Published online Feb 21, 2016. doi: 10.3748/wjg.v22.i7.2342
Peer-review started: October 9, 2015
First decision: November 5, 2015
Revised: November 14, 2015
Accepted: December 12, 2015
Article in press: December 12, 2015
Published online: February 21, 2016
Processing time: 114 Days and 12.9 Hours
AIM: To determine the risk factors of biliary intervention using magnetic resonance cholangiopancreatography (MRCP) after living donor liver transplantation (LDLT).
METHODS: We retrospectively enrolled 196 patients who underwent right lobe LDLT between 2006 and 2010 at a single liver transplantation center. Direct duct-to-duct biliary anastomosis was performed in all 196 patients. MRCP images routinely taken 1 mo after LDLT were analyzed to identify risk factors for biliary intervention during follow-up, such as retrograde cholangiopancreatography or percutaneous transhepatic biliary drainage. Two experienced radiologists evaluated the MRCP findings, including the anastomosis site angle on three-dimensional images, the length of the filling defect on maximum intensity projection, bile duct dilatation, biliary stricture, and leakage.
RESULTS: Eighty-nine patients underwent biliary intervention during follow-up. The anastomosis site angle [hazard ratio (HR) = 0.48; 95% confidence interval (CI), 0.30-0.75, P < 0.001], a filling defect in the anastomosis site (HR = 2.18, 95%CI: 1.41-3.38, P = 0.001), and biliary leakage (HR = 2.52, 95%CI: 1.02-6.20, P = 0.048) on MRCP were identified in the multivariate analysis as significant risk factors for biliary intervention during follow-up. Moreover, a narrower anastomosis site angle (i.e., below the median angle of 113.3°) was associated with earlier biliary intervention (38.5 ± 4.2 mo vs 62. 1 ± 4.1 mo, P < 0.001). Kaplan-Meier analysis comparing biliary intervention-free survival according to the anastomosis site angle revealed that lower survival was associated with a narrower anastomosis site angle (36.3% vs 62.0%, P < 0.001).
CONCLUSION: The biliary anastomosis site angle in MRCP after LDLT may be associated with the need for biliary intervention.
Core tip: Biliary complications and interventions are common after living donor liver transplantation (LDLT). Identifying patients who are at high risk for biliary interventions early after LDLT could help clinicians with patient follow-up. Magnetic resonance cholangiopancreatography (MRCP) imaging was performed 1 mo after LDLT to determine risk factors for biliary intervention. The anastomosis site angle, a filling defect in the anastomosis site, and biliary leakage on MRCP were identified as significant risk factors. Moreover, a narrower anastomosis site angle was related to earlier biliary intervention. Here, for the first time, we have shown that the anastomosis site angle might be associated with the need for biliary intervention.
- Citation: Lee SK, Choi JY, Yeo DM, Lee YJ, Yoon SK, Bae SH, Jang JW, Kim HY, Kim DG, You YK. Risk factors of biliary intervention by imaging after living donor liver transplantation. World J Gastroenterol 2016; 22(7): 2342-2348
- URL: https://www.wjgnet.com/1007-9327/full/v22/i7/2342.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i7.2342
Biliary complications occur commonly after liver transplantation (LT)[1] and are the main cause of morbidity and mortality in LT recipients[2]. Magnetic resonance cholangiopancreatography (MRCP), a non-invasive tool used to visualize the biliary tract, has 88% sensitivity and 94% specificity for detecting biliary complications following LT[3]. Therefore, MRCP is the recommended diagnostic modality for detecting biliary complications after LT[3]. Biliary interventions, such as endoscopic retrograde cholangiopancreatography (ERCP) and percutaneous transhepatic biliary drainage (PTBD) are generally used to manage these complications[4].
Several investigators have documented risk factors influencing the development of biliary complications. Perioperative risk factors include a Model for End-stage Liver Disease (MELD) score > 35, donor age > 60 years, and primary sclerosing cholangitis[5,6]. Intraoperative risk factors include cold ischemic time and anastomosis method (duct-to-duct vs hepaticojejunal methods)[5,6]. Cytomegalovirus infection and hepatic artery thrombosis have been reported as postoperative risk factors[5,6].
