Published online Feb 28, 2016. doi: 10.3748/wjg.v22.i8.2558
Peer-review started: July 9, 2015
First decision: September 29, 2015
Revised: October 13, 2015
Accepted: November 24, 2015
Article in press: November 24, 2015
Published online: February 28, 2016
AIM: To investigate the hepatoprotective effect of improved prescription of Taohechengqi-tang (IPTT) against acute liver failure (ALF) in rats.
METHODS: Seventy specific pathogen free male Wistar rats were randomly divided into four groups: control group (normal rats, n = 10), ALF group (ALF model, n = 20), Stronger Neo-Minophagen C (SNMC) group (ALF model + SNMC, n = 20), and IPTT group (ALF model + IPTT, n = 20). The ALF model group was administered an intraperitoneal injection of D-galactosamine (1.4 g/kg), and the control group received normal saline intraperitoneally. The SNMC and IPTT groups were treated with SMMC (15.6 mg/kg) or IPTT (28.6 g/kg) by gavage at 24 h intervals, and the ALF and control groups were treated with normal saline. At 36 h after injection, serum alanine aminotransferase, aspartate aminotransferase, total bilirubin, albumin, and cholinesterase and prothrombin time were determined, and liver histopathological scores were observed by microscopy after hematoxylin and eosin staining. mRNA expression of high mobility group box (HMGB) 1, toll-like receptor (TLR) 4, nuclear factor kappa B (NF-κB) and caspase-3 were analyzed via fluorescence quantitative reverse transcriptase polymerase chain reaction. Proliferating cell nuclear antigen (PCNA) immunohistochemistry in liver tissue was also performed.
RESULTS: D-galactosamine notably decreased the biochemical and coagulation profiles in serum. IPTT not only improved liver function and histopathology but also normalized the gene expression levels in liver tissue. Compared with the model group, in the IPTT and SNMC groups, HMGB1 mRNA/β-actin (0.06 ± 0.03, 0.11 ± 0.04 vs 0.25 ± 0.04, P < 0.05); TLR4 mRNA/β-actin (0.07 ± 0.02, 0.22 ± 0.08 vs 0.41 ± 0.22, P < 0.05); NF-κB mRNA/β-actin (0.74 ± 0.41, 1.78 ± 0.64 vs 2.68 ± 1.35, P < 0.05); and caspase-3 mRNA/β-actin levels were all significantly reduced (1.61 ± 0.45, 2.57 ± 1.04 vs 3.41 ± 0.85, P < 0.05). The gene expression levels were significantly lower in the IPTT group than in the SNMC group (P < 0.05). Compared with the model group, the PCNA expression in liver tissue was significantly enhanced in the IPTT and SNMC groups (36.34 ± 4.91, 25.57 ± 2.94 vs 17.55 ± 2.40, P < 0.05).
CONCLUSION: IPTT attenuates inflammation in ALF via inhibition of HMGB1 production, which may contribute to limited liver regeneration.
Core tip: Acute liver failure (ALF) is a life-threatening condition. Our prospective cohort study demonstrated that high doses of herbs for clearing heat and resolving stasis have a protective effect on LF. However, the curative mechanism is unclear. Our evidence showed that improved prescription of Taohechengqi-tang attenuated the inflammatory reaction of ALF in rats via inhibition of high mobility group box 1 production, which may contribute to recovery of limited liver regeneration. We provide evidence for the clinical application of Chinese herbs for clearing heat and resolving stasis.
- Citation: Zhang Y, Luo JX, Hu XY, Yang F, Zhong S, Lin W. Improved prescription of taohechengqi-tang alleviates D-galactosamine acute liver failure in rats. World J Gastroenterol 2016; 22(8): 2558-2565
- URL: https://www.wjgnet.com/1007-9327/full/v22/i8/2558.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i8.2558
Acute liver failure (ALF) is a life-threatening condition. It is accompanied by an intense inflammatory response, resulting in massive hepatocyte necrosis and fatal multiorgan failure[1]. Hospital mortality from ALF was as high as 83% in 1973-1978, dropping to 38% in 2004-2008[2]. The only treatment option shown to improve radically the outcome of LF is emergency liver transplantation (LT)[3]. Nevertheless, due to a lack of donated livers, nontransplanted mortality in ALF was > 20% with a waiting time of up to 6 wk[4]. In addition, the complex pathophysiological mechanisms of LT need further study.
