Published online Feb 28, 2016. doi: 10.3748/wjg.v22.i8.2601
Peer-review started: October 14, 2015
First decision: November 5, 2015
Revised: November 18, 2015
Accepted: December 8, 2015
Article in press: December 8, 2015
Published online: February 28, 2016
Processing time: 135 Days and 5.5 Hours
AIM: To evaluate the prognostic factors of hilar cholangiocarcinoma in a large series of patients in a single institution.
METHODS: Eight hundred and fourteen patients with a diagnosis of hilar cholangiocarcinoma that were evaluated and treated between 1990 and 2014, of which 381 patients underwent curative surgery, were included in this study. Potential factors associated with overall survival (OS) and disease-free survival (DFS) were evaluated by univariate and multivariate analyses.
RESULTS: Curative surgery provided the best long-term survival with a median OS of 26.3 mo. The median DFS was 18.1 mo. Multivariate analysis showed that patients with tumor size > 3 cm [hazard ratio (HR) = 1.482, 95%CI: 1.127-1.949; P = 0.005], positive nodal disease (HR = 1.701, 95%CI: 1.346-2.149; P < 0.001), poor differentiation (HR = 2.535, 95%CI: 1.839-3.493; P < 0.001), vascular invasion (HR = 1.542, 95%CI: 1.082-2.197; P = 0.017), and positive margins (HR = 1.798, 95%CI: 1.314-2.461; P < 0.001) had poor OS outcome. The independent factors for DFS were positive nodal disease (HR = 3.383, 95%CI: 2.633-4.348; P < 0.001), poor differentiation (HR = 2.774, 95%CI: 2.012-3.823; P < 0.001), vascular invasion (HR = 2.136, 95%CI: 1.658-3.236; P < 0.001), and positive margins (HR = 1.835, 95%CI: 1.256-2.679; P < 0.001). Multiple logistic regression analysis showed that caudate lobectomy [odds ratio (OR) = 9.771, 95%CI: 4.672-20.433; P < 0.001], tumor diameter (OR = 3.772, 95%CI: 1.914-7.434; P < 0.001), surgical procedures (OR = 10.236, 95%CI: 4.738-22.116; P < 0.001), American Joint Committee On Cancer T stage (OR = 2.010, 95%CI: 1.043-3.870; P = 0.037), and vascular invasion (OR = 2.278, 95%CI: 0.997-5.207; P = 0.051) were independently associated with tumor-free margin, and surgical procedures could indirectly affect survival outcome by influencing the tumor resection margin.
CONCLUSION: Tumor margin, tumor differentiation, vascular invasion, and lymph node status were independent factors for OS and DFS. Surgical procedures can indirectly affect survival outcome by influencing the tumor resection margin.
Core tip: Hilar cholangiocarcinoma remains among the most difficult management problems faced by surgeons. Although curative surgery prolongs the survival time of patients diagnosed with hilar cholangiocarcinoma, outcomes from studies may be contradictory or biased due to differences in study methods and small patient numbers. Furthermore, some large, multi-center reports may induce biases due to the heterogeneity of clinical methods and surgical strategies. Thus, we retrospectively analyzed the prognostic factors of hilar cholangiocarcinoma and factors associated with tumor-free margin in a large sample of hilar cholangiocarcinoma cases from a single institution.
- Citation: Hu HJ, Mao H, Shrestha A, Tan YQ, Ma WJ, Yang Q, Wang JK, Cheng NS, Li FY. Prognostic factors and long-term outcomes of hilar cholangiocarcinoma: A single-institution experience in China. World J Gastroenterol 2016; 22(8): 2601-2610
- URL: https://www.wjgnet.com/1007-9327/full/v22/i8/2601.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i8.2601
Hilar cholangiocarcinoma (HC) is a rare, devastating, and highly malignant disease of the bile duct[1-4]. On the basis of the Bismuth classification, hilar cholangiocarcinoma can be divided into four types: type I represents tumors affecting the common hepatic duct, type II represents tumors affecting the hilus, type III A/B represents tumors invading the right or left hepatic duct, and type IV represents tumors infiltrating both right and left hepatic ducts and the subsegments[1]. A variety of risk factors are reported to increase the odds ratios of HCs, which include primary sclerosing cholangitis, biliary duct cysts, oriental cholangiohepatitis, hepatolithiasis, biliary parasitic disease, and toxins exposure, though the specific etiology is still unclear[5,6].
HC is reported to be a slow-growing and late-metastasizing malignant disease[7-9], but as it is situated at a cabinet and pivotal space, encircled by the portal vein, hepatic artery, liver parenchyma, and the bile duct, it has a strong tendency to extensively invade the portal vein, hepatic artery, perineural tissue, and surrounding liver parenchyma, including the caudate lobe[10-15]. Moreover, the caudate lobe lies in a deep and complex location and has an intimate relationship with the major vascular structures[16,17], resulting in high operative risks and increased postoperative complications[18,19]. In addition, HC always lies in the core part of the biliary system, causing malignant biliary obstruction and cholestatic hepatitis. Thus, major hepatic resection tends to be associated with increased risk of postoperative hepatic insufficiency, and postoperative hepatic failure is reported to be the most frequent cause of in-hospital death following major hepatectomy[14,20]. This is the reason why hepatobiliary surgery for HC is still recognized as the most difficult besides liver transplantation. Nevertheless, surgical resection with extended hepatectomy, caudate lobectomy, lymphadenectomy, vascular resection, and reconstruction remains the cornerstone of the treatment and represents the only potentially radical therapeutic modality to prolong the survival of patients with HC[21-26].