However, no study has investigated the risk factors associated with the future need for intervention based on MRCP findings shortly after living donor LT (LDLT). Identifying patients at high risk for biliary intervention by MRCP, a non-invasive and accurate tool, during the early post-transplant period would be clinically helpful for managing and following patients who have undergone LT.
The purpose of this study was to determine factors, specifically the anastomosis site angle, that increase the requirement for biliary intervention during follow-up in LDLT recipients on MRCP images 1 mo after LT.
We retrospectively reviewed the records of 270 patients who underwent LDLT at Seoul St. Mary’s Hospital between 2006 and 2010. Of these 270 patients, 74 patients were excluded for the following reasons: two subjects underwent ERCP or PTBD within 1 mo after LDLT, 38 had no MRCP images taken 1 mo after LDLT, 13 had no three-dimensional (3D) reconstruction or maximum intensity projection (MIP) images or had poor quality images with severe artefacts, 3 underwent choledochojejunostomy as the biliary anastomosis method, and 18 died < 1 mo after LDLT (bleeding, 4; sepsis, 11; graft failure, 3). Finally, 196 consecutive LDLT recipients who underwent MRCP 1 mo after LT were included in this study. The present study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital (KC13RISI0788).
All 196 patients underwent right lobe living donor transplantation[7]. Biliary anastomosis was performed according to the anatomy of the hepatic duct: single duct-to-duct anastomosis for a single hepatic duct and double duct-to-duct anastomosis, or hepaticoplasty, for double hepatic ducts. Hepaticoplasty for double hepatic ducts was performed to create one lumen in cases of a short distance between the two hepatic ducts[8]. Alternatively, double duct-to-duct anastomosis was performed such that each duct was anastomosed to the left and right hepatic ducts individually.
Biliary intervention was defined as procedures involving ERCP and PTBD. During follow-up, these procedures were performed when the following criteria were met: (1) abnormal laboratory findings of biliary-associated factors, such as serum bilirubin, alkaline phosphatase and γ-glutamyl transferase; and (2) radiologically determined bile duct stricture with dilatation above the stricture site[9].
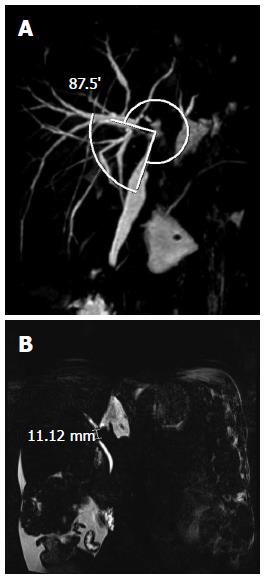
MRCP was performed 1 mo after LDLT. The anastomosis site angle was measured using 3D reconstruction imaging. The anastomosis site angle was defined as the angle between the donor hepatic duct and the recipient’s common hepatic duct (Figure 1A). If there were two anastomosis sites, the smaller one was chosen. To improve the accuracy of measurement of the anastomosis site, we checked the measurements on every 3D image rendered and chose the smallest site. We also identified the presence of a filling defect and the length of the filling defect on MIP images. Every image was reviewed, and the filling defect with the longest length was chosen (Figure 1B). The presence of bile duct dilatation, biliary stricture, and leakage was also verified. MRCP images were reviewed by two experienced radiologists (Lee YJ and Yeo DM) without knowledge of the patient’s clinical status, and the measurements were made in consensus.
The patient characteristics are expressed as the means ± SD (range) or counts, as appropriate. The inter-observer correlation coefficient (ICC) was determined to evaluate agreement between the two radiologists for the anastomosis site angle and the length of the filling defects. The Cox proportional hazards model was used to determine risk factors for biliary intervention, such as ERCP or PTBD, after LDLT. Variables with P values < 0.2 in the univariate Cox regression analysis were considered potential variables for the multivariate Cox regression analysis. A forward selection method was adopted to identify significant risk factors with P values < 0.05. Kaplan-Meier analysis was also performed to estimate biliary intervention-free survival according to the anastomosis site angle. All statistical analyses were performed using SPSS ver. 15.0 (SPSS, Chicago, IL, United States).