According to traditional Chinese medicine (TCR), the syndrome of heat toxin stagnation is a major pattern of LF[5]. The incidence of the pattern was reported to be 58.6% in an epidemiological investigation from China[6]. Taohechengqi-tang (TT) is a well-known traditional Chinese herbal formulation, documented in Shang Han Za Bing Lun (a book of TCR on febrile and miscellaneous diseases in the Han Dynasty, AD 200-210). Based on TCR theory, TT is a classic formulation for clearing heat and resolving stasis and is used for treating not only diabetes mellitus[7] but also liver diseases. A recent study indicated that TT protected against chemically induced liver injury, at least partially through an antioxidant-like mechanism[8].
In our prospective cohort study, high doses of herbs for clearing heat and resolving stasis had a significant protective effect against LF[9]. However, the curative mechanism is still unclear. The present study was performed to investigate the effect of improved prescription of Taohechengqi-tang (IPTT) in ALF rats and its underlying mechanisms.
D-galactosamine (D-GalN) was obtained from Hongbang Medical Technology Co. Ltd. (Shanghai, China). Stronger Neo-Minophagen C (SNMC) was purchased from Minophagen Pharmaceutical Co. Ltd. (Tokyo, Japan) and prepared at a concentration of 1.56 mg/mL with distilled water.
IPTT consists of seven types of TCM ingredients (herbal plants and animal-based materials) (Table 1). All TCMs were purchased from Sichuan Chinese Herbs Co. Ltd. (Chengdu, China) and validated by Prof. Zhu-Yun Yan (Chengdu University of Traditional Chinese Medicine, China). All indicators of the above-mentioned TCMs were in line with the standards of the Chinese Pharmacopoeia (2010). Voucher specimens were deposited at the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine (Chengdu, China).
| Chinese name | Latin name | Origin | Amount in preparation (g) |
| Chi-shao | Radix paeoniae rubra | Sichuan | 100 |
| Tao-ren | Semen persicae | Hebei | 50 |
| Hong-hua | Flos carthami | Sichuan | 50 |
| Shui-zhi | Hirudo | Sichuan | 15 |
| Quan-xie | Scorpio | Henan | 10 |
| Mang-xiao | Natrii sulfas | Sichuan | 10 |
| Da-huang | Radix et rhizoma rhei palmati | Sichuan | 30 |
The extraction process of IPTT was as follows: Radix paeoniae rubra 100 g, Semen persicae 50 g, Flos carthami 50 g, Hirudo 15 g, Scorpio 10 g, Natrii sulfas 10 g, and Radix et rhizoma rhei palmate 30 g were added in 1.5 volumes of water, soaked for 30 min, and decocted three times for 20, 25, and 30 min, respectively. The decoction was mixed and concentrated to 2.86 g/mL (1 mL extract contained 2.86 g herbal mixture).
Male specific pathogen free Wistar rats weighing 150 ± 20 g were obtained from Shanghai Experimental Animal Co. Ltd. (Shanghai, China) and housed in the laboratory animal center at our university. Seventy Sprague-Dawley rats were randomly divided into four groups: control group (n = 10 rats), model group (n = 20), SNMC group (n = 20), and IPTT group (n = 20). For the SNMC group, rats were treated with SNMC 15.6 mg/kg daily by gavage. For the IPTT group, rats were treated with IPTT 28.6 g/kg per day by gavage. The dosages used in rats were calculated from the formula: Doserat = Dosehuman× (habeas indexrat/habeas indexhuman ) × (body weighthuman/body weightrat) × 2/3[10]. First, the translational coefficient 6.25 was produced based on the formula. Then, the dose used in humans was converted to that used in rats through the translational coefficient. For the control and model groups, rats were treated with saline 10 mL/kg per day by gavage. All rats were treated by gavage for 3 d before induction of ALF, once daily, for a total of 5 d.