Uni- and multivariate analyses have identified various prognostic factors for overall survival (OS) and disease-free survival (DFS) of HC[7,27-29]. However, only large-volume patient cases can provide more confidence to guide the treatment. Thus, the current study was planned to determine the following: (1) estimate prognostic factors associated with OS and DFS after successful resection of HC in a large, single-center study; (2) evaluate which factors could contribute to the obtaining of R0 resection to help future surgical decision making; and (3) compare various surgical procedures in treating and prolonging the lifespan of HC patients to guide their treatment and forecast the postoperative prognosis.
All patients with a diagnosis of HC that were evaluated and treated at our institution since 1990 were identified from a hepatobiliary database. Patients were divided into three groups: those who underwent curative surgery (n = 381), those who only received palliative surgery (n = 330), and those who did not receive any surgery (n = 103). Patients with intrahepatic bile duct carcinoma infringing the hilum, those with gallbladder carcinomas, and those who underwent liver transplantation were excluded. Clinical, radiologic, histopathologic, therapy, and survival data were obtained and evaluated.
Resectability was assessed both with preoperative imaging studies and intraoperative evaluation. Patients with any of the following were considered to be unresectable: poor conditions, Child-Pugh C, advanced biliary invasion that excludes complete tumor resection, encasement of major vessel structures that eliminate vascular reconstruction, lymph nodes metastases beyond the hepatoduodenal ligament, and metastatic disease (lung and peritoneum metastases)[30,31]. Biliary drainage was performed in most of patients with obstructive jaundice. In patients with cholangitis, resection was performed after alleviation of inflammation. As for the patients who were considered to have potentially resectable tumors, portal vein embolization was performed when the future remnant liver volume (25%-30%) was deemed insufficient (n = 53 patients). Surgical procedures were selected according to the preoperative and intraoperative evaluation.
After treatment, patients were regularly followed-up at an outpatient clinic with clinical examination, serum carbohydrate antigen (CA) 19-9 level, liver function tests, and hepatic ultrasonography. If recurrence was suspected in some specific patients, further assessments such as abdominal CT or magnetic resonance cholangiopancreatography were used to make a definitive diagnosis. The median follow-up period for resected patients was 21.7 mo (range, 3-85 mo) with a follow-up rate of 95.6%.
Data analysis was performed using SPSS version16.0 (SPSS Inc., Chicago, IL, United States). Frequency and descriptive analysis were used to depict patient characteristics. Parametric statistical analysis was conducted using a Student’s t test, while nonparametric analysis was calculated using a χ2 test. Survival (calculated from the time of surgery) was estimated using Kaplan-Meier methods, differences in survival were reviewed using the log-rank test. Multivariate analysis was performed on all factors with a P value of less than 0.10 in univariate analysis. Univariate and bivariate analyses were used to check the association of several tumor variables with tumor resection margin. Multiple logistic regression analysis was used in the final analysis to adjust for independent variables for tumor-free margin. The hazard ratio (HR) and the 95%CI were estimated, and a P < 0.05 was considered as significant.
The clinical characteristics of the study population are summarized in Table 1. The specific surgical procedures for patients who underwent curative and palliative surgery are presented in Table 2.
| Variable | Curative surgery (n = 381) | Palliative surgery (n = 330) |
| Age (median [range]) | 60 [26-82] | 58 [19-80] |
| Sex, male (%) | 231 (60.6) | 178 (53.9) |
| Presenting symptoms | ||
| Jaundice | 267 (70.1) | 240 (72.7) |
| Weight loss | 26 (6.8) | 22 (6.7) |
| Abdominal pain | 24 (6.3) | 28 (8.5) |
| Nausea and vomiting | 20 (5.2) | 17 (5.1) |
| General fatigue | 44 (11.6) | 23 (7.0) |
| Tumor markers (median [range]) | ||
| Preoperative CA 19-9 level, U/mL | 348 [0.6-1000] | 539.9 [0.6-3015.2] |
| Preoperative CA 125 level, U/mL | 19.84 [1.23-257.70] | 33.94 [1.54-1598.00] |
| Preoperative CEA level, ng/mL | 3.23 [0.20-65.51] | 4.44 [0.47-1000.00] |
| Liver functions (median [range]) | ||
| Preoperative TB level, umol/L | 209.4 [7.1-586.3] | 239.65 [1.90-805.70] |
| Preoperative ALT level, U/L | 95 [10-967] | 78.5 [6.0-1212.0] |
| Preoperative AST level, U/L | 86 [14-1016] | 87 [11-927] |
| Preoperative Albumin level, g/L | 36.7 [18.7-51.8] | 35.95 [21.30-72.50] |
| Preoperative hospital stay (median [range]) | 7 [2-44] | 7 [3-48] |
| Total hospital stay (median [range]) | 19 [9-113] | 16 [4-102] |
| Portal vein embolization | 53 (13.9) | |
| Biliary drainage | 201 (52.8) | |
| Estimated blood loss (median [range]) | 600 [50-2000] | 348 [0.6-1000] |
| Bismuth-Corlette classification | ||
| Type I | 95 (25.2) | 16 (4.8) |
| Type II | 92 (24.2) | 59 (17.9) |
| Type IIIa or IIIb | 102 (26.8) | 102 (30.9) |
| Type IV | 92 (24.2) | 153 (46.4) |
| Surgical procedures | n (%) |
| Curative intent surgery | 381 (53.6) |
| Hilar bile duct resection alone | 50 (13.1) |
| Left hemihepatectomy | 142 (37.3) |
| Right hemihepatectomy | 101 (26.5) |
| Left trisegmentectomy | 46 (12.1) |
| Right trisegmentectomy | 20 (5.2) |
| Mesohepatetctomy | 22 (5.8) |
| Additional procedures | |
| Caudate lobectomy | 300 (78.7) |
| Portal vein resection | 51 (13.4) |
| Pancreatoduodenectomy | 8 (2.1) |
| Palliative intent surgery | 330 (46.4) |
| Surgical palliation | 266 (80.6) |
| Nonsurgical palliation | 64 (19.4) |
| ERCP | 35 (10.6) |
| PTCD | 29 (8.8) |
Curative intent surgery: The perioperative complication rate after major surgical treatment was 29.4% (n = 112), which includes, hemorrhage (15 cases), bile leakage (40 cases), peritoneal cavity infection (16 cases), lung infection (23 cases), sepsis (3 cases), acute cardiac failure (3 cases), hepatic failure (20 cases), renal failure (5 cases), stress ulcer (10 cases), wound dehiscence (4 cases), and hydrothorax or ascites (6 cases). The perioperative mortality rate was 3.8% (n = 10). Both postoperative morbidity and operative mortality were deemed as those occurring within 60 d of the major surgery, or occurred at any time during the postoperative hospital stay.