Of the 196 patients, 136 (70.0%) were men, and the mean recipient age was 49.7 ± 10.1 years. The underlying causes for LT were hepatocellular carcinoma (n = 80, 40.8%), decompensated liver cirrhosis (LC) associated with hepatitis B (n = 51, 26.0%), alcoholic LC (n = 27, 13.8%), and hepatitis C-associated LC (n = 4, 2.0%). Eighty-nine patients (45.4%) underwent biliary intervention (Table 1). At the time of biliary intervention, jaundice (80.5%) and itching sensation (33.3%) were the main manifestations. In laboratory findings, mean total bilirubin was 5.2 (1.7-32.4); mean alkaline phosphatase was 433.1 (130-1465) and mean γ-glutamyl transpeptidase was 502.7 (92.9-2000.0).
| Variable | |
| Recipient age (yr)1 | 49.7 ± 10.1 (13-68) |
| Older age patients (> 65 yr) | 6 (3.1) |
| Recipient sex (M/F) | 138 (70.0)/58 (30.0) |
| Donor age (yr)1 | 34.0 ± 10.9 (16-64) |
| Older donor age (> 60 yr) | 2 (1.0) |
| Donor sex (M/F) | 114 (58.2)/82 (41.8) |
| Age difference (recipient age - donor age) | 15.7 ± 14.4 (-22 to 42) |
| MELD score | 17.4 ± 10.4 (2.1 to 58.1) |
| High score patients (> 35) | 13 (6.6) |
| Cause | |
| LC-B | 51 (26.0) |
| LC-C | 4 (2.0) |
| Alcohol | 27 (13.8) |
| Hepatocellular carcinoma | 80 (40.8) |
| Combined | 5 (2.6) |
| Hepatitis A | 9 (4.6) |
| Other (drug, autoimmune, unknown) | 20 (10.2) |
| Total ischemic time | 91.5 ± 16.0 (60-145) |
| Group 12 | 93.7 ± 17.9 |
| Group 22 | 88.8 ± 15.1 |
| Number of patients with biliary intervention | 89 (45.4) |
| ERCP | 38 |
| PTBD | 12 |
| Both (ERCP and PTBD) | 38 |
| Re-operative intervention | 0 |
| Mean duration without biliary intervention (mo) | 33.5 ± 28.6 (1-89) |
Single duct-to-duct anastomosis was the most common anastomosis type (n = 145, 74.0%). Biliary stricture (n = 91, 46.4%) and a filling defect on an MIP image (n = 90, 45.9%) were the most common findings. Biloma and hematoma were noted in 13 (6.6%) and 10 (5.1%) patients, respectively (Table 2).
| Variable | Number of patients |
| Using T-tube | 13 (6.6) |
| Anastomosis method | |
| Type 11 | 145 (74.0) |
| Type 22 | 51 (26.0) |
| MRI findings | |
| Filling defect on MIP image | 90 (45.9) |
| Diffuse bile duct dilatation | 29 (14.8) |
| Biliary stricture | 91 (46.4) |
| Biliary leakage | 6 (3.1) |
| Biloma | 13 (6.6) |
| Hematoma | 10 (5.1) |
| Thrombus, infarct | 3 (1.5) |
| Common bile duct stone | 2 (1.0) |
The mean anastomosis site angles were 112.9° and 109.2° according to radiologists 1 and 2, respectively, and the ICC was 0.896 (P < 0.001). The lengths of the filling defects on the MIP images were 3.4 mm according to both radiologists, and the ICC was 0.921 (P < 0.001) (Table 3).