For the preparation of rats with D-GalN-induced ALF, rats were administered an intraperitoneal injection of D-GalN (1.4 g/kg). The control group was given intraperitoneally the same dose of saline. Thirty-six hours later, all rats were sacrificed. Blood samples (6 mL) were collected via the femoral artery, centrifuged at 3000 rpm for 10 min, and the sera were kept at -70 °C for detection of biochemical parameters. The livers were rapidly removed, and tissues were removed from the left lobes, fixed in 10% neutral formalin, and retained for further analyses, including hematoxylin and eosin (HE) staining, fluorescence quantitative reverse transcriptase polymerase chain reaction (qRT-PCR), and proliferating cell nuclear antigen (PCNA) immunohistochemistry assay.
Serum biochemical parameters[11] closely associated with liver function, including mainly alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), albumin (ALB), cholinesterase (CHE), and prothrombin time (PT), were determined according to the manufacturers’ instructions.
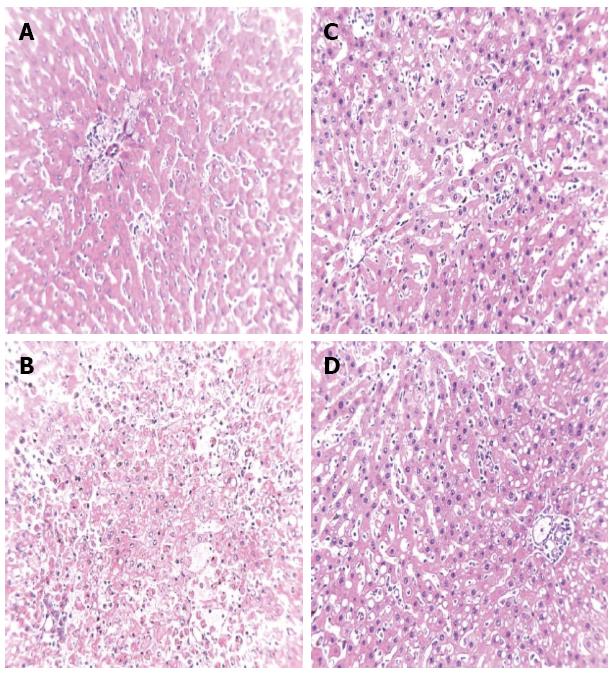
Liver tissues obtained from each rat were fixed, embedded in paraffin, and cut into 3-5 mm sections. HE staining was used to evaluate the degree of hepatocyte necrosis. Microscopic injuries were graded according to a semiquantitative scoring system on a scale of 0 to 4 (0 = no discernable necrosis, 1 = bridging necrosis, 2 = confluent necrosis, 3 = sub-massive necrosis, and 4 = massive necrosis)[1]. In order to minimize bias, each slide was read by three pathologists in a blinded manner and observed three times.
mRNA expression of high mobility group box (HMGB) 1, toll-like receptor (TLR) 4, nuclear factor kappa B (NF-κB) and caspase-3 were analyzed via qRT-PCR and the 2-ΔΔct method. In accordance with the manufacturer’s protocol (Invitrogen, Carlsbad, CA, United States), total RNAs were extracted from pulverized liver tissues with TRIzol reagent, then reverse-transcribed into cDNA by the ABI Step-One Plus Real Time PCR System (Applied Biosystems, Foster City, CA, United States). For HMGB1, the 5’ primer sequence was 5’TGTTCTGAGTACCGCCCAAA3’, and the 3’ primer sequence was 5’TTTCGCTGCATCAGGTTTTC3’. For TLR4, the 5’ primer sequence was 5’CCAGGAAGGCTTCCACAAGA3’, and the 3’ primer sequence was 5’AATTCGACCTGCTGCCTCAG3’. For NF-κB, the 5’ primer sequence was 5’GCACGAGGCTCCTTTTCTCAA3’, and the 3’ primer sequence was 5’CGTTTTTCTTCAATCCGGTGG3’. For caspase-3, the 5’ primer sequence was 5’ACCGATGTCGATGCAGCTAA3’, and the 3’ primer sequence was 5’AGGTCCGTTCGTTCCAAAAA3’. For the housekeeping gene (β-actin), the 5’ primer sequence was 5’AAGGAGGCAAAGGACACCAA3’, and the 3’ primer sequence was 5’AATGGCCCCCTTCACAGTTA3’.