The relationship between postoperative complications and risk factors was evaluated. Patients undergoing hepatectomy had more complications when compared with those who underwent bile duct resection alone (P < 0.001). In patients with postoperative hyperbilirubinemia, the incidence of postoperative complication rate was also higher (P = 0.004).
Palliative intent surgery: The perioperative complication rate after palliative treatment was 11.2% (n = 37), of which the most frequent was lung infection (16 cases), followed by hemorrhage (4 cases), bile leakage (7 cases), peritoneal cavity infection (13 cases), sepsis (2 cases), wound infection (2 cases), hepatic failure (3 cases), renal failure (1 cases), wound dehiscence (1 case), and others (3 cases). The perioperative mortality rate was 4.5% (15 cases), of which the most common cause was multiple organ failure because of liver failure (8 cases), followed by renal failure (3 cases), infective complications (3 cases), and heart failure (1 case).
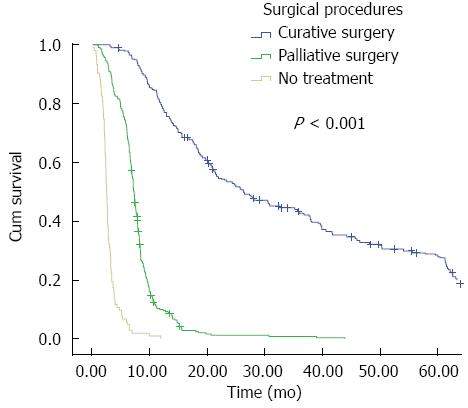
As expected, radical resection provided the best opportunity for OS with a median survival time of 26.3 mo, and the one-, three-, and five-year survival rates were 80%, 43%, and 28%, respectively. This was in contrast to the patients who underwent palliative surgery with a median OS of approximately 7.3 mo, with one-, three-, and five-year survival rates of 10%, 2%, and 0%, respectively (Figure 1, log-rank test, P < 0.001). As for patients who did not receive any surgical treatment, the median OS and the one- and three-year survival rates were 2.6 mo and 1% and 0%, respectively (Figure 1, log-rank test, P < 0.001). The median DFS for the radical resection group was 18.1 mo, and the one-, three-, and five-year DFSs were 78%, 18%, and 10% respectively. Furthermore, we compared the survival rate of those who underwent surgical palliation and nonsurgical palliation; the former had a median survival time and 6-mo survival rate of 7.4 mo and 27% respectively, while the latter had a median survival time of 5.5 mo and 6-mo survival rate of 9% respectively (P < 0.001).
As is shown in Table 3, OS was significantly longer in patients with no lymph node metastasis (P < 0.001), well histologic differentiation (P < 0.001), negative resection margin (P < 0.001), tumor size ≤ 3 cm (P < 0.001), caudate lobectomy (P = 0.04), lower CA 19-9 level (P = 0.039), and no vascular invasion (P = 0.009). Hepatectomy and the lack of perineural infiltration approached statistical differences as positive prognostic factors for OS in univariate analysis (P = 0.072 and 0.084 respectively). The factors associated with disease-free survival were also examined. Resection margins (P < 0.001), tumor differentiation (P < 0.001), lymph nodes metastases (P < 0.001), tumor size (P < 0.001), caudate lobectomy (P < 0.001), CA 19-9 level (P = 0.018), American Joint Committee On Cancer (AJCC) T stage (P = 0.028), and vascular invasion (P < 0.001) were associated with DFS (Table 3).