| Radiologist 1 | Radiologist 2 | Inter-observer agreement | |
| Anastomosis site angle (o) | 112.9 (32.5-168.4) | 109.2 (31-173) | 0.896 (P < 0.001) |
| Length of filling defect (mm) | 3.4 (0-33.9) | 3.4 (0-33) | 0.921 (P < 0.001) |
Factors with P values < 0.20 in the univariate analysis were the anastomosis site angle on a 3D image [hazard ratio (HR) = 0.42, 95% confidence interval (CI), 0.27-0.65, P < 0.001], recipient age > 65 years (HR = 2.10, 95%CI: 0.85-5.20, P = 0.110), a filling defect on an MIP image (HR = 2.44, 95%CI: 1.58-3.75, P < 0.001), length of the filling defect on an MIP image (HR = 1.04, 95%CI: 1.01-1.06, P = 0.010), biliary leakage (HR = 2.49, 95%CI: 1.01-6.14, P = 0.049), hematoma (HR = 1.80, 95%CI: 0.78-4.10, P = 0.179), and diffuse bile duct dilatation (HR = 1.59, 95%CI: 0.93-2.70, P = 0.088) (Table 4).
| Variable | Univariate | Multivariate | ||
| Exp (B) | 95%CI | Exp (B) | 95%CI | |
| Recipient age | 1.01 | 0.99-1.04 | ||
| Older age (> 65 yr) | 2.10 | 0.85-5.20 | ||
| Recipient sex | 1.11 | 0.69-1.70 | ||
| Donor age | 1.00 | 0.98-1.02 | ||
| Older donor age (> 60 yr) | 0.92 | 0.13-6.51 | ||
| Donor sex | 0.84 | 0.55-1.28 | ||
| Age difference1 | 1.01 | 0.99-1.02 | ||
| MELD score2 | 1.00 | 0.99-1.02 | ||
| High MELD score (> 35)2 | 0.97 | 0.42-2.22 | ||
| Anastomosis method3 | ||||
| Type 2 vs 1 | 1.14 | 0.72-1.80 | ||
| T-tube | 0.98 | 0.43-2.24 | ||
| MRI findings | ||||
| Anastomosis site angle4 | ||||
| Group 2 vs group 14 | 0.42 | 0.27-0.65 | 0.48 | 0.30-0.75 |
| Filling defect5 | 2.44 | 1.58-3.75 | 2.18 | 1.41-3.38 |
| Length of filling defect5 | 1.04 | 1.01-1.06 | ||
| Diffuse bile duct dilatation | 1.59 | 0.93-2.70 | ||
| Biliary stricture | 1.03 | 0.68-1.56 | ||
| Biliary leakage | 2.49 | 1.01-6.14 | 2.52 | 1.02-6.20 |
| Biloma | 1.54 | 0.74-3.19 | ||
| Hematoma | 1.80 | 0.78-4.10 | ||
| Thrombus, infarct | 0.64 | 0.09-4.59 | ||
The significant risk factors in the multivariate analysis were a filling defect on an MIP image (HR = 2.18, 95%CI: 1.41-3.38, P = 0.001), biliary leakage (HR = 2.52, 95%CI: 1.02-6.20, P = 0.048), and the anastomosis site angle (HR = 0.48, 95%CI: 0.30-0.75, P < 0.001) (Table 4).
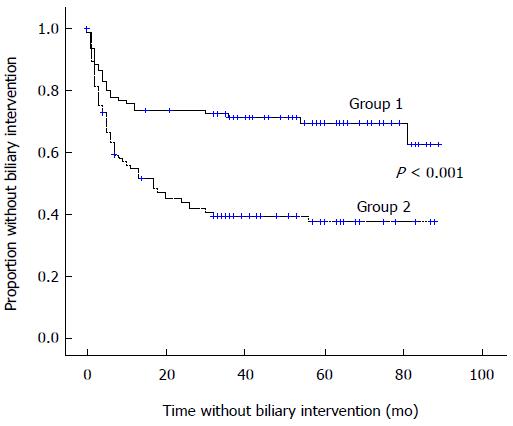
Two groups were created according to the median value of the anastomosis site angle (median angle = 113.3°): group 1, angle > 113.3° and group 2, angle ≤ 113.3°. The biliary intervention rate was significantly lower in group 1 (30.6% vs 60.2% in group 2, P < 0.001), and the mean time to biliary intervention was longer in group 1 (62.1 ± 4.1 vs 38.5 ± 4.2 in group 2, P < 0.001) (Table 5). Kaplan-Meier analysis comparing biliary intervention-free survival between groups 1 and 2 revealed higher survival in group 1 (Figure 2).