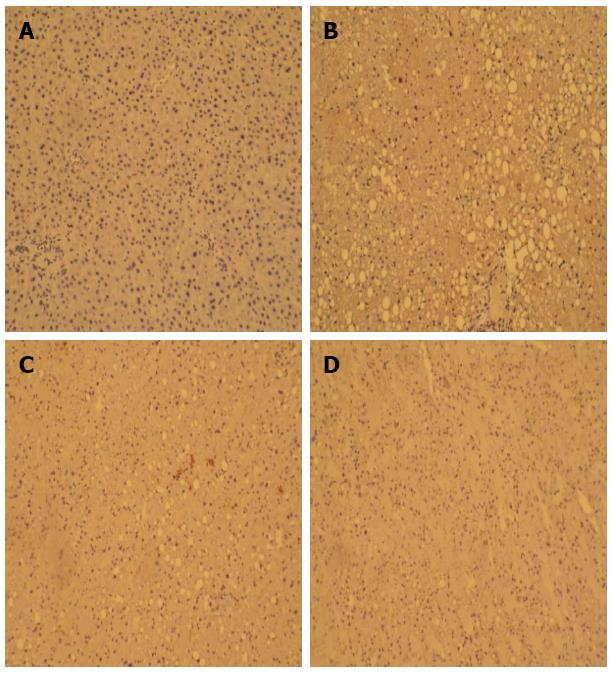
PCNA staining of the formalin-fixed and paraffin-embedded liver tissues was performed with an immunohistochemistry kit purchased from Boster Bioengineering Co. Ltd. (Wuhan, China). The number of PCNA-positive cells was counted in five random fields at 40 × magnification for each section. An average of the percentage of PCNA-positive cells was taken over these fields[12]. Sections were examined microscopically, and photographs were collected using a digital image-capture system (CX40; Olympus, Tokyo, Japan).
Continuous variables were summarized as mean ± SD. The histopathological scores, biochemical parameters, mRNA, and PCNA expression were analyzed by one-way analysis of variance (ANOVA), followed by the Student’s t test. All analyses were performed with SPSS version 17.0 (Chicago, IL, United States). All P values were two-sided, and P < 0.05 was considered statistically significant.
The serum parameters are summarized in Table 2. D-GalN significantly increased serum ALT, AST, TBIL, and PT, while it decreased serum ALB and CHE. The effects were significantly reversed in the IPTT and SNMC groups, especially the IPTT group.
| Group | n | ALT (U/L) | AST (U/L) | TBiL (μmol/L) | ALB (g/L) | CHE (U/L) | PT (s) | Histopathological scores |
| Control | 10 | 35.15 ± 6.01 | 151.61 ± 20.87 | 1.55 ± 0.43 | 35.25 ± 4.19 | 557.40 ± 43.23 | 13.60 ± 1.73 | 0 |
| Model | 20 | 441.10 ± 60.36a | 887.80 ± 128.47a | 38.04 ± 6.84a | 23.67 ± 3.21a | 343.92 ± 68.93a | 31.80 ± 5.02a | 3.69 ± 0.38a |
| SNMC | 20 | 267.18 ± 41.45b | 380.49 ± 55.38b | 24.37 ± 4.03b | 26.65 ± 4.50 | 378.50 ± 83.53 | 29.93 ± 3.83 | 2.78 ± 0.31b |
| IPTT | 20 | 127.45 ± 25.33bc | 264.04 ± 63.432bc | 19.37 ± 3.26bc | 30.22 ± 4.30bc | 488.61 ± 76.41bc | 23.78 ± 4.34bc | 2.06 ± 0.40bc |
The histopathological results are shown in Table 2 and Figure 1. Light microscopy revealed no liver necrosis in the control group, but massive hepatocyte necrosis was observed in rats treated with D-GalN. Compared to the model group, necrosis was markedly reduced in the IPTT and SNMC groups, and IPTT had a more significant impact.
mRNA expression was observed as shown in Table 3. Compared to the model group, mRNA expression of HMGB1, TLR4, NF-κB, and caspase-3 were markedly reduced in the control, IPTT, and SNMC groups. Moreover, IPTT was found to have a more significant impact than SNMC.
| Group | n | HMGB1 | TLR4 | NF-κB | Caspase-3 | PCNA (%) |
| Control | 10 | 0.01 ± 0.01 | 0.04 ± 0.02 | 0.49 ± 0.25 | 1.36 ± 0.26 | 7.48 ± 0.90 |
| Model | 20 | 0.25 ± 0.04a | 0.41 ± 0.22a | 2.68 ± 1.35a | 3.41 ± 0.85a | 17.55 ± 2.40 |
| SNMC | 20 | 0.11 ± 0.04b | 0.22 ± 0.08b | 1.78 ± 0.64b | 2.57 ± 1.04b | 25.57 ± 2.94b |
| IPTT | 20 | 0.06 ± 0.03bc | 0.07 ± 0.02bc | 0.74 ± 0.41bc | 1.61 ± 0.45bc | 36.34 ± 4.91bc |
Table 3 and Figure 2 show that the mean PCNA-positive rates were significantly higher in the model, IPTT, and SNMC groups than in the control group. The PCNA-positive rate in the IPTT group was highest among the three groups.
In our study, IPTT not only remarkably improved the biochemical and coagulation profiles in serum, but it also reduced histopathological scores in ALF induced by D-GalN.
Inflammation plays a centrals role in the development of ALF. The net loss of hepatocytes mainly contributes to overwhelming injury with subsequent cell death rather than a lack of regeneration[13,14]. Similar to severe acute pancreatitis and sepsis, ALF initially starts with a systemic inflammatory response syndrome (SIRS phase) and is followed by a compensatory anti-inflammatory response syndrome[15,16]. There is an imbalance in both the pro- and anti-inflammatory systems, leading to immune-inflammatory dysregulation[17]. The SIRS in ALF, whether or not precipitated by infection, is characterized by prolonged hypercytokinemia, which is associated with the systemic complications (e.g., encephalopathy, infection, and renal failure) and poor outcome[18-20].
Overactivation of the TLR4/NF-κB pathway is correlated with these pathological events in ALF. TLR4 is a member of the TLR family recognizing pathogen-associated molecular patterns[21]. One of its ligands is bacterial lipopolysaccharide (LPS)[22]. Once TLR4 recognizes LPS or LPS-CD14 complex, macrophages and dendritic cells are activated to present antigen to helper T cells. Furthermore, upregulated TLR4 signaling activates NF-κB via a MyD88-dependent pathway and/or MyD88-independent pathway. NF-κB is a downstream intracellular molecule of different receptors and induces the release of proinflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1, and IL-6[23-25]. TNF-α, the core of the cytokine network, still plays an important role in the development of hepatocyte apoptosis. The binding of TNF-α to the TNF receptor 1 triggers the apoptotic cascade mediated by proapoptotic Bcl-2 family proteins[26,27].
HMGB1 is a nuclear protein with cytokine-type functions that is passively released during necrosis or actively secreted during immune cell activation[28]. There are many receptors, including the receptor for advanced glycation endproducts, TLR2, TLR4, and TLR9[29], to which HMGB1 can bind. TLR4 is the critical receptor mediating the inflammatory activity of HMGB1[30,31]. Once the HMGB1/TLR4/NF-κB pathway is activated, overexpression of TNF-α induces hepatocyte necrosis and activates the TNF-α-mediated extrinsic apoptotic pathway[32]. Our results support the anti-inflammatory and antiapoptotic effects of IPTT, as the liver expression of HMGB1, TLR4, NF-κB, and caspase-3 was significantly reduced in ALF rats. Moreover, IPTT improved hepatocyte regeneration.
Acute liver injury may recover via the process of hepatocyte regeneration once the injury is discontinued. After massive liver injury, the capacity of hepatocyte proliferation was not lost, only severely suppressed[33]. In a recent study, HMGB1 was reported to impair hepatocyte regeneration after acetaminophen overdose, and blockade of HMGB1 enhanced liver recovery[34,35]. Our results showed that severe liver damage limited hepatocyte regression. However, the hepatoprotective effect of IPTT might contribute to liver regeneration. Furthermore, IPTT may be sensitive to the stimulation of growth factors, such as HGF and transforming growth factor (TGF) α, and progress into the cell cycle for replication[36].