| Variables | Median OS (mo) | P value | Median DFS (mo) | P value |
| Tumor size | ||||
| ≤ 3 cm | 35.2 | < 0.001 | 19.8 | < 0.001 |
| > 3 cm | 14.7 | 13.0 | ||
| Surgical procedures | ||||
| BDR | 20.8 | 0.072 | 15.0 | 0.065 |
| BDR + hepatectomy | 27.6 | 18.7 | ||
| Lymph node metastasis | ||||
| N0 | 39.9 | < 0.001 | 25.4 | < 0.001 |
| N1-2 | 15.7 | 12.3 | ||
| Tumor differentiation | ||||
| Well | 54.1 | < 0.001 | 25.3 | < 0.001 |
| Moderate | 27.6 | 17.8 | ||
| Poor | 13.5 | 12.1 | ||
| Resection margin | ||||
| R0 | 35.2 | < 0.001 | 20.6 | < 0.001 |
| R1 and R2 | 12.4 | 9.6 | ||
| Presence of vascular invasion | ||||
| No | 26.3 | 0.009 | 19.0 | < 0.001 |
| Yes | 20.9 | 10.6 | ||
| Caudate lobe resection | ||||
| Yes | 35.7 | 0.040 | 21.3 | < 0.001 |
| No | 21.4 | 15.0 | ||
| CA 19-9 > 100 U/L | ||||
| No | 39.7 | 0.039 | 23.6 | 0.018 |
| Yes | 23.0 | 16.7 | ||
| Perineural infiltration | ||||
| No | 27.3 | 0.084 | 22.7 | 0.090 |
| Yes | 20.8 | 15.9 | ||
| T stage (AJCC) | ||||
| T1 and T2 | 27.6 | NS | 19.2 | 0.028 |
| T3 and T4 | 25.7 | 16.9 | ||
In multivariate analysis, tumor size > 3 cm (HR = 1.482, 95%CI: 1.127-1.949; P = 0.005), positive nodal disease (HR = 1.701, 95%CI: 1.346-2.149; P < 0.001), poor differentiation (HR = 2.535, 95%CI: 1.839-3.493; P < 0.001), vascular invasion (HR = 1.542, 95%CI: 1.082-2.197; P = 0.017), and positive margins (HR = 1.798, 95%CI: 1.314-2.461; P < 0.001) remained associated with OS. For DFS, positive nodal disease (HR = 3.383, 95%CI: 2.633-4.348; P < 0.001), poor differentiation (HR = 2.774, 95%CI: 2.012-3.823; P < 0.001), vascular invasion (HR = 2.136, 95%CI: 1.658-3.236; P < 0.001), and positive margins (HR = 1.835, 95%CI: 1.256-2.679; P < 0.001) were correlated as determined by the multivariate analysis.
R0 resection was found to confer to a median survival time of 35.2 mo, while patients with R1 and R2 resections had a median survival time of 12.4 mo, and the palliative surgery only granted a median survival time of 7.3 mo. Thus, further studies emphasized the factors that could affect the tumor-free margin. Univariate analysis showed that tumor differentiation (P = 0.001), tumor diameter (P < 0.001), hepatectomy (P = 0.012), lymph node metastasis (P = 0.001), AJCC T stage (P = 0.001) caudate lobectomy (P = 0.001), Bismuth-Corlette classification (P = 0.038), and vascular invasion (P = 0.010) were associated with tumor-free margin. Multiple logistic regression analysis showed that caudate lobectomy [odds ratio (OR) = 9.771, 95%CI: 4.672-20.433; P < 0.001], tumor diameter (OR = 3.772, 95%CI: 1.914-7.434; P < 0.001), surgical procedures (OR = 10.236, 95%CI: 4.738-22.116; P < 0.001), AJCC T stage (OR = 2.010, 95%CI: 1.043-3.870; P = 0.037), and vascular invasion (OR = 2.278, 95%CI: 0.997-5.207; P = 0.051) were independently associated with tumor-free margin after adjusting for other factors.
HC remains among the most difficult management problems faced by surgeons. The accumulated outcomes from many institutions show confidently that only an excision with negative resection margin can be regarded as radically therapeutic, and in this condition, hepatic resection is often demanded[28,30]. Many authorities have reported various prognostic factors of HC; however, due to the differences in study methods and small patient numbers in other studies, potential biases or even contradictory outcomes may result. Furthermore, some large cases of multi-center reports may also induce biases due to the heterogeneity of clinical methods and surgical strategies. Thus, a large number of HC cases from a single center are urgently needed to standardize the prognostic factors and to supply better guidance and treatment for HC.
We report a median OS of 26.3 mo, with the one-, three-, and five-year survival rates of approximately 80%, 43%, and 28%, which are comparable to some published articles from high-volume hepatobiliary centers reporting a median OS of 16-40 mo, and a five-year survival rate of 11%-40%[4,32-34]. We also report a median DFS of 18.1 mo for the radical resection group, which is within the range of 12 mo to 20 mo covered by other literature[1,4,31,35]. The data in our series has also verified the common notion that curative surgery provides the best opportunity to increase the median and long-term survival rates of patients diagnosed with HC, as the failure of conducting curative surgery resulted in a sharp and signficant decrease in the OS. Compared with patients who did not accept any treatment, palliative surgery resulted in a relatively better outcome, which might be explained as follows. Palliative surgery can remove the root of obstructive jaundice and adequately open the biliary tract, thus, liver function can be improved. We also identified that intraoperative palliation conferred a relatively longer survival than nonsurgical palliation, and can lessen the odds of bile duct obstruction by opening the bile duct as much as possible, facilitating whole biliary decompression, and enhancing liver function. Therefore, we believe that intraoperative palliation is superior to nonsurgical palliation in patients who do not undergo curative resection. However, further studies with larger numbers of cases are needed to confirm this. In the long run, compared with curative surgery, we believe that curative surgery is the best way to prolong survival.
Many clinical, surgical, and pathologic factors have recently been shown to influence OS and DFS after major resection[28,29,31,36]. In our retrospective study, multivariate analysis showed that surgical margin, lymph node, tumor differentiation, and vascular invasion were independent factors for OS and DFS. In addition, tumor size was also an independent factor for OS, but not for DSF.
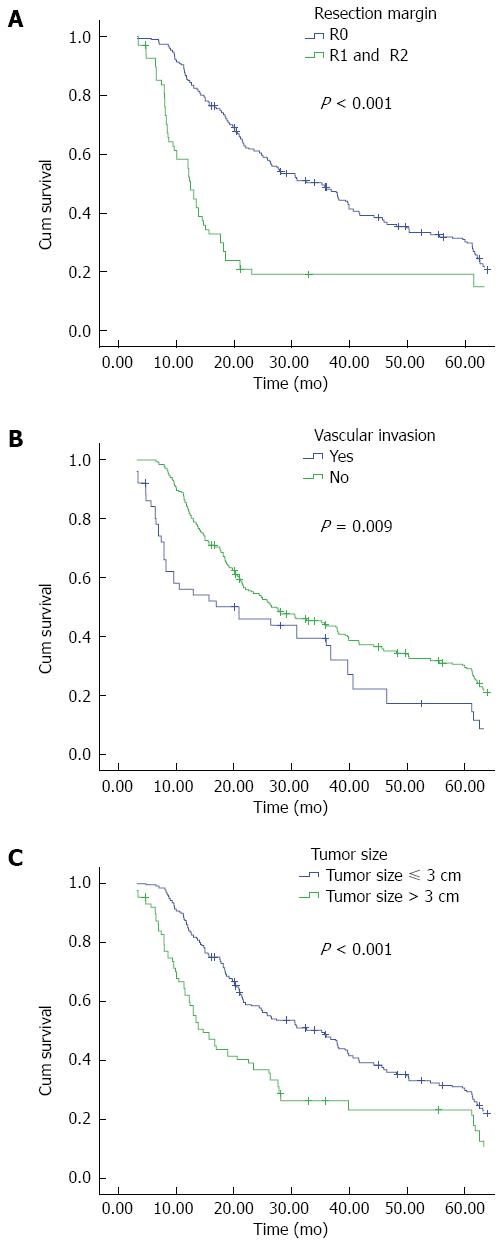
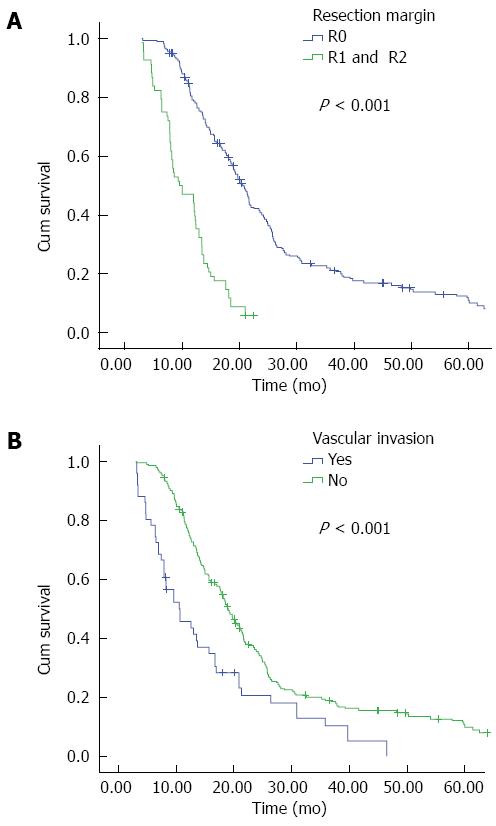
It is widely recognized that among a large succession of prognostic factors for HC, tumor-free margin is the most powerful predictor for both OS and DFS[35,37,38]. In our present study, R0 resection emerged as an independent prognostic factor for both OS (Figure 2A) and DFS (Figure 3A); patients with negative margins had markedly better OS and DFS than those with positive ones. The fact that patients undergoing R1 or R2 resection have better outcomes than patients undergoing palliative surgery has also been confirmed in current research. We further examined factors associated with R0 resection, finding that patients with smaller tumor size, concomitant hepatic resection, caudate lobectomy, T1 and T2 stage, and the lack of vascular invasion had a higher likelihood of obtaining tumor-free margin.
In our study, survival was affected by the existence of lymph node metastasis as testified by univariate and multivariate analyses, with a decrease in the median OS from 39.9 mo to 15.7 mo, and a decrease in the median DFS from 25.4 mo to 12.3 mo. These correspond with times reported by previous studies, in which lymph node metastasis was an important independent prognostic factor for both DFS[31,39,40] and OS[26,28,39,41,42]. The prognostic significance of node status stresses the necessity for radical lymphadenectomy. In this respect, thorough lymph nodes dissection may be important, and should be prospectively evaluated and studied in the future.
Tumor differentiation is a disputable factor in many retrospective studies. Hasegawa et al[42] did not find a survival disadvantage in cases of tumor differentiation, while Saxena et al[4] have shown that tumor differentiation is a biologic marker for measuring tumor invasion and metastasis and predicting long-term survival, which is comparable to our results, as the median OS decreased sharply from 54.1 mo to 13.5 mo when the patients had poor differentiation. The median DFS of patients with well-differentiated tumors was twofold greater than those with poor-differentiated tumors. Thus, in view of the fact that histologic differentiation is an independent prognostic factor for both OS and DFS, we are tempted to conclude that poorly differentiated cancers have a poor prognosis and higher recurrence rate.