| Variable | Total number | Number of events | Rate of events | P value | Mean time interval to events (mo) | P value |
| Group 1 | 98 | 30 | 30.6% | P < 0.001 | 62.1 ± 4.1 | P < 0.001 |
| Group 2 | 98 | 59 | 60.2% | 38.5 ± 4.2 |
Biliary complications after LDLT are an important cause of morbidity and mortality; however, the risk factors for biliary complications that can be determined from an MRCP image after LT have yet to be determined. In the present study, we identified biliary leakage, the presence of a filling defect on an MIP image, and the anastomosis site angle as significant risk factors on MRCP images associated with future biliary intervention.
In our analysis, neither the anastomosis method nor the presence of a T-tube was a risk factor for biliary intervention. Consistent with our results, several studies have demonstrated that the presence of a T-tube is not a risk factor for biliary complications[10,11]; however, some have argued the opposite[2]. Because it is widely accepted to be more physiologically appropriate and has a therapeutic advantage over Roux-en-Y choledochojejunostomy, duct-to-duct anastomosis was performed in our study[12]. We investigated the relationship between increases in the number of duct-to-duct anastomoses and increases in biliary intervention rate. Our findings agree with those of Tsui et al[13]; however, they differ from the results of other studies[14,15].
Our results demonstrate that biliary leakage on MRCP was predictive of biliary intervention after LDLT. Biliary leakage can cause complications, such as infection or biliary stricture[16]. Moreover, ERCP and PTBD are the mainstays for managing biliary leakage[17]. Therefore, our result that biliary leakage was a risk factor for biliary intervention is in accordance with previous findings.
A filling defect on MIP images was also a risk factor for biliary intervention. Several studies have indicated the significance of a filling defect detected on MRCP and have suggested that a filling defect could be a sign of bile duct carcinoma or papillomatosis[18-20]. In addition, intra-ductal debris, sludge, and stones could be causes of a filling defect after LT[21]. For these reasons, a filling defect on MIP images should be considered a risk factor for biliary intervention.
However, donor age > 60 years and a MELD score > 35 were not determined to be significant predictors of biliary intervention. Some studies have shown that these are risk factors for biliary complications[5,6]. These inconsistencies could be due to the lower proportion of patients with a donor age > 60 years or a high MELD score (> 35) in our study.
Finally, the anastomosis site angle on a 3D image was shown to be a risk factor for biliary intervention. We demonstrated that a wider anastomosis site angle (i.e., above the median angle of 113.3°) resulted in a lower and delayed incidence of biliary intervention. No study has investigated the relationship between the anastomosis site angle and biliary complications or interventions after LDLT. Thus, we documented, for the first time, that a decrease in the anastomosis site angle on MRCP after LT could be a risk factor for biliary intervention during the follow-up period.
Several limitations of our study should be discussed. First, the design was retrospective. To improve the accuracy of the results, we reviewed every possible factor blinded to biliary outcome. Further prospective studies are warranted to confirm these results. Second, we could not obtain data on patient cold ischemic time, which is a well-known risk factor for biliary complications in deceased donor liver transplantation (DDLT). Generally, however, cold ischemic time is very short during LDLT. Third, the biliary intervention rate we observed in our study was slightly higher than that reported by previous studies. Unfortunately, the reason for the observed high biliary intervention rate remains unknown.