IPTT has been used for a long time in the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine[9] and is composed of seven different types of TCM. Among them, Radix paeoniae rubra was effective in attenuating hepatocyte apoptosis, and this effect was partly mediated through the activation of the mitochondrial pathway and subsequent regulation of expression of particular proapoptotic genes[37]. Semen persicae protected against cisplatin-induced hepatotoxicity by reducing cisplatin-induced oxidative stress[38]. Flos carthami protected the liver from long-term alcohol injury, which was related to enhanced antioxidant capacity and inhibition of TGF-β1 expression[39]. Radix et rhizoma rhei palmate promoted bioavailability and liver protective effects and prevented and treated hepatic encephalopathy in rats with thioacetamide-induced ALF[40,41]. IPTT combined these Chinese herbal medicines following the principle of clearing heat and resolving stasis. Our data suggested that IPTT protected against ALF through its anti-inflammatory, antiapoptotic, and regeneration promoting effects. It provided evidence for clinical application of Chinese herbs for clearing heat and resolving stasis.
In conclusion, IPTT attenuates inflammatory reaction of ALF in rats via inhibition of HMGB1 production, which may contribute to recovery of limited liver regeneration.
The authors would like to thank the members of the Department of Infectious Diseases, Affiliated Hospital of Chengdu University of Traditional Chinese Medicine, and the Animal Care and Scientific Committee of Chengdu University of Traditional Chinese Medicine for their technical support.
Acute liver failure (ALF) is a severe clinical syndrome with high mortality. In previous studies, Chinese herbal medicine has been shown to improve the outcome of LF. Nevertheless, pharmacological research of Chinese medicine is still lacking.
A systemic inflammatory response syndrome in ALF is characterized by prolonged hypercytokinemia, which is associated with the systemic complications and poor outcome. The high mobility group box (HMGB)1/Toll-like receptor 4/nuclear factor-κB pathway has been the recent research focus in inflammation.
The present study demonstrates that improved prescription of Taohechengqi-tang (IPTT) attenuated the inflammatory reaction of ALF. One of the mechanisms is likely related to inhibition of HMGB1 production, which contributes to recovery of liver regeneration.
The data from this study provide a rational basis for IPTT for treatment of LF in clinical practice. It also provides evidence for clinical application of Chinese herbs for clearing heat and resolving stasis.
ALF is a clinical syndrome in which abrupt onset, manifesting as jaundice and coagulopathy, is complicated within 2 wk by grade II + encephalopathy in a patient with undiagnosed chronic liver disease.
The experiments appear to be well conducted and the conclusions drawn are justified. The manuscript has novelty and significance.
P- Reviewer: Kuan YH S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Organization Committee of 13th Asia-Pacific Congress of Clinical Microbiology and Infection. 13th Asia-Pacific Congress of Clinical Microbiology and Infection Consensus Guidelines for diagnosis and treatment of liver failure. Hepatobiliary Pancreat Dis Int. 2013;12:346-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Bernal W, Hyyrylainen A, Gera A, Audimoolam VK, McPhail MJ, Auzinger G, Rela M, Heaton N, O’Grady JG, Wendon J. Lessons from look-back in acute liver failure? A single centre experience of 3300 patients. J Hepatol. 2013;59:74-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 254] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 3. | Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947-954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1562] [Cited by in F6Publishing: 1386] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 4. | Urrunaga NH, Rachakonda VP, Magder LS, Mindikoglu AL. Outcomes of living versus deceased donor liver transplantation for acute liver failure in the United States. Transplant Proc. 2014;46:219-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Zhang JL, Zeng H, Wang XB. [Discussion of Chinese syndrome typing in acute hepatic failure model]. Zhongguo Zhongxiyi Jiehe Zazhi. 2011;31:659-662. [PubMed] [Cited in This Article: ] |