Vascular invasion is no longer a contraindication for the excision of HC. As this procedure has a high mortality and risk, we performed vascular resection and reconstruction only in patients with clinically suspected vascular infiltration. Our report demonstrates that patients with vascular invasion are more likely to have a poor OS and DFS (Figures 2B and 3B); similarly, vascular invasion was also associated with a higher tendency of R1 and R2 status, though it only approached statistical difference multivariate logistic regression. Thus, in those highly suspected patients, vascular resection is recommended.
Perhaps more importantly, we found that tumor size was a significant prognostic parameter for OS and DFS in the univariate analysis. It was also an independent factor associated with OS in multivariate analysis (Figure 2C). So far, no other reports have shown this association as clearly as the results of the present study. The findings also confirmed that T stage of the DeOliveira staging system, in which the 3-cm cutoff was regarded as T3, is important. Moreover, tumor size influenced R0 resection in multivariate logistic regression analysis. Thus, from this point of view, tumor size needs to be taken into consideration when evaluating the long-term survival of HC patients.
Many studies have shown that caudate lobectomy appears to have a positive effect on tumor-free margin and survival after resection of Klatskin tumors[26,43,44]. In our study, caudate lobes were routinely removed, which was not associated with postoperative complications, and the OS and DFS were significantly longer in these patients as compared with those without caudate lobectomy in the univariate analysis. At the same time, caudate lobectomy approached statistical significance as a positive prognostic factor for OS on multivariate analysis. Furthermore, it was also an independent factor for tumor-free margin in our current series. Thus, we firmly believe that this procedure should be considered as a part of the standard surgical resection.
In contrast to previous studies, a survival advantage was not observed in case of hepatectomy. We only found that curative surgery accompanied with hepatectomy achieved more complete tumor-free margins, which is consistent with many previous studies[27,29,43,45]. Tumor-free margin, in turn, could promote OS and is a powerful predictor of survival, both reported in our series and in other literature. In our research, hepatectomy was the most important factor that could affect the tumor-free margin. Thus hepatectomy is an indirect prognostic factor that can promote OS by influencing the tumor resection margin.
In conclusion, radical surgical resection is the best treatment option for HC. R0 resection along with negative lymph nodes metastases, well differentiation, and lack of vascular invasion indicate better OS and DFS. Smaller tumor size also predicts better OS, but not DFS. Multivariate logistic regression analysis shows that hepatectomy, tumor diameter, T stage, caudate lobectomy, and vascular invasion are independently associated with tumor-free margin. Hepatectomy can help achieve more tumor-free resection margins and then indirectly affect OS; it is an indirect prognostic factor for survival.
Many authorities have reported various prognostic factors for hilar cholangiocarcinoma (HC); however, due to the difference in study methods and small patient numbers, these studies may be biased or have contradictory outcomes. Furthermore, some large multi-center reports could be biased due to the heterogeneity of clinical methods and surgical strategies.
This large, single-center study estimates prognostic factors associated with overall survival (OS) and disease-free survival (DFS) after successful resection of HC to identify those that could contribute to R0 resection. These findings will help future surgical decision making in the treatment of HC.
Based on our study, R0 resection along with negative lymph nodes metastases, well differentiation, and lack of vascular invasion indicated better OS and DFS. Smaller tumor size also predicts better OS, but not DFS. Hepatectomy, tumor diameter, T stage, caudate lobectomy, and vascular invasion were independently associated with tumor-free margin. Hepatectomy can help achieve more tumor-free resection margins and indirectly affect OS; it was an indirect prognostic factor for survival.
HC remains among the most difficult management problems faced by surgeons. These results may help future surgical decision making to better guide the treatment of HC.
Curative resection is important for the prognosis of HC, and R0 resection plays a significant role in prolonging the survival time of patients. R0 resection refers to those with microscopically negative tumor resection margin.
The authors examined the potential risk factors correlated with HC and factors that could affect tumor-free margin in a large, single-center study. The study was well performed and its findings are interesting and informative.