Biliary complications after LDLT are commonly compared with those following DDLT[5]. Thus, predicting a future need for biliary intervention using a non-invasive method, such as MRCP, could be useful for hematologists and liver transplant surgeons.
In summary, we suggest that MRCP findings of a filling defect on MIP images, biliary leakage, and anastomosis site angle results 1 mo after LDLT can be used to determine the need for future biliary intervention. A further prospective clinical study will be needed to confirm the clinical implications of MRCP 1 mo after LDLT.
Biliary complications commonly occur after liver transplantation (LT) and are the main cause of morbidity and mortality in LT recipients. Magnetic resonance cholangiopancreatography (MRCP), a non-invasive and accurate tool, is the recommended diagnostic modality for detecting biliary complications after LT. Biliary interventions, such as endoscopic retrograde cholangiopancreatography (ERCP) and percutaneous transhepatic biliary drainage (PTBD), are generally used to manage these complications. Several investigators have documented risk factors influencing the development of biliary complications. However, no study has investigated the risk factors associated with the future need for intervention based on MRCP findings shortly after living donor LT (LDLT). The purpose of this study was to determine factors, specifically the anastomosis site angle, that increase the requirement for biliary intervention during follow-up in LDLT recipients on MRCP images 1 mo after LT.
In this study, the authors documented several risk factors for biliary complication by MRCP. They suggest that MRCP findings of a filling defect on maximum intensity projection (MIP) images, biliary leakage, and anastomosis site angle results 1 mo after LDLT can be used to determine the need for future biliary intervention.
No study has investigated the relationship between the anastomosis site angle and biliary complications or interventions after LDLT. Thus, current study documented, for the first time, that a decrease in the anastomosis site angle on MRCP after LT could be a risk factor for biliary intervention during the follow-up period.
Biliary complications after LDLT are an important cause of morbidity and mortality. Thus, predicting a future biliary intervention using a non-invasive method, such as MRCP, could be useful for hematologists and liver transplant surgeons.
MRCP: A non-invasive magnetic resonance imaging tool used to visualize the biliary tract with high sensitivity and specificity. ERCP: An endoscopic procedure specialized for viewing the biliary system and treating biliary complications such as stone and stricture. PTBD: An invasive procedure used to approach the biliary system and treat biliary complications via a percutaneous route.
Lee et al analyzed MRCP imaging performed 1 mo after LDLT to determine risk factors for biliary intervention. The anastomosis site angle, a filling defect in the anastomosis site, and biliary leakage on MRCP were identified as significant risk factors. Moreover, a narrower anastomosis site angle was related to earlier biliary intervention. For the first time, they showed that the anastomosis site angle may be associated with the need for biliary intervention. A further prospective clinical study will be needed to confirm the clinical implications of MRCP mo after LDLT.
P- Reviewer: Berlakovich GA, Liu B, Marino IR S- Editor: Yu J L- Editor: A E- Editor: Liu XM
| 1. | Ryu CH, Lee SK. Biliary strictures after liver transplantation. Gut Liver. 2011;5:133-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 2. | Gastaca M. Biliary complications after orthotopic liver transplantation: a review of incidence and risk factors. Transplant Proc. 2012;44:1545-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Katz LH, Benjaminov O, Belinki A, Geler A, Braun M, Knizhnik M, Aizner S, Shaharabani E, Sulkes J, Shabtai E. Magnetic resonance cholangiopancreatography for the accurate diagnosis of biliary complications after liver transplantation: comparison with endoscopic retrograde cholangiography and percutaneous transhepatic cholangiography - long-term follow-up. Clin Transplant. 2010;24:E163-E169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Arain MA, Attam R, Freeman ML. Advances in endoscopic management of biliary tract complications after liver transplantation. Liver Transpl. 