| 6. | Hu X, Zhang Y, Chen G, Li Y, Zhong S. Distribution of traditional Chinese medicine patterns in 324 cases with hepatitis B-related acute-on-chronic liver failure: a prospective, cross-sectional survey. J Tradit Chin Med. 2012;32:538-544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Xiong MQ, Lin AZ, Zhu ZZ. [Effects of supplemented taohe chengqi decoction in treating insulin resistance in rats with non-insulin dependent diabetes mellitus]. Zhongguo Zhongxiyi Jiehe Zazhi. 1997;17:165-168. [PubMed] [Cited in This Article: ] |
| 8. | Lai TY, Weng YJ, Kuo WW, Chen LM, Chung YT, Lin YM, Tsai FJ, Lee CH, Choong YM, Lai EY. Taohe Chengqi Tang ameliorates acute liver injury induced by carbon tetrachloride in rats. Zhongxiyi Jiehe Xuebao. 2010;8:49-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Hu XY, Zhang Y, Chen G, Zhong S, Fan XJ. [A prospective cohort study on the influence of high doses of herbs for clearing heat and resolving stasis on survival rates in patients with hepatitis B-related acute-on-chronic liver failure]. Zhongxiyi Jiehe Xuebao. 2012;10:176-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Li JS, Li Y, Li SY, Wang YY, Deng L, Tian YG, Jiang SL, Wang Y. Long-term effects of Tiaobu Feishen therapies on systemic and local inflammation responses in rats with stable chronic obstructive pulmonary disease. Zhongxiyi Jiehe Xuebao. 2012;10:1039-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Chinese Society of Hepatology of Chinese Medical Association; Chinese Society of Gastroenterology of Chinese Medical Association, Jia JD, Cheng LF. [Consensus on the clinical significance and evaluation of common liver biochemistry tests]. Zhonghua Ganzangbing Zazhi. 2010;18:387-393. [PubMed] [Cited in This Article: ] |
| 12. | Zeng H, Yuan Z, Zhu H, Li L, Shi H, Wang Z, Fan Y, Deng Q, Zeng J, He Y. Expression of hPNAS-4 radiosensitizes Lewis lung cancer. Int J Radiat Oncol Biol Phys. 2012;84:e533-e540. [PubMed] [Cited in This Article: ] |
| 13. | Wolf HK, Michalopoulos GK. Hepatocyte regeneration in acute fulminant and nonfulminant hepatitis: a study of proliferating cell nuclear antigen expression. Hepatology. 1992;15:707-713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 93] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Park JH, Kim KH, Lee WR, Han SM, Park KK. Protective effect of melittin on inflammation and apoptosis in acute liver failure. Apoptosis. 2012;17:61-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3146] [Cited by in F6Publishing: 3058] [Article Influence: 278.0] [Reference Citation Analysis (0)] |
| 16. | Sarin SK, Kumar A, Almeida JA, Chawla YK, Fan ST, Garg H, de Silva HJ, Hamid SS, Jalan R, Komolmit P. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int. 2009;3:269-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 630] [Cited by in F6Publishing: 610] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 17. | Jalan R, Gines P, Olson JC, Mookerjee RP, Moreau R, Garcia-Tsao G, Arroyo V, Kamath PS. Acute-on chronic liver failure. J Hepatol. 2012;57:1336-1348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 447] [Cited by in F6Publishing: 426] [Article Influence: 35.5] [Reference Citation Analysis (1)] |
| 18. | Rolando N, Wade J, Davalos M, Wendon J, Philpott-Howard J, Williams R. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000;32:734-739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 535] [Cited by in F6Publishing: 481] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 19. | Vaquero J, Polson J, Chung C, Helenowski I, Schiodt FV, Reisch J, Lee WM, Blei AT. Infection and the progression of hepatic encephalopathy in acute liver failure. Gastroenterology. 2003;125:755-764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 310] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 20. | Stravitz RT, Bowling R, Bradford RL, Key NS, Glover S, Thacker LR, Gabriel DA. Role of procoagulant microparticles in mediating complications and outcome of acute liver injury/acute liver failure. Hepatology. 2013;58:304-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 21. | Karvellas CJ, Pink F, McPhail M, Cross T, Auzinger G, Bernal W, Sizer E, Kutsogiannis DJ, Eltringham I, Wendon JA. Predictors of bacteraemia and mortality in patients with acute liver failure. Intensive Care Med. 2009;35:1390-1396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Fisher JE, McKenzie TJ, Lillegard JB, Yu Y, Juskewitch JE, Nedredal GI, Brunn GJ, Yi ES, Malhi H, Smyrk TC. Role of Kupffer cells and toll-like receptor 4 in acetaminophen-induced acute liver failure. J Surg Res. 2013;180:147-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | O’Neill LA. TAMpering with toll-like receptor signaling. Cell. 2007;131:1039-1041. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Takayashiki T, Yoshidome H, Kimura F, Ohtsuka M, Shimizu Y, Kato A, Ito H, Shimizu H, Ambiru S, Togawa A. Increased expression of toll-like receptor 4 enhances endotoxin-induced hepatic failure in partially hepatectomized mice. J Hepatol. 2004;41:621-628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Zhang S, Yang N, Ni S, Li W, Xu L, Dong P, Lu M. Pretreatment of lipopolysaccharide (LPS) ameliorates D-GalN/LPS induced acute liver failure through TLR4 signaling pathway. Int J Clin Exp Pathol. 2014;7:6626-6634. [PubMed] [Cited in This Article: ] |
| 26. | Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634-1635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1322] [Cited by in F6Publishing: 1328] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 27. | Yang F, Li X, Wang LK, Wang LW, Han XQ, Zhang H, Gong ZJ. Inhibitions of NF-κB and TNF-α result in differential effects in rats with acute on chronic liver failure induced by d-Gal and LPS. Inflammation. 2014;37:848-857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Laliena A, San Miguel B, Crespo I, Alvarez M, González-Gallego J, Tuñón MJ. Melatonin attenuates inflammation and promotes regeneration in rabbits with fulminant hepatitis of viral origin. J Pineal Res. 2012;53:270-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Wang LW, Wang LK, Chen H, Fan C, Li X, He CM, Gong ZJ. Ethyl pyruvate protects against experimental acute-on-chronic liver failure in rats. World J Gastroenterol. 2012;18:5709-5718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 25] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, Bianchi ME. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22:5551-5560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 950] [Cited by in F6Publishing: 977] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 31. | Liu S, Stolz DB, Sappington PL, Macias CA, Killeen ME, Tenhunen JJ, Delude RL, Fink MP. HMGB1 is secreted by immunostimulated enterocytes and contributes to cytomix-induced hyperpermeability of Caco-2 monolayers. Am J Physiol Cell Physiol. 2006;290:C990-C999. [PubMed] [Cited in This Article: ] |
| 32. | Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1860] [Cited by in F6Publishing: 2136] [Article Influence: 133.5] [Reference Citation Analysis (0)] |
| 33. | Borzio M, Trerè D, Borzio F, Ferrari AR, Bruno S, Roncalli M, Colloredo G, Leandro G, Oliveri F, Derenzini M. Hepatocyte proliferation rate is a powerful parameter for predicting hepatocellular carcinoma development in liver cirrhosis. Mol Pathol. 1998;51:96-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Yang R, Zhang S, Cotoia A, Oksala N, Zhu S, Tenhunen J. High mobility group B1 impairs hepatocyte regeneration in acetaminophen hepatotoxicity. BMC Gastroenterol. 2012;12:45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 35. | Cataldegirmen G, Zeng S, Feirt N, Ippagunta N, Dun H, Qu W, Lu Y, Rong LL, Hofmann MA, Kislinger T. RAGE limits regeneration after massive liver injury by coordinated suppression of TNF-alpha and NF-kappaB. J Exp Med. 2005;201:473-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 36. | Jia C. Advances in the regulation of liver regeneration. Expert Rev Gastroenterol Hepatol. 2011;5:105-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 37. | Jiang ZQ, Yan XJ, Bi L, Chen JP, Zhou Q, Chen WP. Mechanism for hepato-protective action of Liangxue Huayu Recipe (LHR): blockade of mitochondrial cytochrome c release and caspase activation. J Ethnopharmacol. 2013;148:851-860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Lee CK, Park KK, Hwang JK, Lee SK, Chung WY. The pericarp extract of Prunus persica attenuates chemotherapy-induced acute nephrotoxicity and hepatotoxicity in mice. J Med Food. 2008;11:302-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | He Y, Liu Q, Li Y, Yang X, Wang W, Li T, Zhang W, Cui Y, Wang C, Lin R. Protective effects of hydroxysafflor yellow A (HSYA) on alcohol-induced liver injury in rats. J Physiol Biochem. 2015;71:69-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Yang CH, Ting WJ, Shen CY, Hsu HH, Lin YM, Kuo CH, Tsai FJ, Tsai CH, Tsai Y, Huang CY. Anti-apoptotic effect of San Huang Shel Shin Tang cyclodextrin complex (SHSSTc) on CCl4 -induced hepatotoxicity in rats. Environ Toxicol. 2014;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 41. | Zhang Q, Cheng H, Wang C, Zhang C, Wang J. [Preventive and therapeutic effects of extract from Rheum palmatum on hepatic encephalopathy in rats with acute liver failure]. Zhong Yao Cai. 2002;25:573-575. [PubMed] [Cited in This Article: ] |