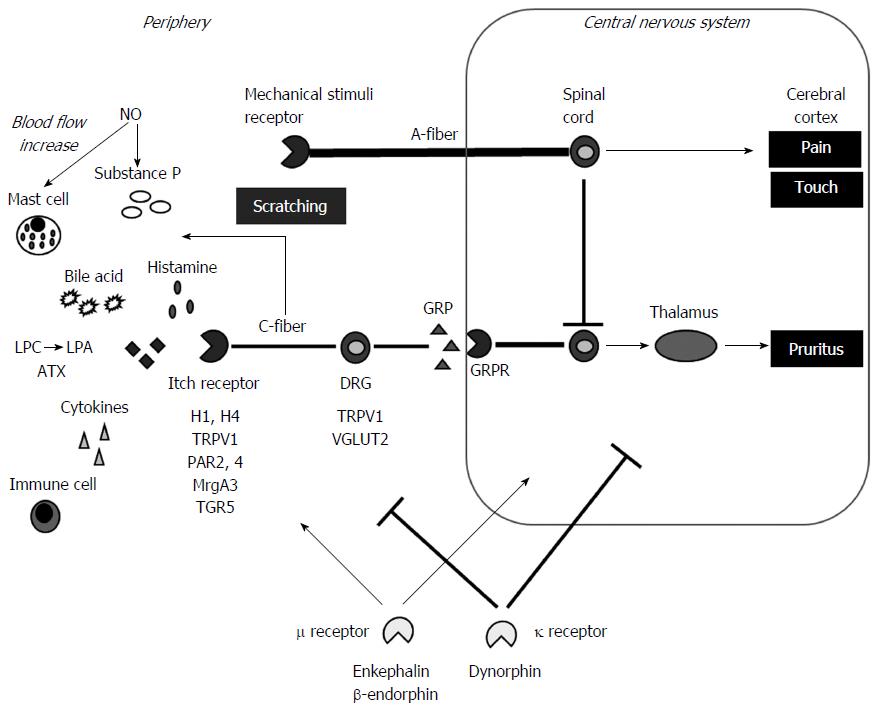
Research in recent decades has clarified mechanisms of pruritus induction in the periphery (Figure 1). The first involves an itch-selective pathway, consisting of slow-conducting C-fibers insensitive to mechanical stimuli, which convey itch signals that are distinct from pain transmission[17,18]. Mechanical stimuli such as pain and touch are transmitted through myelinated fast-conducting fibers with larger diameter and competitively inhibit itch-transmission[19-21], thus explaining reason why itching is diminished by scratching[22]. Scratching damages the skin barrier, inducing the release of substance P or calcitonin gene-related peptide, thereby increasing pruritus[23].
Several pruritogens and their receptors specific to itch signaling have been identified. Histamine and the histamine H1 and H4 receptors are considered the main contributors to itch signaling[24]. The H1 receptor interacts with phospholipase Cβ3 (PLCβ3)[25] and transient receptor potential (TRP) vanilloid receptor subfamily V1 (TRPV1), which constitute a nociceptive ion channel[26]. Protease-activated receptors (PAR) 2 and 4 are thought to be involved in itch signaling[27], and PAR2 activation may sensitize TRPV1, thereby contributing to itch[28,29]. Serotonin is another pruritogen, which, together with PLCβ3, acts on G-protein-coupled receptors (GPCR)[26], such as NK-1, a GPCR shown to play a critical role in serotonin-mediated itch[30]. The Mrg subtype A3 (MrgA3), a type of GPCR, is also involved in itch signaling[31,32] by interacting with TRPV1[32]. Thus, the mechanisms underlying itch signaling is complicated. Other pruritogens may indirectly trigger itch signaling. For example, mast cells are associated with itch not only by releasing histamine but other pruritogens[33]. Cytokines produced by immune cells are also involved in itch[22]. Keratinocytes (KCs) may also be associated with itching. These cells express the potential itch-associated molecules TRPV3 and 4[34], with TRPV3 expression associated with allergic dermatitis[35]. Skin inflammation suggests the involvement of immune cells[22].
Gastrin-releasing peptide (GRP) may act as an itch transmitter[36]. GRP is an itch-selective neurotransmitter of dorsal root ganglia (DRG) neurons that activates the GRP receptor (GRPR) on spinal neurons specific to itch not but to pain[37]. The vesicular glutamate transporter (VGLUT) 2 was also shown to be involved in itch-selective neurotransmission[38,39].
Lysophosphatidic acid (LPA) has been regarded as a specific target pruritogen/neurotransmitter in patients with cholestasis[11,12]. LPA is generated from lysophosphatidylcholine (LPC) by autotaxin (ATX)[40,41]. LPA and ATX were shown to be increased in cholestatic patients, suggesting they may be potential therapeutic targets[11,12,42]. Serum ATX level was also shown to be increased by the administration of oral contraceptives to healthy females, and was a potential good indicator of intrahepatic cholestasis of pregnancy (ICP)[43]. The metabolism of sex hormones is impaired in cirrhotic livers, accompanied by overt feminization[44], thereby partly explaining the mechanism of ATX-induced pruritus in cirrhotic patients. In addition, the G-protein-coupled bile acid receptor 1, TGR5, encoded by GPBAR1 and expressed on sensory nerves, was recently shown to be involved in pruritus by stimulating the release of neuropeptides in the spinal cord[45]. TGR5 was also found to activate the transient receptor potential ankyrin 1 (TRPA1) and to induce pruritus[46]. Thus many pruritogens, especially those specific to cholestatic conditions, have been identified.
Pruritus also involves the central nervous system (CNS). For example, the most common adverse event of the μ-opioid receptor agonist morphine is pruritus[47,48]. The μ-opioid receptor antagonist naloxone, however, inhibits morphine-induced pruritus[49], and suppresses pruritus in patients with chronic cholestasis[50]. Plasma concentrations of the μ-opioid receptor agonists methionine enkephalin and β-endorphin were shown to be increased in patients with cirrhosis, as ascites increased due to decreased hepatic elimination[51,52]. The liver plays a major role in the elimination of blood-derived opioid peptides[53]. These findings suggest that the μ-opioid receptor system is involved in pruritus sensations in patients with liver diseases[54]. In contrast, the κ-opioid receptor was shown to suppress pruritus. The κ-receptor agonist, nalfurafine (TRK-820) [(E)-N-[17-(cyclopropylmethyl)-4,5α-epoxy-3,14- dihydroxymorphinan-6β−yl]-3-(furan-3-yl)-N-methylpop-2-enamide monohydrochloride], was shown to suppress anti-histamine-resistant pruritus in a mouse model[54], whereas pruritus was not neutralized by the peripheral administration of the κ-opioid receptor antagonist nor-binaltorphimine[54]. Nalfurafine was also found to suppress pruritus induced by the intracisternal administration of morphine[55]. Similar results were observed in a rat model of cholestasis induced by treatment with ethynylestradiol (EE), in which the levels of expression of the κ-receptor agonist dynorphin and nitric oxide (NO) were decreased[56]. Nalfurafine showed anti-pruritic activity in this model, an activity partly mediated by NO systems[56]. These model indicates that NO is involved in mediating the antipruritic effect of κ-receptor action[56].
NO expression is enhanced in patients with cirrhosis and primary biliary cholangitis, with NO being a main contributor to hyperdynamic circulation in patients with cirrhosis[16,57]. NO was shown to enhance substance P-induced scratching in the periphery, whereas a NO synthase inhibitor suppressed this scratching in a dose dependent manner[58]. NO induces vasodilatation[59], suggesting it increases peripheral blood flow. Thus the contribution of NO to pruritus remains still controversial and requires further investigation. Furthermore β and κ receptors have been shown to be distributed also in peripheral nerves and contribute to the development of pruritus[60,61]. Thus their mechanisms of action are complicated between the periphery and CNS, especially in patients with chronic liver diseases, making understanding of the pathogenesis of pruritus in these patients difficult and making them refractory to treatment.
Primary biliary cholangitis (PBC), formerly called primary biliary cirrhosis, is a representative chronic cholestatic liver disease manifesting pruritus. Pruritus is found in about 70% of patients with PBC[2,62] and precedes the diagnosis of PBC in about 75%[62]. Pruritus has been shown to impair quality of life, such as sleep, in patients with PBC[62,63].
Primary sclerosing cholangitis (PSC) is also associated with pruritus during the course of disease progression. In contrast to PBC, most patients with PSC are asymptomatic at the time of diagnosis; therefore the exact prevalence of pruritus in patients with PSC remains unclear[64].
Pruritus in patients with PBC and PSC manifests frequently in the limbs, particularly in the palms and soles[1,65]. Multivariate analysis showed that serum alkaline phosphatase activity and Mayo risk score were independent predictors of pruritus in patients with PBC[66]. Severe pruritus limits daily life activities and causes fatigue, depression and even suicidal tendencies, becoming an indication for liver transplantation in some patients[8,67,68].
Pruritus was observed in four of 49 (8%) patients with chronic hepatitis B and 42 of 210 (20%) with chronic hepatitis C[69]. Studies of large cohorts found that the proportion of HCV-infected patients with pruritus ranged from 2.5% of 1060 patients[70] to 15% of 1614 patients[71]. Pruritus in these patients was not caused by cholestasis[70], whereas liver fibrosis progression was a risk factor contributing to pruritus[71].
Pruritus is a defining symptom of intrahepatic cholestasis of pregnancy (ICP), a condition characterized by increases in serum bile acid concentrations and increased rates of adverse fetal outcomes[72]. Pruritus in ICP is usually localized to the palms and soles[73]. The incidence of ICP was reported to be 1.5%, with increased fetal complications occurring at serum bile acid concentrations > 40[74].
Familial intrahepatic cholestasis, such as benign recurrent intrahepatic cholestasis (BRIC), is an autosomal recessive disorder associated with canalicular transport defects resulting from mutations in ATP8B1, ABCB11 and ABCB4. The phenotype is ranging from BRIC to progressive familial intrahepatic cholestasis according to the severity of disease[75]. BRIC is characterized by intermittent jaundice and pruritus, and the clinical symptoms may be severe, last from several weeks to months and usually resolve spontaneously[75,76].
Benign obstructive jaundice has been associated with a lower rate of pruritus than malignant obstruction. For example, pruritus was observed in 16% of patients with benign biliary obstruction such as choledocholithiasis, but in up to 45% of patients with malignant obstruction such as carcinoma of the pancreatic head[77].
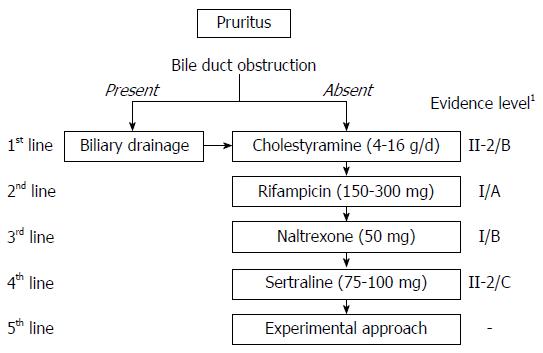
The guidelines of the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL) include criteria for the management of cholestatic pruritus in patients with PBC and PSC[78,79]. Recommendations to all patients should include the use of moisturizing and cooling ointments and shortening of the fingernails to avoid secondary skin damage. The principal goals of treatment include[5]: (1) removal of pruritogens such as cholestyramine or biliary drainage in the absence of cholestasis; (2) alteration of the metabolism of presumed pruritogens such as rifampicin; (3) modulation of itch signaling such as opioid receptor acting agents; and (4) removal of potential pruritogens by anion absorption, plasmapheresis or extracorporeal albumin dialysis (Figure 2).
Ursodeoxycholic acid (UDCA) is an established drug for the management of PBC and PSC[80,81]. Although associated with biological and histological improvements and improving overall survival[80,81], UDCA was ineffective in relieving cholestatic pruritus in both PBC and PSC[66,82]. In patients with ICP, however, UDCA not only improved biological parameters such as aspartate aminotransferase and alkaline phosphatase concentrations, but ameliorated pruritus[74,83]. UDCA is used for BRIC to stimulate hepatobiliary secretion of bile salts. Antiapoptotic effects of UDCA are also expected to protect hepatocytes in the treatment for BRIC[75].
Cholestyramine, a bile acid resin, has been recommended as the treatment of choice for patients with cholestatic pruritus, as it was shown effective in randomized studies with small numbers of patients (eight and 10, respectively)[84,85]. Although generally well-tolerated, cholestyramine has several side effects, including unpleasant taste, fat malabsorption, constipation, anorexia and gastrointestinal discomfort[86].
A meta-analysis of five prospective randomized control trials showed that rifampicin, a pregnane X receptor (PXR) agonist commonly used to treat mycobacterial infection, was effective in treating chronic pruritus[87]. Rifampicin was shown to reduce ATX expression in vitro by a PXR-dependent mechanism[42]. Although safe as short-term treatment of chronic pruritus, rifampicin was associated with hepatotoxicity in up to 13% of patients after treatment for several weeks or months[88]. Adverse effects that may lead to discontinuation of therapy include nausea, loss of appetite, hemolytic anemia, renal failure and thrombocytopenia[87,89]. Careful monitoring of blood count and liver function tests is required during administration of rifampicin for cholestatic pruritus, and administration for more than 2 wk is not recommended[90].
Prospective placebo-controlled showed that the μ-opioid receptor antagonist naltrexone was effective in treating cholestatic pruritus[91-94]. Common side effects of opioid antagonists include opiate withdrawal reactions, particularly during the first days of therapy. Contraindications to naltrexone include acute liver injury and severe liver insufficiency. Opioid antagonists should be avoided in patients with drug addictions and those taking opioid containing medications[95]. In Japan, nalfurafine, a κ-opioid receptor agonist, is available for the treatment of pruritus in chronic liver diseases (2.5-5 μg/d). Nalfurafine is metabolized predominantly by cytochrome P450[96], but its main metabolite has no pharmacological activity, suggesting its availability and effectiveness for treatment of patients with advanced liver diseases[97]. Recently a randomized controlled trial showed the effectiveness of nalfurafine by small dose (2.5 or 5 μg/d) for refractory pruritus with chronic liver diseases[98].
Sertraline, a selective serotonin re-uptake inhibitor (SSRI), is a fourth-line therapeutic option for patients with cholestatic pruritus. Sertraline (75-100 mg/d) was well-tolerated and showed moderate anti-pruritic effects in a randomized trial with a small number of patients[99]. Because sertraline is largely metabolized in the liver, careful administration (e.g., lower or less frequent dosing) should be considered in patients with advanced liver diseases. Sertraline should not be administered to patients who treated with monoamine oxidase inhibitors for the previous 14 d or those concurrently taking pimozide, and oral sertraline concentrate should not be administered together with disulfiram[90].
The AASLD and EASL guidelines both recommend that patients who show no improvement on these standard therapies be treated by experimental approaches. Case studies have described methods such as plasmapheresis[100,101], albumin dialysis using a molecular absorbent recirculating system (MARS)[102-104], plasma separation and anion absorption[105], ultraviolet B phototherapy[106], nasobiliary drainage[107] and surgical intervention such as partial biliary diversion[75]. Little evidence is available for the effectiveness of these approaches, suggesting a need for validation prior to standard use. Furthermore therapeutic options recommended by guidelines lack strong evidence, except for rifampicin as second line-treatment. However, rifampicin cannot be administered for longer than 2 wk. Because pruritus is found in patients with chronic liver diseases, especially cirrhosis, therapeutic modalities tolerable for longer times are needed. A large-scale (n = 337), placebo-controlled study showed that nalfurafine was effective and safe in hemodialysis patients with uremic pruritus resistant to conventional treatments[108] and that this treatment was tolerable for 52 wk[109]. Its effectiveness in treating pruritus in patients with chronic liver diseases was also shown by a randomized controlled study (n = 318)[98]. Its tolerability is under investigation. The effectiveness and tolerability in non-Japanese or non-Asian people are desired.
Recent studies have assessed the association of nuclear receptors with the homeostasis of bile acids, with farnesoid X receptor (FXR) shown to regulate bile acid synthesis[110,111]. Obeticolic acid, a FXR agonist, showed significant improvements in biochemical parameters, but increased pruritus rates[112]. The incidence and severity of pruritus were reported to be independent of PBC disease stage[113,114], and the mechanism of FXR-induced pruritus remains unknown. The effect of FXR agonist on ATX level should be evaluated.
The mechanism of cholestatic pruritus in chronic liver diseases is complex. The various mechanisms at the periphery and in the central nervous system result complicate determinations of its pathogenesis and treatment strategies. Pruritus occurs frequently in patients with chronic liver diseases, especially those with chronic cholestatic diseases, and impairs patient quality of life. Current treatments for cholestatic pruritus are inadequate, and additional, more effective therapeutic options are required.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chamuleau RAFM, Feuerstein JD S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
| 1. | Bergasa NV, Mehlman JK, Jones EA. Pruritus and fatigue in primary biliary cirrhosis. Baillieres Best Pract Res Clin Gastroenterol. 2000;14:643-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Koulentaki M, Ioannidou D, Stefanidou M, Maraki S, Drigiannakis I, Dimoulios P, Melono JM, Tosca A, Kouroumalis EA. Dermatological manifestations in primary biliary cirrhosis patients: a case control study. Am J Gastroenterol. 2006;101:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Younossi ZM, Kiwi ML, Boparai N, Price LL, Guyatt G. Cholestatic liver diseases and health-related quality of life. Am J Gastroenterol. 2000;95:497-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Kremer AE, Beuers U, Oude-Elferink RP, Pusl T. Pathogenesis and treatment of pruritus in cholestasis. Drugs. 2008;68:2163-2182. [PubMed] |
| 5. | Kremer AE, Namer B, Bolier R, Fischer MJ, Oude Elferink RP, Beuers U. Pathogenesis and Management of Pruritus in PBC and PSC. Dig Dis. 2015;33 Suppl 2:164-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Bergasa NV. The pruritus of cholestasis. J Hepatol. 2005;43:1078-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 7. | Jin XY, Khan TM. Quality of life among patients suffering from cholestatic liver disease-induced pruritus: A systematic review. J Formos Med Assoc. 2016;115:689-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Elias E, Burra P. Primary biliary cirrhosis: symptomatic treatment. J Gastroenterol Hepatol. 1991;6:570-573. [PubMed] |
| 9. | Elias E. Liver transplantation. J R Coll Physicians Lond. 1993;27:224-232. [PubMed] |
| 10. | Quist RG, Ton-Nu HT, Lillienau J, Hofmann AF, Barrett KE. Activation of mast cells by bile acids. Gastroenterology. 1991;101:446-456. [PubMed] |
| 11. | Kremer AE, Martens JJ, Kulik W, Ruëff F, Kuiper EM, van Buuren HR, van Erpecum KJ, Kondrackiene J, Prieto J, Rust C. Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology. 2010;139:1008-1018, 1018.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 284] [Article Influence: 18.9] [Reference Citation Analysis (1)] |
| 12. | Oude Elferink RP, Kremer AE, Martens JJ, Beuers UH. The molecular mechanism of cholestatic pruritus. Dig Dis. 2011;29:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Spivey JR, Jorgensen RA, Gores GJ, Lindor KD. Methionine-enkephalin concentrations correlate with stage of disease but not pruritus in patients with primary biliary cirrhosis. Am J Gastroenterol. 1994;89:2028-2032. [PubMed] |
| 14. | Bergasa NV, Schmitt JM, Talbot TL, Alling DW, Swain MG, Turner ML, Jenkins JB, Jones EA. Open-label trial of oral nalmefene therapy for the pruritus of cholestasis. Hepatology. 1998;27:679-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 136] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Bergasa NV, Alling DW, Talbot TL, Wells MC, Jones EA. Oral nalmefene therapy reduces scratching activity due to the pruritus of cholestasis: a controlled study. J Am Acad Dermatol. 1999;41:431-434. [PubMed] |
| 16. | Vallance P, Moncada S. Hyperdynamic circulation in cirrhosis: a role for nitric oxide? Lancet. 1991;337:776-778. [PubMed] |
| 17. | Andrew D, Craig AD. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nat Neurosci. 2001;4:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 347] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 18. | Schmelz M. A neural pathway for itch. Nat Neurosci. 2001;4:9-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 19. | Patel KN, Dong X. An itch to be scratched. Neuron. 2010;68:334-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Braz J, Solorzano C, Wang X, Basbaum AI. Transmitting pain and itch messages: a contemporary view of the spinal cord circuits that generate gate control. Neuron. 2014;82:522-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 319] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 21. | LaMotte RH, Dong X, Ringkamp M. Sensory neurons and circuits mediating itch. Nat Rev Neurosci. 2014;15:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 224] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 22. | Ikoma A, Steinhoff M, Ständer S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci. 2006;7:535-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 651] [Cited by in RCA: 665] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 23. | Yamaoka J, Di ZH, Sun W, Kawana S. Changes in cutaneous sensory nerve fibers induced by skin-scratching in mice. J Dermatol Sci. 2007;46:41-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Bell JK, McQueen DS, Rees JL. Involvement of histamine H4 and H1 receptors in scratching induced by histamine receptor agonists in Balb C mice. Br J Pharmacol. 2004;142:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 171] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 25. | Han SK, Mancino V, Simon MI. Phospholipase Cbeta 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron. 2006;52:691-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 143] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 26. | Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci USA. 2009;106:11330-11335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 334] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 27. | Reddy VB, Iuga AO, Shimada SG, LaMotte RH, Lerner EA. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci. 2008;28:4331-4335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 162] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 28. | Dai Y, Moriyama T, Higashi T, Togashi K, Kobayashi K, Yamanaka H, Tominaga M, Noguchi K. Proteinase-activated receptor 2-mediated potentiation of transient receptor potential vanilloid subfamily 1 activity reveals a mechanism for proteinase-induced inflammatory pain. J Neurosci. 2004;24:4293-4299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 242] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 29. | Amadesi S, Nie J, Vergnolle N, Cottrell GS, Grady EF, Trevisani M, Manni C, Geppetti P, McRoberts JA, Ennes H. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J Neurosci. 2004;24:4300-4312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 320] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 30. | Carstens EE, Carstens MI, Simons CT, Jinks SL. Dorsal horn neurons expressing NK-1 receptors mediate scratching in rats. Neuroreport. 2010;21:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 31. | Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 623] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 32. | Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci. 2013;16:174-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 448] [Cited by in RCA: 432] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 33. | Harvima IT, Nilsson G, Suttle MM, Naukkarinen A. Is there a role for mast cells in psoriasis? Arch Dermatol Res. 2008;300:461-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ. TRPV3 and TRPV4 mediate warmth-evoked currents in primary mouse keratinocytes. J Biol Chem. 2004;279:21569-21575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 229] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 35. | Yoshioka T, Imura K, Asakawa M, Suzuki M, Oshima I, Hirasawa T, Sakata T, Horikawa T, Arimura A. Impact of the Gly573Ser substitution in TRPV3 on the development of allergic and pruritic dermatitis in mice. J Invest Dermatol. 2009;129:714-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 36. | Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 574] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 37. | Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science. 2009;325:1531-1534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 503] [Cited by in RCA: 456] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 38. | Lagerström MC, Rogoz K, Abrahamsen B, Persson E, Reinius B, Nordenankar K, Olund C, Smith C, Mendez JA, Chen ZF. VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron. 2010;68:529-542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 39. | Liu Y, Abdel Samad O, Zhang L, Duan B, Tong Q, Lopes C, Ji RR, Lowell BB, Ma Q. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron. 2010;68:543-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 210] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 40. | Tokumura A, Majima E, Kariya Y, Tominaga K, Kogure K, Yasuda K, Fukuzawa K. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol Chem. 2002;277:39436-39442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 602] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 41. | Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, Yamori T, Mills GB, Inoue K, Aoki J. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol. 2002;158:227-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 741] [Cited by in RCA: 769] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 42. | Kremer AE, van Dijk R, Leckie P, Schaap FG, Kuiper EM, Mettang T, Reiners KS, Raap U, van Buuren HR, van Erpecum KJ. Serum autotaxin is increased in pruritus of cholestasis, but not of other origin, and responds to therapeutic interventions. Hepatology. 2012;56:1391-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 186] [Article Influence: 14.3] [Reference Citation Analysis (1)] |
| 43. | Kremer AE, Bolier R, Dixon PH, Geenes V, Chambers J, Tolenaars D, Ris-Stalpers C, Kaess BM, Rust C, van der Post JA. Autotaxin activity has a high accuracy to diagnose intrahepatic cholestasis of pregnancy. J Hepatol. 2015;62:897-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 45. | Alemi F, Kwon E, Poole DP, Lieu T, Lyo V, Cattaruzza F, Cevikbas F, Steinhoff M, Nassini R, Materazzi S. The TGR5 receptor mediates bile acid-induced itch and analgesia. J Clin Invest. 2013;123:1513-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 284] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 46. | Lieu T, Jayaweera G, Zhao P, Poole DP, Jensen D, Grace M, McIntyre P, Bron R, Wilson YM, Krappitz M. The bile acid receptor TGR5 activates the TRPA1 channel to induce itch in mice. Gastroenterology. 2014;147:1417-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 47. | Cousins MJ, Mather LE. Intrathecal and epidural administration of opioids. Anesthesiology. 1984;61:276-310. [PubMed] |
| 48. | Ballantyne JC, Loach AB, Carr DB. Itching after epidural and spinal opiates. Pain. 1988;33:149-160. [PubMed] |
| 49. | Bernstein JE, Swift RM, Soltani K, Lorincz AL. Antipruritic effect of an opiate antagonist, naloxone hydrochloride. J Invest Dermatol. 1982;78:82-83. [PubMed] |
| 50. | Bergasa NV, Talbot TL, Alling DW, Schmitt JM, Walker EC, Baker BL, Korenman JC, Park Y, Hoofnagle JH, Jones EA. A controlled trial of naloxone infusions for the pruritus of chronic cholestasis. Gastroenterology. 1992;102:544-549. [PubMed] |
| 51. | Thornton JR, Dean H, Losowsky MS. Is ascites caused by impaired hepatic inactivation of blood borne endogenous opioid peptides? Gut. 1988;29:1167-1172. [PubMed] |
| 52. | Thornton JR, Losowsky MS. Plasma beta endorphin in cirrhosis and renal failure. Gut. 1991;32:306-308. [PubMed] |
| 53. | Thornton JR, Losowsky MS. Methionine enkephalin is increased in plasma in acute liver disease and is present in bile and urine. J Hepatol. 1989;8:53-59. [PubMed] |
| 54. | Togashi Y, Umeuchi H, Okano K, Ando N, Yoshizawa Y, Honda T, Kawamura K, Endoh T, Utsumi J, Kamei J. Antipruritic activity of the kappa-opioid receptor agonist, TRK-820. Eur J Pharmacol. 2002;435:259-264. [PubMed] |
| 55. | Umeuchi H, Togashi Y, Honda T, Nakao K, Okano K, Tanaka T, Nagase H. Involvement of central mu-opioid system in the scratching behavior in mice, and the suppression of it by the activation of kappa-opioid system. Eur J Pharmacol. 2003;477:29-35. [PubMed] |
| 56. | Inan S, Cowan A. Nalfurafine, a kappa opioid receptor agonist, inhibits scratching behavior secondary to cholestasis induced by chronic ethynylestradiol injections in rats. Pharmacol Biochem Behav. 2006;85:39-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 57. | Dimoulios P, Kolios G, Notas G, Matrella E, Xidakis C, Koulentaki M, Tsagarakis N, Kouroumalis A, Kouroumalis E. Ursodeoxycholic acid reduces increased circulating endothelin 2 in primary biliary cirrhosis. Aliment Pharmacol Ther. 2005;21:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 58. | Andoh T, Kuraishi Y. Nitric oxide enhances substance P-induced itch-associated responses in mice. Br J Pharmacol. 2003;138:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 59. | Tajiri K, Miyakawa H, Izumi N, Marumo F, Sato C. Systemic hypotension and diuresis by L-arginine in cirrhotic patients with ascites: role of nitric oxide. Hepatology. 1995;22:1430-1435. [PubMed] |
| 60. | Bigliardi PL, Bigliardi-Qi M, Buechner S, Rufli T. Expression of mu-opiate receptor in human epidermis and keratinocytes. J Invest Dermatol. 1998;111:297-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 61. | Tominaga M, Ogawa H, Takamori K. Possible roles of epidermal opioid systems in pruritus of atopic dermatitis. J Invest Dermatol. 2007;127:2228-2235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 62. | Rishe E, Azarm A, Bergasa NV. Itch in primary biliary cirrhosis: a patients’ perspective. Acta Derm Venereol. 2008;88:34-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 63. | Mells GF, Pells G, Newton JL, Bathgate AJ, Burroughs AK, Heneghan MA, Neuberger JM, Day DB, Ducker SJ, Sandford RN. Impact of primary biliary cirrhosis on perceived quality of life: the UK-PBC national study. Hepatology. 2013;58:273-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 64. | Zein CO, Lindor KD. Latest and emerging therapies for primary biliary cirrhosis and primary sclerosing cholangitis. Curr Gastroenterol Rep. 2010;12:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 65. | Pusl T, Beuers U. Ursodeoxycholic acid treatment of vanishing bile duct syndromes. World J Gastroenterol. 2006;12:3487-3495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 66. | Talwalkar JA, Souto E, Jorgensen RA, Lindor KD. Natural history of pruritus in primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2003;1:297-302. [PubMed] |
| 67. | Heathcote EJ. Management of primary biliary cirrhosis. The American Association for the Study of Liver Diseases practice guidelines. Hepatology. 2000;31:1005-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 275] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 68. | Neuberger J, Jones EA. Liver transplantation for intractable pruritus is contraindicated before an adequate trial of opiate antagonist therapy. Eur J Gastroenterol Hepatol. 2001;13:1393-1394. [PubMed] |
| 69. | Bonacini M. Pruritus in patients with chronic human immunodeficiency virus, hepatitis B and C virus infections. Dig Liver Dis. 2000;32:621-625. [PubMed] |
| 70. | Dega H, Francès C, Dupin N, Lebre C, Simantov A, Callot C, Laporte JL, Blot C, Opolon P, Poynard T. [Pruritus and the hepatitis C virus. The MULTIVIRC Unit]. Ann Dermatol Venereol. 1998;125:9-12. [PubMed] |
| 71. | Cacoub P, Poynard T, Ghillani P, Charlotte F, Olivi M, Piette JC, Opolon P. Extrahepatic manifestations of chronic hepatitis C. MULTIVIRC Group. Multidepartment Virus C. Arthritis Rheum. 1999;42:2204-2212. [PubMed] [DOI] [Full Text] |
| 72. | Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15:2049-2066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 367] [Cited by in RCA: 383] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 73. | Pathak B, Sheibani L, Lee RH. Cholestasis of pregnancy. Obstet Gynecol Clin North Am. 2010;37:269-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 74. | Glantz A, Marschall HU, Mattsson LA. Intrahepatic cholestasis of pregnancy: Relationships between bile acid levels and fetal complication rates. Hepatology. 2004;40:467-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 494] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 75. | van der Woerd WL, Houwen RH, van de Graaf SF. Current and future therapies for inherited cholestatic liver diseases. World J Gastroenterol. 2017;23:763-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 76. | Summerskill WH, Walshe JM. Benign recurrent intrahepatic “obstructive” jaundice. Lancet. 1959;2:686-690. [PubMed] |
| 77. | Mcphedran NT, Henderson RD. Pruritus and jaundice. Can Med Assoc J. 1965;92:1258-1260. [PubMed] |
| 78. | Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ. Primary biliary cirrhosis. Hepatology. 2009;50:291-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 894] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 79. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 1198] [Article Influence: 74.9] [Reference Citation Analysis (1)] |
| 80. | Parés A, Caballería L, Rodés J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic Acid. Gastroenterology. 2006;130:715-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 538] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 81. | Kuiper EM, Hansen BE, de Vries RA, den Ouden-Muller JW, van Ditzhuijsen TJ, Haagsma EB, Houben MH, Witteman BJ, van Erpecum KJ, van Buuren HR. Improved prognosis of patients with primary biliary cirrhosis that have a biochemical response to ursodeoxycholic acid. Gastroenterology. 2009;136:1281-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 348] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 82. | Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. N Engl J Med. 1997;336:691-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 379] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 83. | Chappell LC, Gurung V, Seed PT, Chambers J, Williamson C, Thornton JG. Ursodeoxycholic acid versus placebo, and early term delivery versus expectant management, in women with intrahepatic cholestasis of pregnancy: semifactorial randomised clinical trial. BMJ. 2012;344:e3799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 84. | Duncan JS, Kennedy HJ, Triger DR. Treatment of pruritus due to chronic obstructive liver disease. Br Med J (Clin Res Ed). 1984;289:22. [PubMed] |
| 85. | Di Padova C, Tritapepe R, Rovagnati P, Rossetti S. Double-blind placebo-controlled clinical trial of microporous cholestyramine in the treatment of intra- and extra-hepatic cholestasis: relationship between itching and serum bile acids. Methods Find Exp Clin Pharmacol. 1984;6:773-776. [PubMed] |
| 86. | Mela M, Mancuso A, Burroughs AK. Review article: pruritus in cholestatic and other liver diseases. Aliment Pharmacol Ther. 2003;17:857-870. [PubMed] |
| 87. | Khurana S, Singh P. Rifampin is safe for treatment of pruritus due to chronic cholestasis: a meta-analysis of prospective randomized-controlled trials. Liver Int. 2006;26:943-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 111] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 88. | Bachs L, Parés A, Elena M, Piera C, Rodés J. Effects of long-term rifampicin administration in primary biliary cirrhosis. Gastroenterology. 1992;102:2077-2080. [PubMed] |
| 89. | Martínez E, Collazos J, Mayo J. Hypersensitivity reactions to rifampin. Pathogenetic mechanisms, clinical manifestations, management strategies, and review of the anaphylactic-like reactions. Medicine (Baltimore). 1999;78:361-369. [PubMed] |
| 90. | Imam MH, Gossard AA, Sinakos E, Lindor KD. Pathogenesis and management of pruritus in cholestatic liver disease. J Gastroenterol Hepatol. 2012;27:1150-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 91. | Bergasa NV, Alling DW, Talbot TL, Swain MG, Yurdaydin C, Turner ML, Schmitt JM, Walker EC, Jones EA. Effects of naloxone infusions in patients with the pruritus of cholestasis. A double-blind, randomized, controlled trial. Ann Intern Med. 1995;123:161-167. [PubMed] |
| 92. | Wolfhagen FH, Sternieri E, Hop WC, Vitale G, Bertolotti M, Van Buuren HR. Oral naltrexone treatment for cholestatic pruritus: a double-blind, placebo-controlled study. Gastroenterology. 1997;113:1264-1269. [PubMed] |
| 93. | Terg R, Coronel E, Sordá J, Muñoz AE, Findor J. Efficacy and safety of oral naltrexone treatment for pruritus of cholestasis, a crossover, double blind, placebo-controlled study. J Hepatol. 2002;37:717-722. [PubMed] |
| 94. | Mansour-Ghanaei F, Taheri A, Froutan H, Ghofrani H, Nasiri-Toosi M, Bagherzadeh AH, Farahvash MJ, Mirmomen S, Ebrahimi-Dariani N, Farhangi E. Effect of oral naltrexone on pruritus in cholestatic patients. World J Gastroenterol. 2006;12:1125-1128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 95. | Phan NQ, Bernhard JD, Luger TA, Ständer S. Antipruritic treatment with systemic μ-opioid receptor antagonists: a review. J Am Acad Dermatol. 2010;63:680-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 96. | Ando A, Oshida K, Fukuyama S, Watanabe A, Hashimoto H, Miyamoto Y. Identification of human cytochrome P450 enzymes involved in the metabolism of a novel к-opioid receptor agonist, nalfurafine hydrochloride. Biopharm Drug Dispos. 2012;33:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 97. | Nakao K, Togashi Y, Honda T, Momen S, Umeuchi H, Sakakibara S, Tanaka T, Okano K, Mochizuki H. In vitro and in vivo pharmacological characterization of the main metabolites of nalfurafine hydrochloride. Eur J Pharmacol. 2012;695:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 98. | Kumada H, Miyakawa H, Muramatsu T, Ando N, Oh T, Takamori K, Nakamoto H. Efficacy of nalfurafine hydrochloride in patients with chronic liver disease with refractory pruritus: A randomized, double-blind trial. Hepatol Res. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 99. | Mayo MJ, Handem I, Saldana S, Jacobe H, Getachew Y, Rush AJ. Sertraline as a first-line treatment for cholestatic pruritus. Hepatology. 2007;45:666-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 186] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 100. | Alallam A, Barth D, Heathcote EJ. Role of plasmapheresis in the treatment of severe pruritus in pregnant patients with primary biliary cirrhosis: case reports. Can J Gastroenterol. 2008;22:505-507. [PubMed] |
| 101. | Fuhrmann V, Drolz A, Trauner M. Extracorporeal artificial liver support systems in the management of intractable cholestatic pruritus. Liver Int. 2011;31 Suppl 3:31-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 102. | Parés A, Cisneros L, Salmerón JM, Caballería L, Mas A, Torras A, Rodés J. Extracorporeal albumin dialysis: a procedure for prolonged relief of intractable pruritus in patients with primary biliary cirrhosis. Am J Gastroenterol. 2004;99:1105-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 103. | Leckie P, Tritto G, Mookerjee R, Davies N, Jones D, Jalan R. ‘Out-patient’ albumin dialysis for cholestatic patients with intractable pruritus. Aliment Pharmacol Ther. 2012;35:696-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 104. | Parés A, Herrera M, Avilés J, Sanz M, Mas A. Treatment of resistant pruritus from cholestasis with albumin dialysis: combined analysis of patients from three centers. J Hepatol. 2010;53:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 105. | Pusl T, Denk GU, Parhofer KG, Beuers U. Plasma separation and anion adsorption transiently relieve intractable pruritus in primary biliary cirrhosis. J Hepatol. 2006;45:887-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 106. | Decock S, Roelandts R, Steenbergen WV, Laleman W, Cassiman D, Verslype C, Fevery J, Pelt JV, Nevens F. Cholestasis-induced pruritus treated with ultraviolet B phototherapy: an observational case series study. J Hepatol. 2012;57:637-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 107. | Beuers U, Gerken G, Pusl T. Biliary drainage transiently relieves intractable pruritus in primary biliary cirrhosis. Hepatology. 2006;44:280-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 108. | Kumagai H, Ebata T, Takamori K, Muramatsu T, Nakamoto H, Suzuki H. Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: a Phase III, randomized, double-blind, placebo-controlled study. Nephrol Dial Transplant. 2010;25:1251-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 243] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 109. | Kumagai H, Ebata T, Takamori K, Miyasato K, Muramatsu T, Nakamoto H, Kurihara M, Yanagita T, Suzuki H. Efficacy and safety of a novel ĸ-agonist for managing intractable pruritus in dialysis patients. Am J Nephrol. 2012;36:175-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 110. | Pellicciari R, Fiorucci S, Camaioni E, Clerici C, Costantino G, Maloney PR, Morelli A, Parks DJ, Willson TM. 6alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J Med Chem. 2002;45:3569-3572. [PubMed] |
| 111. | Lindor KD. Farnesoid X receptor agonists for primary biliary cirrhosis. Curr Opin Gastroenterol. 2011;27:285-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 112. | Nevens F, Andreone P, Mazzella G, Strasser SI, Bowlus C, Invernizzi P, Drenth JP, Pockros PJ, Regula J, Beuers U. A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis. N Engl J Med. 2016;375:631-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 791] [Article Influence: 87.9] [Reference Citation Analysis (0)] |
| 113. | Bolier AR, Peri S, Oude Elferink RP, Beuers U. The challenge of cholestatic pruritus. Acta Gastroenterol Belg. 2012;75:399-404. [PubMed] |
| 114. | Jones EA, Bergasa NV. The pruritus of cholestasis. Hepatology. 1999;29:1003-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 104] [Article Influence: 4.0] [Reference Citation Analysis (0)] |