2013;19:482-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Akamatsu N, Sugawara Y, Hashimoto D. Biliary reconstruction, its complications and management of biliary complications after adult liver transplantation: a systematic review of the incidence, risk factors and outcome. Transpl Int. 2011;24:379-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 246] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 6. | Nemes B, Gámán G, Doros A. Biliary complications after liver transplantation. Expert Rev Gastroenterol Hepatol. 2015;9:447-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Wachs ME, Bak TE, Karrer FM, Everson GT, Shrestha R, Trouillot TE, Mandell MS, Steinberg TG, Kam I. Adult living donor liver transplantation using a right hepatic lobe. Transplantation. 1998;66:1313-1316. [PubMed] |
| 8. | Testa G, Malagó M, Valentín-Gamazo C, Lindell G, Broelsch CE. Biliary anastomosis in living related liver transplantation using the right liver lobe: techniques and complications. Liver Transpl. 2000;6:710-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 140] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Na GH, Kim DG, Choi HJ, Han JH, Hong TH, You YK. Interventional treatment of a biliary stricture after adult right-lobe living-donor liver transplantation with duct-to-duct anastomosis. HPB (Oxford). 2014;16:312-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Riediger C, Müller MW, Michalski CW, Hüser N, Schuster T, Kleeff J, Friess H. T-Tube or no T-tube in the reconstruction of the biliary tract during orthotopic liver transplantation: systematic review and meta-analysis. Liver Transpl. 2010;16:705-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Sotiropoulos GC, Sgourakis G, Radtke A, Molmenti EP, Goumas K, Mylona S, Fouzas I, Karaliotas C, Lang H. Orthotopic liver transplantation: T-tube or not T-tube? Systematic review and meta-analysis of results. Transplantation. 2009;87:1672-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Kirimlioglu V, Tatli F, Ince V, Aydin C, Ersan V, Ara C, Aladag M, Kutlu R, Kirimlioglu H, Yilmaz S. Biliary complications in 106 consecutive duct-to-duct biliary reconstruction in right-lobe living donor liver transplantation performed in 1 year in a single center: a new surgical technique. Transplant Proc. 2011;43:917-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Tsui TY, Schlitt HJ, Obed A. Prospective evaluation of biliary reconstruction with duct-to-duct continuous suture in adult live donor liver transplantation. Langenbecks Arch Surg. 2011;396:209-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Gondolesi GE, Varotti G, Florman SS, Muñoz L, Fishbein TM, Emre SH, Schwartz ME, Miller C. Biliary complications in 96 consecutive right lobe living donor transplant recipients. Transplantation. 2004;77:1842-1848. [PubMed] |
| 15. | Wang SF, Huang ZY, Chen XP. Biliary complications after living donor liver transplantation. Liver Transpl. 2011;17:1127-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 16. | Park CS, Jung BH, Hwang S, Park YH, Kang SH, Park GC, Song GW, Jung DH, Ahn CS, Kim KH. External biliary drainage in living donor liver transplantation using duct-to-duct anastomosis. Transplant Proc. 2014;46:678-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Mizuno S, Inoue H, Tanemura A, Murata Y, Kuriyama N, Azumi Y, Kishiwada M, Usui M, Sakurai H, Tabata M. Biliary complications in 108 consecutive recipients with duct-to-duct biliary reconstruction in living-donor liver transplantation. Transplant Proc. 2014;46:850-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Sai JK, Suyama M, Kubokawa Y, Watanabe S, Maehara T. Early detection of extrahepatic bile-duct carcinomas in the nonicteric stage by using MRCP followed by EUS. Gastrointest Endosc. 2009;70:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Chung YE, Kim MJ, Park MS, Choi JY, Kim H, Kim SK, Lee M, Kim HJ, Choi JS, Song SY. Differential features of pancreatobiliary- and intestinal-type ampullary carcinomas at MR imaging. Radiology. 2010;257:384-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Yoon JH. Biliary papillomatosis: correlation of radiologic findings with percutaneous transhepatic cholangioscopy. J Gastrointestin Liver Dis. 2013;22:427-433. [PubMed] |
| 21. | Girometti R, Cereser L, Como G, Zuiani C, Bazzocchi M. Biliary complications after orthotopic liver transplantation: MRCP findings. Abdom Imaging. 2008;33:542-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |