Published online Apr 7, 2017. doi: 10.3748/wjg.v23.i13.2404
Peer-review started: December 6, 2016
First decision: January 10, 2017
Revised: January 28, 2017
Accepted: February 17, 2017
Article in press: February 17, 2017
Published online: April 7, 2017
Processing time: 123 Days and 5 Hours
To investigate serum mean platelet volume (MPV) levels in acute pancreatitis (AP) patients and assess whether MPV effectively predicts the disease severity of AP.
We included 117 consecutive patients with AP as the AP group and 34 consecutive patients with colorectal polyps (before endoscopic treatment) as the control group. Complete blood counts, liver function, platelet indices (MPV), coagulation parameters, lactate dehydrogenase (LDH) and C-reactive protein (CRP) were measured on days 1, 2, 3 and 7 after admission. Receiver operating characteristic curves were used to compare the sensitivity and specificity of MPV, white blood cell (WBC), LDH and CRP in predicting AP severity. The Modified Glasgow Prognostic Score (mGPS) and the 2012 revised Atlanta criteria were used to evaluate disease severity in AP.
MPV levels were significantly lower in the AP group than in the control group on day 1 (P = 0.000), day 2 (P = 0.029) and day 3 (P = 0.001) after admission. In addition, MPV values were lower on day 1 after admission than on day 2 (P = 0.012), day 3 (P = 0.000) and day 7 (P = 0.002) in all AP patients. Based on the mGPS, 78 patients (66.7%) were diagnosed with mild and 39 patients (33.3%) with severe AP. There was no significant difference in mean MPV levels between patients diagnosed with mild and severe AP based on the mGPS (P = 0.424). According to the 2012 revised Atlanta criteria, there were 98 patients (83.8%) without persistent organ failure (OF) [non-severe acute pancreatitis (non-SAP) group] and 19 patients (16.2%) with persistent OF (SAP group). MPV levels were significantly lower in the SAP group than in the non-SAP group on day 1 after admission (P = 0.002). On day 1 after admission using a cut-off value of 6.65 fL, the overall accuracy of MPV for predicting SAP according to the 2012 revised Atlanta criteria (AUC = 0.716) had a sensitivity of 91.8% and a specificity of 47.4% and was superior to the accuracy of the traditional markers WBC (AUC = 0.700) and LDH (AUC = 0.697).
MPV can be used at no additional cost as a useful, non-invasive biomarker that distinguishes AP with persistent OF from AP without persistent OF on day 1 of hospital admission.
Core tip: Mean platelet volume (MPV) is a machine-calculated measurement of average platelet size that is easily obtained using automatic blood count equipment at no additional cost and is often overlooked by clinicians. However, the relationship between MPV and acute pancreatitis (AP) remains unclear, and previous studies have been limited and produced conflicting results. In the present study, we demonstrated that the MPV was significantly lower in AP patients than in controls during the first three days after admission, particularly on day 1 after admission. Moreover, on day 1 of hospital admission, white blood cell count, lactate dehydrogenase and C-reactive protein measures were not as sensitive as MPV for predicting persistent organ failure in AP patients.
- Citation: Lei JJ, Zhou L, Liu Q, Xiong C, Xu CF. Can mean platelet volume play a role in evaluating the severity of acute pancreatitis? World J Gastroenterol 2017; 23(13): 2404-2413
- URL: https://www.wjgnet.com/1007-9327/full/v23/i13/2404.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i13.2404
Severe acute pancreatitis (SAP) is a critical illness in which both the inflammatory and coagulation systems are considered ticking time bombs. The most extreme cases can result in multiple organ dysfunction and disseminated intravascular coagulation. Platelet activation appears to play an important role in both the inflamed pancreas itself and remote organ failure (OF)[1]. Indeed, complex interactions occur between inflammation and hemostasis. Inflammation increases procoagulant factors, and coagulation augments inflammation. Because SAP is associated with systemic complications and high mortality, and approximately half of SAP patients show no clinical signs of OF during the first hours or even days of hospitalization[2-4], it is important to identify the mechanisms that induce the switch from mild to severe AP and the point at which this switch occurs. The adjunctive use of additional markers may add significant benefit to the prediction of disease severity and improve diagnostic accuracy.
Mean platelet volume (MPV) is a parameter in complete blood count analysis that measures average platelet size. As an indicator of thrombocytic activity, MPV has been investigated in various proinflammatory and prothrombotic clinical states[5]. Increased MPV has been associated with the risk of thrombosis and observed in patients with acute myocardial infarction, acute cerebral ischemia, and transient ischemic attack[6-9]. High-grade inflammatory conditions such as inflammatory bowel disease[10], ulcerative colitis[11], acute appendicitis[12-14], acute cholecystitis[15], chronic hepatitis B[16], rheumatoid arthritis and familial Mediterranean fever are characterized by small platelets, and disease remission is characterized by large platelets[7,17-19]. However, the relationship between MPV and AP remains unclear. In addition, there are few previous studies in this area, and their results have been conflicting[20-22]. Therefore, the objective of the present study was to evaluate serum MPV levels in AP and determine whether MPV is more useful than previously established single biochemical markers in predicting AP severity.
In this study, we included 117 patients who were diagnosed with a first attack of AP (AP group) and 34 consecutive patients with colorectal polyps who had not yet undergone endoscopic treatment (control group). Extensive demographic, radiographic, and laboratory data were prospectively collected for all included patients. The following exclusion criteria were applied: (1) pancreatic tumor; (2) a > 5-year history of heavy drinking (> 50 g/d); (3) age younger than 18 years; (4) admitted more than 24 h after the onset of the disease; and (5) pre-existing chronic pancreatitis and a previous history of AP. This study was approved by the Institutional Review Board of the Affiliated Baiyun Hospital of Guizhou Medical University and conformed to the requirements of the Declaration of Helsinki. Informed written consent was obtained from all participants.