P- Reviewer: Balaban YH, Chetty R, Dang SS, Raoul JL, Sergi C S- Editor: Qi Y L- Editor: Filipodia E- Editor: Zhang DN
| 1. | Baton O, Azoulay D, Adam DV, Castaing D. Major hepatectomy for hilar cholangiocarcinoma type 3 and 4: prognostic factors and longterm outcomes. J Am Coll Surg. 2007;204:250-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Xiong J, Nunes QM, Huang W, Wei A, Ke N, Mai G, Liu X, Hu W. Major hepatectomy in Bismuth types I and II hilar cholangiocarcinoma. J Surg Res. 2015;194:194-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Figueras J, Codina-Barreras A, López-Ben S, Soriano J, Pardina B, Falgueras L, Castro E, Torres-Bahi S, Ortiz R, Diaz E. [Major hepatectomies are safe in patients with cholangiocarcinoma and jaundice]. Cir Esp. 2009;86:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Saxena A, Chua TC, Chu FC, Morris DL. Improved outcomes after aggressive surgical resection of hilar cholangiocarcinoma: a critical analysis of recurrence and survival. Am J Surg. 2011;202:310-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | D’Angelica MI, Jarnagin WR, Blumgart LH. Resectable hilar cholangiocarcinoma: surgical treatment and long-term outcome. Surg Today. 2004;34:885-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Khan SA, Toledano MB, Taylor-Robinson SD. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB (Oxford). 2008;10:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 343] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 7. | Seyama Y, Kubota K, Sano K, Noie T, Takayama T, Kosuge T, Makuuchi M. Long-term outcome of extended hemihepatectomy for hilar bile duct cancer with no mortality and high survival rate. Ann Surg. 2003;238:73-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 246] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 8. | Lillemoe KD, Cameron JL. Surgery for hilar cholangiocarcinoma: the Johns Hopkins approach. J Hepatobiliary Pancreat Surg. 2000;7:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Rea DJ, Munoz-Juarez M, Farnell MB, Donohue JH, Que FG, Crownhart B, Larson D, Nagorney DM. Major hepatic resection for hilar cholangiocarcinoma: analysis of 46 patients. Arch Surg. 2004;139:514-523; discussion 523-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 154] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Gerhards MF, van Gulik TM, Bosma A, ten Hoopen-Neumann H, Verbeek PC, Gonzalez Gonzalez D, de Wit LT, Gouma DJ. Long-term survival after resection of proximal bile duct carcinoma (Klatskin tumors). World J Surg. 1999;23:91-96. [PubMed] |
| 11. | Lee SG, Lee YJ, Park KM, Hwang S, Min PC. One hundred and eleven liver resections for hilar bile duct cancer. J Hepatobiliary Pancreat Surg. 2000;7:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Tsao JI, Nimura Y, Kamiya J, Hayakawa N, Kondo S, Nagino M, Miyachi M, Kanai M, Uesaka K, Oda K. Management of hilar cholangiocarcinoma: comparison of an American and a Japanese experience. Ann Surg. 2000;232:166-174. [PubMed] |
| 13. | Gazzaniga GM, Filauro M, Bagarolo C, Mori L. Surgery for hilar cholangiocarcinoma: an Italian experience. J Hepatobiliary Pancreat Surg. 2000;7:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Yi B, Xu AM, Lai EC, Qu ZQ, Cheng QB, Liu C, Luo XJ, Yu Y, Qiu YH, Wang XY. Preoperative portal vein embolization for hilar cholangiocarcinoma--a comparative study. Hepatogastroenterology. 2010;57:1341-1346. [PubMed] |
| 15. | Burke EC, Jarnagin WR, Hochwald SN, Pisters PW, Fong Y, Blumgart LH. Hilar Cholangiocarcinoma: patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann Surg. 1998;228:385-394. [PubMed] |
| 16. | Qiu ZQ, Tan WF, Yan PN, Luo XJ, Zhang BH, Wu MC, Jiang XQ, Lau WY. Early control of short hepatic portal veins in isolated or combined hepatic caudate lobectomy. Hepatobiliary Pancreat Dis Int. 2012;11:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Ahanatha Pillai S, Sathyanesan J, Perumal S, Ulagendra Perumal S, Lakshmanan A, Ramaswami S, Ramasamy R, Palaniappan R, Rajagopal S. Isolated caudate lobe resection: technical challenges. Ann Gastroenterol. 2013;26:150-155. [PubMed] |
| 18. | Sakoda M, Ueno S, Kubo F, Hiwatashi K, Tateno T, Kurahara H, Mataki Y, Shinchi H, Natsugoe S. Surgery for hepatocellular carcinoma located in the caudate lobe. World J Surg. 2009;33:1922-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Abdel Wahab M, Lawal AR, EL Hanafy E, Salah T, Hamdy E, Sultan AM. Caudate lobe resection: an Egyptian center experience. Langenbecks Arch Surg. 2009;394:1057-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Hirano S, Kondo S, Tanaka E, Shichinohe T, Tsuchikawa T, Kato K, Matsumoto J, Kawasaki R. Outcome of surgical treatment of hilar cholangiocarcinoma: a special reference to postoperative morbidity and mortality. J Hepatobiliary Pancreat Sci. 2010;17:455-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | Ramacciato G, Nigri G, Bellagamba R, Petrucciani N, Ravaioli M, Cescon M, Del Gaudio M, Ercolani G, Di Benedetto F, Cautero N. Univariate and multivariate analysis of prognostic factors in the surgical treatment of hilar cholangiocarcinoma. Am Surg. 2010;76:1260-1268. [PubMed] |
| 22. | Neuhaus P, Jonas S, Settmacher U, Thelen A, Benckert C, Lopez-Hänninen E, Hintze RE. Surgical management of proximal bile duct cancer: extended right lobe resection increases resectability and radicality. Langenbecks Arch Surg. 2003;388:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 111] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Regimbeau JM, Fuks D, Pessaux P, Bachellier P, Chatelain D, Diouf M, Raventos A, Mantion G, Gigot JF, Chiche L. Tumour size over 3 cm predicts poor short-term outcomes after major liver resection for hilar cholangiocarcinoma. By the HC-AFC-2009 group. HPB (Oxford). 2015;17:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Miyazawa M, Toshimitsu Y, Torii T, Okada K, Koyama I. Extended right hepatectomy for hilar cholangiocarcinoma with resection of the left hepatic duct prior to hepatic resection. J Surg Oncol. 2006;93:72-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Miyazaki M, Kimura F, Shimizu H, Yoshidome H, Ohtsuka M, Kato A, Yoshitomi H, Nozawa S, Furukawa K, Mitsuhashi N. Recent advance in the treatment of hilar cholangiocarcinoma: hepatectomy with vascular resection. J Hepatobiliary Pancreat Surg. 2007;14:463-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Kow AW, Wook CD, Song SC, Kim WS, Kim MJ, Park HJ, Heo JS, Choi SH. Role of caudate lobectomy in type III A and III B hilar cholangiocarcinoma: a 15-year experience in a tertiary institution. World J Surg. 2012;36:1112-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Klempnauer J, Ridder GJ, Werner M, Weimann A, Pichlmayr R. What constitutes long-term survival after surgery for hilar cholangiocarcinoma? Cancer. 1997;79:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Li H, Qin Y, Cui Y, Chen H, Hao X, Li Q. Analysis of the surgical outcome and prognostic factors for hilar cholangiocarcinoma: a Chinese experience. Dig Surg. 2011;28:226-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Dumitrascu T, Chirita D, Ionescu M, Popescu I. Resection for hilar cholangiocarcinoma: analysis of prognostic factors and the impact of systemic inflammation on long-term outcome. J Gastrointest Surg. 2013;17:913-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BS J, Youssef BA M, Klimstra D, Blumgart LH. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507-17; discussion 517-9. [PubMed] |
| 31. | Ito F, Agni R, Rettammel RJ, Been MJ, Cho CS, Mahvi DM, Rikkers LF, Weber SM. Resection of hilar cholangiocarcinoma: concomitant liver resection decreases hepatic recurrence. Ann Surg. 2008;248:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 32. | Igami T, Nishio H, Ebata T, Yokoyama Y, Sugawara G, Nimura Y, Nagino M. Surgical treatment of hilar cholangiocarcinoma in the “new era”: the Nagoya University experience. J Hepatobiliary Pancreat Sci. 2010;17:449-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 33. | Ercolani G, Zanello M, Grazi GL, Cescon M, Ravaioli M, Del Gaudio M, Vetrone G, Cucchetti A, Brandi G, Ramacciato G. Changes in the surgical approach to hilar cholangiocarcinoma during an 18-year period in a Western single center. J Hepatobiliary Pancreat Sci. 2010;17:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Sano T, Shimada K, Sakamoto Y, Yamamoto J, Yamasaki S, Kosuge T. One hundred two consecutive hepatobiliary resections for perihilar cholangiocarcinoma with zero mortality. Ann Surg. 2006;244:240-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 188] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 35. | Matsuo K, Rocha FG, Ito K, D’Angelica MI, Allen PJ, Fong Y, Dematteo RP, Gonen M, Endo I, Jarnagin WR. The Blumgart preoperative staging system for hilar cholangiocarcinoma: analysis of resectability and outcomes in 380 patients. J Am Coll Surg. 2012;215:343-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 36. | Ruys AT, van Haelst S, Busch OR, Rauws EA, Gouma DJ, van Gulik TM. Long-term survival in hilar cholangiocarcinoma also possible in unresectable patients. World J Surg. 2012;36:2179-2186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Ebata T, Nagino M, Kamiya J, Uesaka K, Nagasaka T, Nimura Y. Hepatectomy with portal vein resection for hilar cholangiocarcinoma: audit of 52 consecutive cases. Ann Surg. 2003;238:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 262] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 38. | Lee SG, Song GW, Hwang S, Ha TY, Moon DB, Jung DH, Kim KH, Ahn CS, Kim MH, Lee SK. Surgical treatment of hilar cholangiocarcinoma in the new era: the Asan experience. J Hepatobiliary Pancreat Sci. 2010;17:476-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 193] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 39. | Nuzzo G, Giuliante F, Ardito F, Giovannini I, Aldrighetti L, Belli G, Bresadola F, Calise F, Dalla Valle R, D’Amico DF. Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: results of an Italian multicenter analysis of 440 patients. Arch Surg. 2012;147:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 205] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 40. | Kobayashi A, Miwa S, Nakata T, Miyagawa S. Disease recurrence patterns after R0 resection of hilar cholangiocarcinoma. Br J Surg. 2010;97:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 41. | DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, Choti MA, Yeo CJ, Schulick RD. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 882] [Cited by in RCA: 1017] [Article Influence: 56.5] [Reference Citation Analysis (1)] |
| 42. | Hasegawa S, Ikai I, Fujii H, Hatano E, Shimahara Y. Surgical resection of hilar cholangiocarcinoma: analysis of survival and postoperative complications. World J Surg. 2007;31:1256-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 43. | Dinant S, Gerhards MF, Rauws EA, Busch OR, Gouma DJ, van Gulik TM. Improved outcome of resection of hilar cholangiocarcinoma (Klatskin tumor). Ann Surg Oncol. 2006;13:872-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 44. | Cheng QB, Yi B, Wang JH, Jiang XQ, Luo XJ, Liu C, Ran RZ, Yan PN, Zhang BH. Resection with total caudate lobectomy confers survival benefit in hilar cholangiocarcinoma of Bismuth type III and IV. Eur J Surg Oncol. 2012;38:1197-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 45. | Hemming AW, Reed AI, Fujita S, Foley DP, Howard RJ. Surgical management of hilar cholangiocarcinoma. Ann Surg. 2005;241:693-699; discussion 699-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 250] [Article Influence: 12.5] [Reference Citation Analysis (0)] |