AP was diagnosed if a patient presented at least two of the three following findings: (1) abdominal pain characteristic of AP (i.e., acute onset of persistent and severe epigastric pain that often radiated to the back); (2) elevated serum amylase and/or lipase levels higher than three times the upper normal limit; and (3) characteristic findings in imaging studies, including abdominal ultrasonography or computed tomography (CT), consistent with AP[23]. Hyperlipidemic AP was considered when serum triglyceride levels were higher than 11.3 mmol/L in parallel with clinical manifestations or when blood triglyceride levels were 5.56-11.30 mmol/L in cases where chylous effusion was confirmed and other diseases were excluded[24]. Biliary AP was diagnosed when a gallstone or biliary sludge was observed on abdominal ultrasonography or CT. Alcohol was considered a cause of AP in patients who had a history of alcohol consumption within 48 h before symptom onset and in whom other possible causes were ruled out. Etiology was considered idiopathic when causative factors could not be identified from a detailed clinical and drug history or after initial investigations.
A Modified Glasgow Prognostic Score (mGPS) and the 2012 revised Atlanta criteria were used to evaluate disease severity in AP.
According to the mGPS[25], eight variables [age > 55 years; white blood cell (WBC) count > 15 × 109/L; blood glucose > 10 mmol/L; blood urea > 16 mmol/L; arterial oxygen partial pressure < 8.0 kPa; serum albumin < 32 g/L; serum calcium < 2.0 mmol/L; and lactate dehydrogenase (LDH) > 600 U/L] were analyzed, and patients were subsequently graded as having mild AP (score < 3) or severe (score ≥ 3) AP.
Patients were categorized into the following three groups based on the most recent 2012 revised Atlanta Classification[23]: MAP: patients without OF and without local complications; MSAP: patients with OF for less than 48 h or local complications; and SAP: patients with OF for more than 48 h. Since the main purpose of this study was to distinguish SAP in the early stage of the disease, MSAP and MAP were merged with the non-SAP group, while AP with persistent OF was considered the SAP group.
The following criteria were used for OF: (1) respiratory failure: an oxygenation index (OI) lower than 300; (2) renal failure: a serum creatinine level higher than 170 μmol/L or 1.9 mg/dL; and (3) cardiac failure: systolic blood pressure lower than 90 mmHg and no response to fluid resuscitation.
The withdrawal criterion was that the patient himself/herself or the authorized person requested to be withdrawn from the study. Indications for discontinuing therapy included the following: (1) disappearance of specific abdominal symptoms, (2) Marshall score < 2, and (3) triglycerides < 5.6 mmol/L[23]. Contrast-enhanced computed tomography was performed in required cases on day 4 after admission to identify pancreatic necrosis (PNec), local complications, and possible AP etiology.
Clinical data, including the patients’ gender, age, body temperature, pulse, blood pressure, respiratory rate, complete blood cell count, platelet count, chemical examination results, monitoring indicators, and hematocrit, glucose, creatinine, blood urea nitrogen, and electrolyte levels, were collected on days 1, 2, 3 and 7 after admission.
Data were collected and entered into a Microsoft Excel database. After data collection was completed, the data were imported into SPSS for Windows (21.0, SPSS, Chicago, IL, United States). Data are presented as the mean ± SD for normally distributed continuous variables. Two-group comparisons were made using the paired samples t-test and Mann-Whitney U test. One-way ANOVA was used to identify differences among multiple groups with normally distributed variables. Diagnostic accuracy was portrayed as the area under the curve (AUC) for receiver-operator curves (ROCs). When a significant cut-off value was observed, the sensitivity, specificity, and positive and negative predictive values are presented. Two-sided P values < 0.05 were considered statistically significant. Categorical variables are expressed as absolute numbers and proportions. Pearson’s χ2 test or Fisher’s exact test was used to compare categorical variables. A P value < 0.05 was considered statistically significant.
A total of 117 patients with AP and 34 control subjects were enrolled in the present study. The clinical differences and laboratory results of the study participants are presented in Table 1. There were no significant differences in sex and age between the AP and control groups. Serum MPV and antithrombin III (AT-III) levels were significantly lower in the AP patients than in the control group, and WBC, serum fibrinogen (FIB) and D-dimer (D-D) levels were significantly higher in the AP patients than in the control group.
| Acute pancreatitis (n = 117) | Control group (n = 34) | P value | |
| Age (yr) | 46.98 ± 14.42 | 50.53 ± 12.18 | 0.1171 |
| Gender (M/F) | 72 (61.5%)/45 (38.5%) | 19 (55.9%)/15 (44.1%) | 0.5572 |
| MPV (fL) | 8.72 ± 2.37 | 10.98 ± 1.40 | 0.00061 |
| WBC (× 109/L) | 13.28 ± 3.84 | 5.99 ± 1.64 | 0.0001 |
| Platelet (× 109/L) | 180.00 ± 65.04 | 203.56 ± 52.73 | 0.0601 |
| AT-III (%) | 90.93 ± 19.00 | 101.44 ± 10.36 | 0.0001 |
| APTT (S) | 37.96 ± 14.12 | 36.79 ± 4.14 | 0.6351 |
| PT (S) | 12.85 ± 1.14 | 12.63 ± 0.62 | 0.1441 |
| FIB (g/L) | 3.68 ± 1.45 | 2.91 ± 0.51 | 0.0001 |
| D-D (μg/mL) | 1.16 ± 1.14 | 0.27 ± 0.16 | 0.0001 |
| Hb (g/L) | 143.69 ± 26.04 | 147.53 ± 14.76 | 0.4211 |
| Hct (%) | 42.91 ± 6.46 | 44.75 ± 4.51 | 0.1281 |
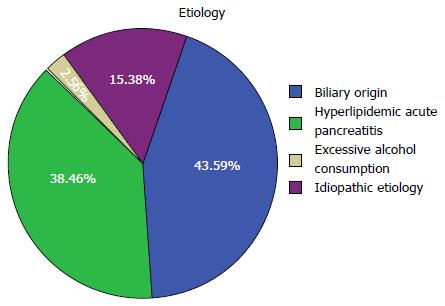
The most common cause of AP was biliary origin in 51 (43.6%) patients, followed by hyperlipidemic acute pancreatitis in 45 (38.5%), idiopathic etiology in 18 (15.4%) and excessive alcohol consumption in 3 (2.6%) patients. No patient whose disease was induced by drugs or endoscopic retrograde cholangiopancreatography was included in this study (Figure 1).
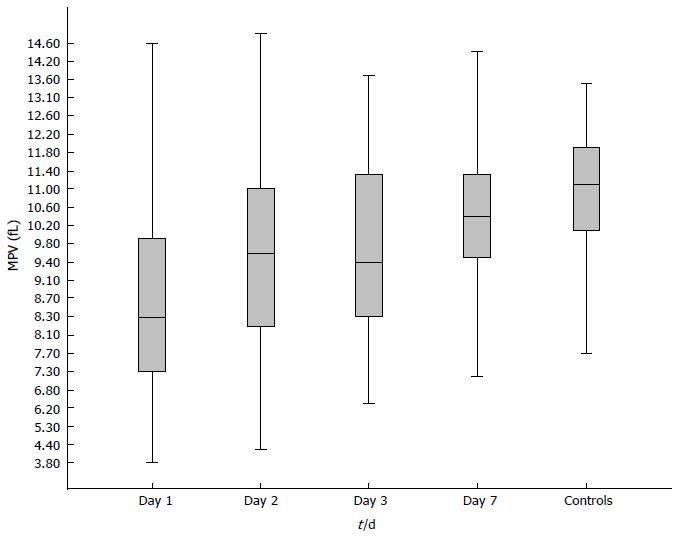
MPV levels were significantly lower in patients with AP than in the control group (F = 13.92, P = 0.000). Table 2 and Figure 2 show the mean MPV values in the AP and control patients on days 1, 2, 3 and 7 after admission. MPV levels were significantly lower in the AP patients than in the control group on day 1 (P = 0.000), day 2 (P = 0.029) and day 3 (P = 0.001) after admission. In addition, MPV levels were significantly lower in AP patients on day 1 than on day 2 (P = 0.012), day 3 (P = 0.000) and day 7 (P = 0.002).
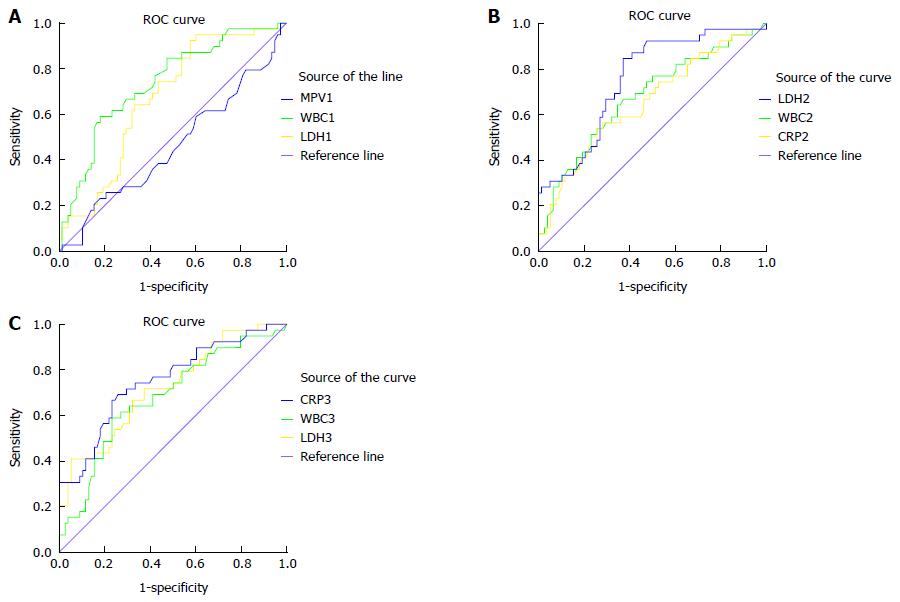
According to the mGPS, 78 AP patients (66.7%) were classified as MAP, and 39 AP patients (33.3%) were classified as SAP. Serum MPV, WBC, LDH and C-reactive protein (CRP) levels in the MAP and SAP patients according to the mGPS were calculated on days 1, 2, 3, and 7 after admission, as shown in Table 3. There was no significant difference in mean MPV levels between the MAP and SAP groups according to the mGPS on days 1, 2, 3 and 7 after admission. CRP levels were significantly higher in the SAP group than in the MAP group on days 2, 3 and 7 after admission. WBC and LDH levels were significantly higher in the SAP group than in the MAP group on days 1, 2, 3 and 7 after admission. ROC curve analysis showed that the overall accuracy of MPV in predicting SAP (AUC = 0.540) was lower than that of traditional WBC and LDH (AUC = 0.737, 0.669 respectively) on day 1 after admission, lower than that of CRP (AUC = 0.651), LDH (AUC = 0.753) and WBC (AUC = 0.675) on day 2 after admission, and lower than that of WBC (AUC = 0.681), LDH (AUC = 0.724) and CRP (AUC = 0.754) on day 3 after admission (Figure 3 and Table 4).
| Mild pancreatitis (n = 78) | Severe pancreatitis (n = 39) | t | P value | |
| MPV1 (fL) | 8.85 ± 2.32 | 8.47 ± 2.49 | 0.802 | 0.424 |
| MPV2 (fL) | 9.66 ± 2.32 | 9.67 ± 2.49 | -0.024 | 0.981 |
| MPV3 (fL) | 9.68 ± 1.84 | 9.96 ± 1.84 | -0.791 | 0.430 |
| MPV7 (fL) | 10.40 ± 1.59 | 10.52 ± 1.80 | -0.357 | 0.721 |
| WBC1 (× 109/L) | 12.26 ± 3.62 | 15.33 ± 3.47 | -4.387 | 0.000 |
| WBC2 (× 109/L) | 10.52 ± 3.63 | 13.26 ± 4.78 | -3.456 | 0.001 |
| WBC3 (× 109/L) | 8.51 ± 0.19 | 10.69 ± 3.56 | -3.356 | 0.001 |
| WBC7 (× 109/L) | 6.82 ± 2.26 | 9.53 ± 3.80 | -4.831 | 0.001 |
| LDH1 (U/L) | 230.70 ± 79.79 | 280.15 ± 102.28 | -2.870 | 0.005 |
| LDH2 (U/L) | 187.49 ± 51.72 | 258.95 ± 103.09 | -4.080 | 0.000 |
| LDH3 (U/L) | 181.17 ± 44.77 | 242.05 ± 84.61 | -4.209 | 0.000 |
| LDH7 (U/L) | 180.29 ± 46.22 | 227.25 ± 58.69 | -4.366 | 0.000 |
| CRP1 (mg/L) | 42.53 ± 71.33 | 66.85 ± 96.00 | -1.398 | 0.167 |
| CRP2 (mg/L) | 76.83 ± 69.50 | 129.77 ± 111.28 | -2.718 | 0.009 |
| CRP3 (mg/L) | 68.62 ± 61.01 | 154.05 ± 114.50 | -4.361 | 0.000 |
| CRP7 (mg/L) | 23.12 ± 31.93 | 66.49 ± 57.40 | -4.391 | 0.000 |
| Cut-off value | AUC | Sensitivity | Specificity | PPV | NPV | Overall accuracy (%) | |
| MPV1 (fL) | 7.45 | 0.540 | 73.1 | 38.5 | 41.67 | 70.37 | 61.54 |
| WBC1 (× 109/L) | 15.20 | 0.737 | 59.0 | 82.1 | 62.16 | 80.00 | 74.36 |
| LDH1 (U/L) | 192.45 | 0.669 | 92.3 | 42.3 | 44.44 | 91.67 | 58.97 |
| WBC2 (× 109/L) | 11.47 | 0.675 | 64.1 | 65.4 | 48.08 | 78.46 | 64.96 |
| LDH2 (U/L) | 189.60 | 0.753 | 84.6 | 62.8 | 55.53 | 89.09 | 70.09 |
| CRP2 (mg/L) | 98.00 | 0.651 | 56.4 | 71.8 | 50.00 | 76.71 | 66.67 |
| WBC3 (× 109/L) | 9.755 | 0.681 | 59.0 | 76.9 | 56.10 | 78.95 | 70.94 |
| LDH3 (U/L) | 255.96 | 0.724 | 41.0 | 94.9 | 80.00 | 76.28 | 76.92 |
| CRP3 (mg/L) | 101.00 | 0.754 | 69.2 | 74.4 | 57.45 | 82.65 | 72.65 |
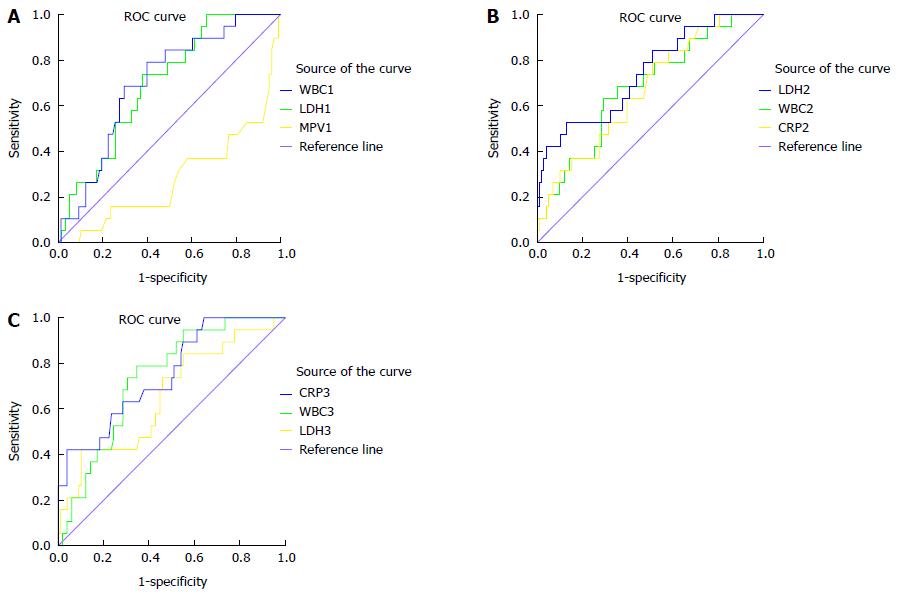
The AP patients were divided into the three groups according to the 2012 revised Atlanta criteria: 24 (20.5%) patients were classified as mild, 74 (63.2%) patients were classified as moderate, and 19 (16.2%) patients were classified as severe. Because SAP is characterized by persistent OF (≥ 48 h) and has different prognoses and high mortality, patients with persistent OF were viewed as a single group, while patients with mild and moderate AP were merged into another group. A subgroup analysis was performed between these two groups (i.e., the non-SAP and SAP groups). There were 98 patients (83.8%) in the non-SAP group and 19 patients (16.2%) in the SAP group. The results of comparisons of MPV, WBC, LDH and CRP between the SAP (according to the 2012 revised Atlanta criteria) and non-SAP groups on days 1, 2, 3 and 7 after admission are shown in Table 5. Mean MPV levels were significantly lower in the SAP group than in the non-SAP on day 1 after admission. In addition, the overall accuracy of MPV in predicting persistent OF (according to the 2012 revised Atlanta criteria) (AUC = 0.716) was superior to that of traditional WBC (AUC = 0.700), LDH (AUC = 0.697) on day 1 after admission, superior to that of CRP (AUC = 0.667), and WBC (AUC = 0.676) on day 2 after admission, and superior to that of LDH (AUC = 0.655) on day 3 after admission. However, the accuracy of MPV was inferior to that of LDH (AUC = 0.740) on day 2 after admission and to those of WBC (AUC = 0.735) and CRP (AUC = 0.749) on day 3 after admission (Figure 4 and Table 6).
| Non-SAP group (n = 98) | SAP (n = 19) | t | P value | |
| MPV1 (fL) | 9.02 ± 2.27 | 7.19 ± 2.34 | 3.198 | 0.002 |
| MPV2 (fL) | 9.84 ± 2.30 | 8.77 ± 2.56 | 1.825 | 0.071 |
| MPV3 (fL) | 9.74 ± 1.88 | 9.92 ± 1.61 | -0.387 | 0.699 |
| MPV7 (fL) | 10.39 ± 1.66 | 10.72 ± 1.67 | -0.798 | 0.426 |
| WBC1 (× 109/L) | 12.85 ± 3.79 | 15.52 ± 3.36 | -2.864 | 0.005 |
| WBC2 (× 109/L) | 10.95 ± 3.89 | 13.94 ± 5.08 | -2.909 | 0.004 |
| WBC3 (× 109/L) | 8.81 ± 3.38 | 11.46 ± 3.02 | -3.174 | 0.002 |
| WBC7 (× 109/L) | 7.22 ± 2.66 | 10.31 ± 4.05 | -3.187 | 0.004 |
| LDH1 (U/L) | 238.82 ± 90.06 | 290.32 ± 82.45 | -2.311 | 0.023 |
| LDH2 (U/L) | 196.39 ± 57.82 | 288.30 ± 125.57 | -3.127 | 0.005 |
| LDH3 (U/L) | 193.37 ± 57.37 | 243.25 ± 95.41 | -2.203 | 0.039 |
| LDH7 (U/L) | 190.58 ± 52.58 | 223.59 ± 61.12 | -2.438 | 0.016 |
| CRP1 (mg/L) | 48.29 ± 79.96 | 63.05 ± 86.57 | -0.726 | 0.469 |
| CRP2 (mg/L) | 84.56 ± 76.89 | 145.63 ± 125.27 | -2.051 | 0.053 |
| CRP3 (mg/L) | 80.71 ± 68.79 | 181.58 ± 140.16 | -3.066 | 0.006 |
| CRP7 (mg/L) | 29.96 ± 37.14 | 76.84 ± 68.26 | -2.912 | 0.009 |
| Cut-off value | AUC | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Overall accuracy (%) | |
| MPV1 (fL) | 6.65 | 0.716 | 91.8 | 47.4 | 52.49 | 70.00 | 67.52 |
| WBC1 (× 109/L) | 13.55 | 0.700 | 60.2 | 78.9 | 27.78 | 93.65 | 63.25 |
| LDH1 (U/L) | 239.08 | 0.697 | 73.7 | 62.2 | 27.45 | 92.42 | 64.10 |
| WBC2 (× 109/L) | 12.36 | 0.676 | 63.2 | 70.4 | 29.27 | 90.80 | 69.23 |
| LDH2 (U/L) | 263.28 | 0.740 | 52.6 | 86.7 | 43.48 | 90.43 | 81.20 |
| CRP2 (mg/L) | 63.50 | 0.667 | 78.9 | 49.0 | 38.46 | 92.31 | 53.00 |
| WBC3 (× 109/L) | 9.32 | 0.735 | 78.9 | 65.3 | 30.61 | 94.12 | 67.52 |
| LDH3 (U/L) | 260.87 | 0.655 | 42.1 | 89.7 | 44.44 | 88.89 | 82.05 |
| CRP3 (mg/L) | 206.50 | 0.749 | 42.1 | 95.9 | 66.67 | 89.52 | 87.17 |
MPV is a machine-calculated measurement of average platelet size that is easily measured with automatic blood count equipment at no additional cost and is often overlooked by clinicians. Many studies have reported that MPV is a commonly used marker of platelet production and function, and MPV has also been shown to reflect inflammatory burden. Evidence, especially data from prospective studies and a meta-analysis, have suggested that there is a correlation between an increase in MPV and the risk of thrombosis and between a decrease in MPV in patients with inflammation and a reversal in the course of anti-inflammatory treatment[5]. AP presents as acute inflammation that is accompanied by thrombosis and bleeding disorders, and platelet activation plays an important role in AP. Because established serum biomarkers are only modestly useful in reflecting disease severity in AP, alternative, cheap, easily applicable and non-invasive markers are needed. We therefore performed this study to evaluate the role of MPV in predicting AP severity and to compare its efficacy with that of other serological markers, such as WBC, LDH and CRP.
Several previous studies have explored MPV in AP, with conflicting results. Okuturlar et al[20] found that MPV levels were significantly lower in both biliary and non-biliary AP patients; Mimidis et al[21], in a study of 54 AP patients, found that MPV values were lower at onset (9.1 fL) than at remission (9.5 fL). However, Akbal et al[22] reported that MPV was significantly higher at admission in acute edematous pancreatitis patients (8.6 ± 1.4 fL) than in controls (7.6 ± 0.7 fL) (P < 0.005). The results of our study revealed that serum MPV levels were lower in AP patients than in controls during the first week after admission. Furthermore, MPV levels were higher after treatment, consistent with the results described by Okuturlar et al[20] and Mimidis et al[21] but in conflict with the results described by Akbal et al[22]. The exact reason why MPV is lower in AP patients remains unclear, but it has been speculated that platelets not only control thrombosis and hemostasis but also regulate inflammatory processes. The lower MPV in AP may reflect an increase in the consumption of large platelets at sites of pancreatitis and distant organ inflammation, which may occur before clinical manifestation of AP attacks[5]. A higher MPV in AP is thought to reflect a hypercoagulable state in acute edematous pancreatitis, as reported by Akbal et al[22] and Boos et al[26], possibly due to inappropriate blood sampling and storing.
Only two reports[27,28] have explored the role of MPV in the severity of AP. Beyazit et al[27] reported that according to the mGPS, the overall accuracy of MPV for identifying severe AP was 72.7% with a sensitivity, specificity, NPV and PPV of 70.6%, 73.9%, 81.9%, and 60.0%, respectively (AUC = 0.762). Erbis et al[28] found that MPV was lower in acute necrotizing pancreatitis (ANP) (7.2 ± 0.52 fL) than in edematous pancreatitis (AEP) (7.9 ± 0.53 fL; P < 0.001). When they compared the study groups using a ROC analysis, the results demonstrated that the cut-off value for necrotizing pancreatitis patients was 7.8 fL (AUC = 0.857), with a sensitivity of 86.1% and specificity of 72.5%. In the present study, MPV was significantly lower in pancreatitis patients with persistent OF than in patients without persistent OF, with a cut-off value of 6.65 fL, which yielded a sensitivity of 91.8% and specificity of 47.4% (AUC = 0.716) in predicting AP with persistent OF. Sensitivity and specificity represent the proportions of severe and mild attacks in AP, respectively. Good sensitivity ensures that high-risk patients are distinguished from those with mild, self-limiting disease in the early stage of AP, which is important to allow clinicians to identify potential OF patients and initiate appropriate supportive treatments and interventions. However, in this study, we found that there was no advantage in predicting the severity of AP according to the mGPS. The potential reasons for this discrepancy include the following: first, our study is prospective, but the results reported by Beyazit et al[27] were obtained from retrospective studies; the second most common cause of AP was alcohol consumption in the study by Beyazit et al[27], but hypertriglyceridemia in our study.
LDH, a glycolytic enzyme, is present in the cytoplasm of all living cells but is found at higher concentrations in the heart, kidneys, and skeletal muscles. We found that LDH levels were significantly higher in the SAP group (according to mGPS) than in the MAP group during the first week after admission, and serum LDH levels were also significantly higher in the SAP group than in the non-SAP group during the first week after admission based on the 2012 revised Atlanta criteria. Moreover, the cut-off LDH level on day 1 after admission for SAP according to mGPS was 192.45 IU/L, with a sensitivity of 92.3% and specificity of 42.3%, whereas the cut-off LDH level on day 3 after admission for severe AP according to mGPS was 255.96 U/L, with a sensitivity of 41.0% and specificity of 94.9%. These results suggest that dynamic monitoring of serum LDH levels is essential to making a correct diagnosis. The cut-off LDH level on day 1 after admission for persistent OF according to the 2012 revised Atlanta criteria was 239.08 U/L, with a sensitivity of 73.7% and specificity of 62.2%. On days 2 and 3 after admission, the LDH cut-off values were 263.28 U/L and 260.87 U/L, respectively, with a relatively higher specificity (86.7% and 89.7%, respectively) and a lower sensitivity (52.6% and 42.1%, respectively) for predicting persistent OF. The normal reference value for LDH is 9-245 U/L, suggesting that if LDH levels do not significantly increase, the probability that persistent OF and SAP will occur is small. Our results are consistent with those of Tasić et al[29] and Zrnić et al[30]. Those authors observed that LDH levels were significantly higher in patients with severe pancreatitis than in patients with moderate pancreatitis (P < 0.01).They also found that specificity and diagnostic accuracy were highest for LDH on the first day (67.74%; 57%) when predicting complications of AP.
CRP is a neutrophil-activating peptide (acute phase protein) that is synthesized in hepatocytes in multiple cell lines. Its production is induced by the release of interleukins (ILs) 1 and 6. Our results showed that on day 1 after admission, plasma CRP levels were not significantly different between MAP and SAP according to mGPS, and there was no significant difference between the non-SAP group and SAP defined based on the 2012 revised Atlanta criteria, suggesting that in AP patients, CRP levels are not likely to reflect disease severity when measured during its early phase after onset. However, on day 3 after admission, CRP levels peaked (AUC = 0.754) in the ROC curve analysis and were superior to WBC and LDH for predicting SAP according to mGPS (Figure 3C and Table 4) and persistent OF (AUC = 0.749) according to the 2012 revised Atlanta criteria (Figure 4C and Table 6). These results suggest that despite its delayed increase, in which CRP peaks no earlier than 72 h after symptom onset, CRP remains among the most useful[31] serum biochemical markers for predicting the severity and progression of AP.
In conclusion, in the present study, we demonstrated that MPV levels were lower in AP patients than in the control group during the first week after admission. In addition, MPV levels were lower in patients with persistent OF than in patients without persistent OF. Furthermore, MPV had higher sensitivity than WBC, LDH and CRP for predicting AP with persistent OF on day 1 after admission. Serum MPV levels may be a useful tool for predicting SAP as defined by the latest 2012 Atlanta Classification during the early stage of the disease.
Many studies have suggested that there is a correlation between an increase in mean platelet volume (MPV) and the risk of thrombosis and between a decrease in MPV in patients with acute inflammation and the reversal of the course of anti-inflammation treatment. Acute pancreatitis (AP) is an acute inflammatory condition that is accompanied by thrombosis and bleeding disorders, and platelet activation therefore plays an important role in AP. Currently established serum biomarkers are only modestly useful for predicting disease activity of AP. Hence, cheap, easily applicable and non-invasive markers are needed. Accordingly, this study was performed to evaluate the efficacy of using MPV to predicting AP severity compared to other serological markers, such as white blood cell (WBC), lactate dehydrogenase (LDH) and C-reactive protein (CRP).
AP is a potentially life-threatening disease with a wide spectrum of severity. The overall mortality rate for AP is approximately 5% and is as high as 20%-30% in patients with severe AP. It is generally recognized that predicting disease severity is important for managing individual patients, but making such predictions is very difficult. Although CRP is considered the most useful biochemical serum marker for predicting the severity and progression of AP, it can only predict severity at 48 h after admission. This timeframe may be too late because early aggressive fluid resuscitation is a cornerstone of AP therapy. One focus of research on this topic should be the introduction of MPV as a useful, non-invasive biomarker that can be evaluated with no additional cost and that can distinguish AP with persistent organ failure (OF) from that without OF on day 1 of hospital admission.
Studies have been performed over the last few decades in an attempt to identify new biochemical markers that accurately predict the severity of pancreatitis. However, no gold standard has emerged for predicting the course of AP. The present study is the first prospective clinical study to measure MPV in AP patients in an attempt to predict persistent OF in the early stage of AP.
The results of this study suggest that on day 1 after admission, the overall accuracy of MPV for predicting SAP (defined according to the 2012 revised Atlanta criteria) is superior to those of the traditional markers WBC and LDH. MPV may therefore be a useful biochemical marker for predicting persistent OF during the early stage of AP. Furthermore, this study also provides readers with important information about the predictive value of LDH on day 2 after admission and of WBC and CRP on day 3 after admission.
MPV is a machine-calculated measurement of average platelet size that is easily measured at no additional cost using automatic blood count equipment and is often overlooked by clinicians. MPV is a commonly used marker of platelet production and function and has also been shown to reflect inflammatory burden.
In this observational, prospective clinical study article, the authors evaluate the efficacy of measuring MPV to predicting the severity of AP. This is an important study because conflicting results have been reported regarding the levels of MPV in AP patients. The authors demonstrated that lower MPV levels were observed in AP patients than in controls during the first week of hospital admission. Moreover, the results indicated that the MPV level was lower in patients with persistent OF than in those without persistent OF on day 1 of hospital admission. These results may assist clinicians in achieving more accurate early diagnoses of AP patients with persistent OF.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Inal V, Ulmasov B S- Editor: Yu J L- Editor: Ma JY E- Editor: Zhang FF
| 1. | Aksu K, Donmez A, Keser G. Inflammation-induced thrombosis: mechanisms, disease associations and management. Curr Pharm Des. 2012;18:1478-1493. [PubMed] |
| 2. | Johnson CD, Kingsnorth AN, Imrie CW, McMahon MJ, Neoptolemos JP, McKay C, Toh SK, Skaife P, Leeder PC, Wilson P. Double blind, randomised, placebo controlled study of a platelet activating factor antagonist, lexipafant, in the treatment and prevention of organ failure in predicted severe acute pancreatitis. Gut. 2001;48:62-69. [PubMed] |
| 3. | Maksimow M, Kyhälä L, Nieminen A, Kylänpää L, Aalto K, Elima K, Mentula P, Lehti M, Puolakkainen P, Yegutkin GG. Early prediction of persistent organ failure by soluble CD73 in patients with acute pancreatitis*. Crit Care Med. 2014;42:2556-2564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Nieminen A, Maksimow M, Mentula P, Kyhälä L, Kylänpää L, Puolakkainen P, Kemppainen E, Repo H, Salmi M. Circulating cytokines in predicting development of severe acute pancreatitis. Crit Care. 2014;18:R104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 5. | Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des. 2011;17:47-58. [PubMed] |
| 6. | Giles H, Smith RE, Martin JF. Platelet glycoprotein IIb-IIIa and size are increased in acute myocardial infarction. Eur J Clin Invest. 1994;24:69-72. [PubMed] |
| 7. | Leader A, Pereg D, Lishner M. Are platelet volume indices of clinical use? A multidisciplinary review. Ann Med. 2012;44:805-816. [PubMed] |
| 8. | Chu SG, Becker RC, Berger PB, Bhatt DL, Eikelboom JW, Konkle B, Mohler ER, Reilly MP, Berger JS. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost. 2010;8:148-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Balcik ÖS, Bilen S, Ulusoy EK, Akdeniz D, Uysal S, Ikizek M, Ak F, Kosar A. Thrombopoietin and mean platelet volume in patients with ischemic stroke. Clin Appl Thromb Hemost. 2001;19:92-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Kapsoritakis AN, Koukourakis MI, Sfiridaki A, Potamianos SP, Kosmadaki MG, Koutroubakis IE, Kouroumalis EA. Mean platelet volume: a useful marker of inflammatory bowel disease activity. Am J Gastroenterol. 2001;96:776-781. [PubMed] |
| 11. | Yüksel O, Helvaci K, Başar O, Köklü S, Caner S, Helvaci N, Abayli E, Altiparmak E. An overlooked indicator of disease activity in ulcerative colitis: mean platelet volume. Platelets. 2009;20:277-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 12. | Albayrak Y, Albayrak A, Albayrak F, Yildirim R, Aylu B, Uyanik A, Kabalar E, Güzel IC. Mean platelet volume: a new predictor in confirming acute appendicitis diagnosis. Clin Appl Thromb Hemost. 2011;17:362-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Fan Z, Pan J, Zhang Y, Wang Z, Zhu M, Yang B, Shi L, Jing H. Mean Platelet Volume and Platelet Distribution Width as Markers in the Diagnosis of Acute Gangrenous Appendicitis. Dis Markers. 2015;2015:542013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Kucuk E, Kucuk I. Mean Platelet Volume is Reduced in Acute Appendicitis. Turk J Emerg Med. 2015;15:23-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Sayit AT, Gunbey PH, Terzi Y. Is the Mean Platelet Volume in Patients with Acute Cholecystitis an Inflammatory Marker? J Clin Diagn Res. 2015;9:TC05-TC07. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Turhan O, Coban E, Inan D, Yalcin AN. Increased mean platelet volume in chronic hepatitis B patients with inactive disease. Med Sci Monit. 2010;16:CR202-CR205. [PubMed] |
| 17. | Kisacik B, Tufan A, Kalyoncu U, Karadag O, Akdogan A, Ozturk MA, Kiraz S, Ertenli I, Calguneri M. Mean platelet volume (MPV) as an inflammatory marker in ankylosing spondylitis and rheumatoid arthritis. Joint Bone Spine. 2008;75:291-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 238] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 18. | Gasparyan AY, Sandoo A, Stavropoulos-Kalinoglou A, Kitas GD. Mean platelet volume in patients with rheumatoid arthritis: the effect of anti-TNF-α therapy. Rheumatol Int. 2010;30:1125-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Yazici S, Yazici M, Erer B, Erer B, Calik Y, Bulur S, Ozhan H, Ataoglu S. The platelet functions in patients with ankylosing spondylitis: anti-TNF-alpha therapy decreases the mean platelet volume and platelet mass. Platelets. 2010;21:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Okuturlar Y, Soylu A, Dogan H, Cakmak S, Kirac Utku I, Oztosun B, Akarsu C, Ocak Serin S, Avci A, Kones O. Mean platelet volume in patients with biliary and non-biliary acute pancreatitis. Int J Clin Exp Pathol. 2015;8:2051-2056. [PubMed] |
| 21. | Mimidis K, Papadopoulos V, Kotsianidis J, Filippou D, Spanoudakis E, Bourikas G, Dervenis C, Kartalis G. Alterations of platelet function, number and indexes during acute pancreatitis. Pancreatology. 2004;4:22-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Akbal E, Demirci S, Koçak E, Köklü S, Başar O, Tuna Y. Alterations of platelet function and coagulation parameters during acute pancreatitis. Blood Coagul Fibrinolysis. 2013;24:243-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4328] [Article Influence: 360.7] [Reference Citation Analysis (45)] |
| 24. | Takaishi K, Miyoshi J, Matsumura T, Honda R, Ohba T, Katabuchi H. Hypertriglyceridemic acute pancreatitis during pregnancy: prevention with diet therapy and omega-3 fatty acids in the following pregnancy. Nutrition. 1984;25:1094-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Blamey SL, Imrie CW, O’Neill J, Gilmour WH, Carter DC. Prognostic factors in acute pancreatitis. Gut. 1984;25:1340-1346. [PubMed] |
| 26. | Boos CJ, Balakrishnan B, Lip GY. The effects of coronary artery disease severity on time-dependent changes in platelet activation indices in stored whole blood. J Thromb Thrombolysis. 2008;25:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Beyazit Y, Sayilir A, Torun S, Suvak B, Yesil Y, Purnak T, Oztas E, Kurt M, Kekilli M, Ibis M. Mean platelet volume as an indicator of disease severity in patients with acute pancreatitis. Clin Res Hepatol Gastroenterol. 2012;36:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Erbis H, Aliosmanoglu I, Turkoglu MA, Ay E, Turkoglu A, Ulger BV. Evaluating mean platelet volume as a new indicator for confirming the diagnosis of necrotizing pancreatitis. Ann Ital Chir. 2014;86:132-136. [PubMed] |
| 29. | Tasić T, Grgov S, Nagorni A, Benedeto-Stojanov D. [Comparison of biohumoral and morphological parameters in acute pancreatitis]. Srp Arh Celok Lek. 2007;142:29-33. [PubMed] |
| 30. | Zrnić IK, Milić S, Fisić E, Radić M, Stimac D. [C-reactive protein and lactate dehydrogenase as single prognostic factors of severity in acute pancreatitis]. Lijec Vjesn. 2010;129:1-4. [PubMed] |
| 31. | Dambrauskas Z, Gulbinas A, Pundzius J, Barauskas G. Value of the different prognostic systems and biological markers for predicting severity and progression of acute pancreatitis. Scand J Gastroenterol. 2010;45:959-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |