Published online Feb 14, 2016. doi: 10.3748/wjg.v22.i6.2142
Peer-review started: May 25, 2015
First decision: June 25, 2015
Revised: August 19, 2015
Accepted: November 19, 2015
Article in press: November 19, 2015
Published online: February 14, 2016
Processing time: 243 Days and 19.2 Hours
AIM: To investigate the advantages of inferoposterior duodenal approach (IPDA) for laparoscopic pancreaticoduodenectomy (LPD).
METHODS: A total of 36 patients subjected to LPD were admitted to the Affiliated Yijishan Hospital of Wannan Medical College from December 2009 to February 2015. These patients were diagnosed with an ampullary tumour or a pancreatic head tumour through computed tomography, magnetic resonance imaging or endoscopic retrograde cholangiopancreatography preoperatively. The cases were selected on the basis of the following criteria: tumour diameter < 4 cm; no signs of peripheral vascular invasion; evident lymph node swelling; and distant metastasis. Of the 36 cases, 20 were subjected to anterior approach (AA; AA group) and 16 were subjected to IPDA (IPDA group). Specimen removal time, intraoperative blood loss and postoperative complications in the two groups were observed, and their differences were compared.
RESULTS: During the operation, 2 cases in the AA group and 2 cases in the IPDA group were converted to laparotomy; these cases were excluded from statistical analysis. The remaining 32 cases successfully completed the surgery. The AA group and IPDA group exhibited the specimen removal time of 205 ± 52 and 160 ± 35 min, respectively, and the difference was significant (P < 0.01). The AA group and IPDA group revealed the intraoperative blood loss of 360 ± 210 mL and 310 ± 180 mL, respectively, but these values were not significantly different. Postoperative pathological results revealed 4 cases of inferior common bile duct cancer, 8 cases of duodenal papillary cancer, 6 cases of ampullary cancer, 13 cases of pancreatic cancer, 3 cases of chronic pancreatitis accompanied with cyst formation or duct expansion, and 2 cases of mucinous cystic tumour in the pancreatic head. The postoperative complications were pulmonary Staphylococcus aureus infection, incision faulty union, ascites induced poor drainage accompanied with infection, bile leakage, pancreatic leakage and delayed abdominal bleeding.
CONCLUSION: In IPDA, probing for important steps can be performed in early stages, surgical procedures can be optimised and operation time can be shortened.
Core tip: This study investigated the advantages of inferoposterior duodenal approach for laparoscopic pancreaticoduodenectomy. Results revealed that the inferoposterior duodenal approach can be performed not only to probe for important steps in early stages but also to optimise surgical procedures and shorten operation time.
- Citation: Wang XM, Sun WD, Hu MH, Wang GN, Jiang YQ, Fang XS, Han M. Inferoposterior duodenal approach for laparoscopic pancreaticoduodenectomy. World J Gastroenterol 2016; 22(6): 2142-2148
- URL: https://www.wjgnet.com/1007-9327/full/v22/i6/2142.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i6.2142
In 1994, Gagner[1] completed the first laparoscopic pancreaticoduodenectomy (LPD) worldwide; after 20 years of efforts, LPD has been gradually performed globally, and its safety has been confirmed[2-6]. However, compared with other fields of abdominal surgery, LPD is in the exploratory stage, and numerous problems, such as surgical approaches, remain unresolved. In LPD, an open abdominal approach or the anterior approach (AA) is commonly used[7,8]. In this approach, the hepatic flexure of the colon is freed and Kocher incision is implemented, and this procedure is performed from the front parts to the rear parts and from the top sections to the bottom sections. However, the freedom degree of LPD cannot be compared with that of open abdominal surgery because LPD is limited by visual angles and operating holes, as well as insufficient exposure effects by hands; as such, the open abdominal surgical approach cannot be fully applied into LPD. Despite these drawbacks, LPD exhibits good characteristics and provides advantages. For instance, small spaces or surgical fields that cannot be reached through laparotomy can be accessed through LPD. LPD provides unique caudal and dorsal visual angles. Dissection and freeing towards specific parts can be completed from specific angles. LPD can also be applied to perform amplification, which can yield a clear surgical field and high-throughput operations. Therefore, studies have developed new suitable surgical approaches for endoscopic surgeries; such novel approaches have been considered as the basis to optimise the advantages of LPD and to improve LPD. We have acquired experiences in AA-LPD; on the basis of these experiences, we developed a new surgical approach, namely, inferoposterior duodenal approach (IPDA). Here we describe this surgical approach and its advantages in detail.
A total of 36 cases (21 males and 15 females, aged 35 to 75 years) subjected to LPD were admitted to our hospital from December 2009 to February 2015. These patients were diagnosed with an ampullary tumour or a pancreatic head tumour through computed tomography, magnetic resonance imaging or endoscopic retrograde cholangiopancreatography preoperatively. The cases were selected in accordance with the following criteria: tumour diameter < 4 cm; no signs of peripheral vascular invasion; evident lymph node swelling; and distant metastasis. Of the 36 cases, 20 were included in the AA group and 16 were included in the IPDA group.
Anaesthesia and position: The patients received general anaesthesia and tracheal cannulation. The patients were then placed in a supine position and the two legs were in a split position. A laparoscope was inserted through one small incision at the inferior navel ring edge (or 3-5 cm below the belly button); four small incisions were made as primary and secondary operating holes below the front rib margin of the left and right armpits and mildly over the umbilical level of the left and right clavicular midline. The surgeons remained on the patients’ left side, and the assistant stood on the patients’ right side.
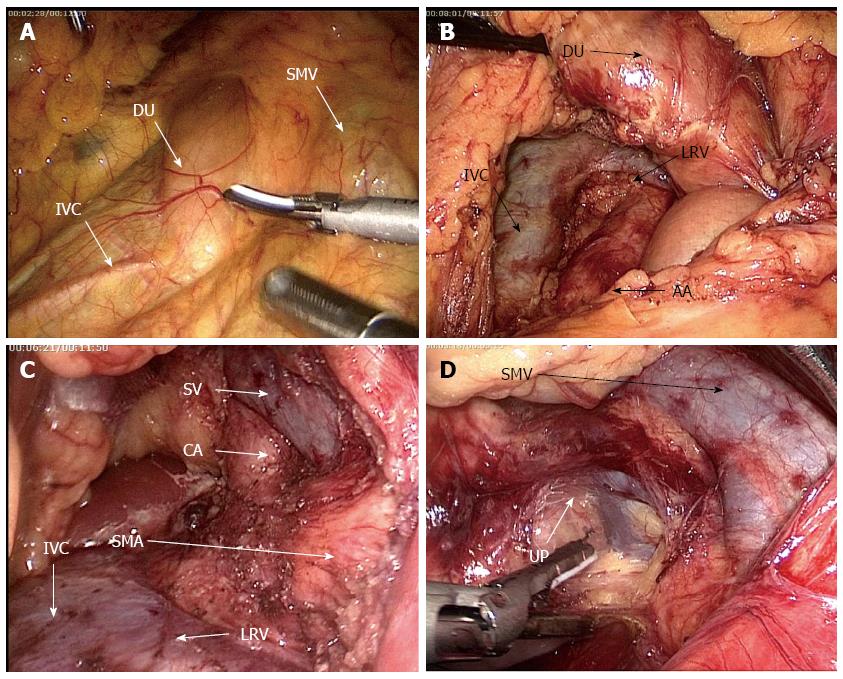
Probing: The liver, abdominal cavity and omentum were conventionally explored for several situations, such as metastasis, cholestasis in the liver or bile duct dilation. The transverse mesocolon was lifted, and the inferior duodenal flexure was exposed from the right side of its root (Figure 1A). The rear part along the inferior duodenal flexure was freed, Toldt’s gap was penetrated, the inferior vena cava was exposed, and whether the lesion invaded this site was probed. The abdominal aorta was then revealed and the para- aortic lymph nodes were harvested for the frozen section (Figure 1B). The surgery was terminated if lymph node metastasis occurred. Subsequently, The left renal vein (LRV) was revealed and the superior mesenteric artery (SMA) was exposed just above the LRV and dissected along its trunk under the unique dorsal view of laparoscopy until the horizontal part of duodenum, probing whether the tumor invaded the SMA or not. The root of celiac trunk was also revealed and the surrounding lymph nodes were cleaned (Figure 1C). The SMV was revealed at the inferior duodenal part (Figure 1D), the vascular sheath was opened and freed upwards, and the right gastroepiploic vein branches were anatomically dissected. The lower pancreatic edge was freed and lifted, sneak dissection was performed from the rear pancreas to the abouchement point of the splenic vein, and whether the tumour invaded the SMV was then determined.
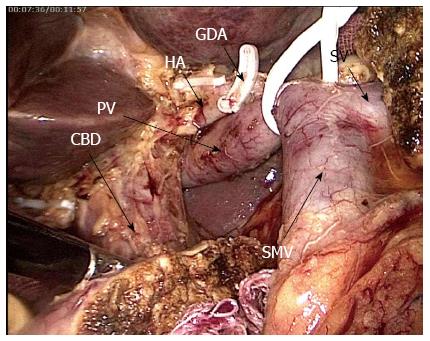
Specimen dissection: After probing confirmed that the tumour was resectable, the visual field was shifted to the left of the transverse mesocolon root and the jejunum was transected 15 cm away from Treitz ligament. The proximal jejunum was pulled to the right through the rear part of the mesenteric vessels. The gastrocolic ligament was transected, the greater and lesser gastric curvatures were freed, and the gastric body was transected. The pancreatic neck was transected and the common hepatic artery was revealed on its upper edge. A tape, which was a “sling” from the pancreatic head and the inferior uncinate process, was suspended and pulled rightwards, while the SMV was pushed leftwards, and the right side wall of superior mesenteric artery was revealed. The arterial sheath was opened and the SMV and branches from the SMA to the pancreatic uncinate process from bottom to top were dissected, and the uncinated process was completely freed. The surrounding lymph adipose tissues were cleaned. The hepatoduodenal ligament was penetrated, and the GDA was dissected, freed towards the hepatic portal along the surface of the portal vein, and separated from the hepatic artery and the bile duct. The hepatic artery was fully anatomised and the surrounding fat lymphoid tissues were cleaned (Figure 2). The gallbladder was finally removed and the common hepatic duct was transected. The specimen was then removed.
Reconstruction: The digestive tract was reconstructed in accordance with Child surgical procedures through endoscopy or with small incision assistance.
Specimen removal time, intraoperative blood loss and postoperative complications in the two groups were observed.
Statistical analyses were carried out using SPSS 17.0 (SPSS Inc., Chicago, IL, United States). Data are presented as mean ± SD. Comparisons between the two groups were performed using t-test. P < 0.05 was considered statistically significant.
Of the 36 patients, 4 were converted to laparotomy; the lesions of the 3 cases were closely related to the vessels and difficult to be endoscopically separated, and the chronic pancreatic inflammation of 1 case exhibited heavy adhesion to the surrounding tissues and easily caused bleeding during separation. Of these 4 cases, 2 were in the AA group and 2 were in the IPDA group, and these 4 cases were not included in the statistical analyses. The remaining 32 cases successfully completed the surgery. The difference in the specimen removal time was significant (P < 0.01), with 205 ± 52 min in the AA group and 160 ± 35 min in the IPDA group. The blood loss was 360 ± 210 mL in the AA group and 310 ± 180 mL in the IPDA group, and this finding did not significantly differ (P > 0.05).
Of the 36 cases, the following conditions were observed: 4 cases of inferior common bile duct cancer; 8 cases of duodenal papillary cancer; 6 cases of ampullary cancer; 13 cases of pancreatic cancer (9 cases of adenocarcinoma, 2 cases of adenosquamous cancer, 1 case of neuroendocrine tumour, and 1 case of solid false papilloma); 3 cases of chronic pancreatitis accompanied with cyst formation or duct expansion; and 2 cases of mucinous cystic tumour in the pancreatic head.
Several postoperative complications were encountered. One case of postoperative pulmonary Staphylococcus aureus infection was observed; as such, the antibacterial treatment was strengthened. One case of incision faulty union was documented, and the phase II suture was performed to repair this problem. One case of ascites-induced poor drainage accompanied with infection was recorded; as such, re-surgical drainage was performed. Three cases of bile leakage occurred, but this condition spontaneously healed after drainage. Five cases of pancreatic leakage, including one case of secondary abdominal bleeding, were found. Therefore, re-operation was conducted, and a dehiscent wound was detected on the partial anterior wall of the pancreatic anastomotic site. The wound was then re-sutured, and the patient spontaneously healed after the wound was rinsed with double cannula. Four cases of pancreatic leakage were documented, and this condition spontaneously healed after drainage. One case of delayed abdominal bleeding was found, and surgical exploration revealed that this haemorrhagic condition was caused by a hepatic artery rupture; therefore, the patient was subjected to partial arterial resection and reconstruction. However, the anastomotic site bled postoperatively; as such, the patient was subjected to an artery ligation again. Afterwards, the patient recovered well and was discharged.
In this study, LPD was performed with the duodenum as the centre. In general, the traditional anterior approach is initiated by freeing the descending part of the duodenum. However, the intestinal segment is found deep into the retroperitoneum, in which the transverse colon and its mesenteria are covered; as such, these parts cannot be easily exposed or reached. Furthermore, the operation is difficult and limited by a trocar hole. However, we found that the location at the junction of the descending duodenum and its inferior part (i.e., inferior duodenal flexure) was relatively superficial, and only one layer of the peritoneum was covered. The site was located on the right of transverse mesocolon root and thus could be exposed when the transverse mesocolon was lifted. The anteromedial side of the intestinal canal was the SMV, and the posterolateral side was the inferior vena cava, which is the major hub of pancreaticoduodenectomy. We used this part as the initial step of the surgery, which was combined with the “window”, and the inferoposterior approach of LPD was developed. The outcomes were good, and the approach provided the following advantages.
First, the inferoposterior approach of LPD maximised the advantages of laparoscopy; in particular, small spaces could be reached. The probe could enter the dorsal part of the pancreatic head and the duodenum through the rear part of the “window”, and relevant exploration could be accomplished to determine the surgical methods. In the biopsy of the para-abdominal aortic lymph nodes, pancreatic cancer causes a high rate of lymph node metastasis; for instance, 54% to 86% of patients likely suffer from lymph node metastasis after they undergo surgery[9,10]. Even a small pancreatic tumour (diameter < 2 cm) exhibits a lymphatic metastatic rate of up to 37.2%[11]. In addition, the involvement of para-abdominal aortic lymph nodes is manifested as distant metastasis (M1), which often corresponds to poor prognosis. Although these patients are subjected to enlarged lymph node dissection, the long-term survival is significantly worse than those with negative lymphatic metastasis[12-15], and this finding does not significantly differ from the patients who did not opt for the removal of their tumours[16-18]. Therefore, the intraoperative biopsy towards the para-abdominal aortic lymph nodes helps guide this surgical approach; during surgery, a surgeon can further evaluate the patients’ prognosis and determine appropriate surgical methods. In AA-LPD, lymph nodes cannot be easily obtained in this region. For this reason, the hepatic flexure of the colon must be dissected, Kocher incision must be made and the pancreatic head and the duodenum must be fully freed. IPDA could be performed to directly enter the region after the hepatic flexure of the duodenum was freed; thus, the lymph nodes could be obtained with the shortest distance and the fastest speed to assess lymph node metastasis in early stages. Lymph node metastasis confirmed through rapid intraoperative pathological assessment could indicate poor prognosis, and surgical resection unlikely yielded positive outcomes and further trauma could be avoided. In the exploration to the superior mesenteric artery, the resection and reconstruction of SMV are safe and feasible through pancreaticoduodenectomy[19,20]. By comparison, the invasion of tumour towards the SMA is a counterindication for surgery because the resection and reconstruction of the SMA likely cause a high mortality rate and induce complications after surgery; these procedures could not prolong the survival period of patients[21]. In traditional PD surgery, the exploration towards the SMV and the portal venous system is set in early stages, and the SMA damaged by tumour is often determined in the last stage of resection. In particular, the SMA is determined during the disruption of the pancreatic neck, and the surgeon does not have other options. Thus, the surgeon must resect the specimens. As a result, positive margins may be detected, and these patients likely show a poor prognosis. Furthermore, the long-term survival rate is very low. Pessaux et al[22] proposed the artery-leading surgical approach in 2006; SMA is developed and thus has allowed surgeons to find the damaged arteries in early stages; further resection is not performed, so it could avoid the embarrassment of not being able to regret. Since then, scholars have published similar reports; on the basis of different locations and conditions of tumours, these scholars proposed a number of artery-leading surgical approaches[23-27]. In this study, the pancreatic head and the duodenum were lifted forward after we obtained the para-abdominal aortic lymph nodes, and the SMA root was then exposed from the site where the left renal vein spanned the upper edge of the abdominal aorta. The unique dorsal visual angle of laparoscopy was then used to dissect along its direction to confirm whether the SMA was invaded by tumours. Further freeing along this path and towards the caput could help elucidate the relationship of cancer with celiac trunk. Therefore, this approach could reveal the relationships between tumour and arteries in earlier stages than traditional surgical approaches; as such, appropriate surgical procedures can be determined and selected.
Second, in this approach, the SMV was dissected from the anteromedial side of the “window”; thus, the SMV probing could be completed safely. SMV probing is one of the difficulties in LPD. Traditional probing begins from the uncinate process segment of the blood vessels, and the lower edge of the pancreas is exposed; the mid colon vein or the right gastroepiploic vein is considered as an indicator[28,29]. The branch vessels followed by the SMV trunk are processed. However, this SMV segment is the shortest and contains the highest number of branches, such as superoanterior pancreaticoduodenal vein and other branches besides the right gastroepiploic vein. These vessels are imported into the SMV with different vessels and from different levels; therefore, the anatomical levels are difficult to be determined during separation. Once the damage occurs, uncontrollable bleeding is inevitable; as such, this procedure likely causes bleeding. In this study, the SMV probing was initiated from the anteromedial side of the “window”, and this SMV segment was longer and located entirely within the small bowel mesentery. The segment did not show vascular association with the inferior duodenal part. Thus, the segment was convenient and safe for the exposure. Opening the vascular sheath from this site and freeing upwards along the intrathecal space, surgeons could quickly locate and process the blood vessel branches in the uncinate process and could reach the rear pancreatic vessels for further exploration. This trunk-first-branch-later approach could reduce the risk of bleeding and increase the safety of the surgery.
Lastly, this approach was more conducive to the lymph node dissection of the hepatoduodenal ligament, which is another technical difficulty in LPD. Traditional dissection methods begin from the top parts to the bottom parts of the hepatic portal, and this procedure is difficult to perform through endoscopy. When the common hepatic duct is transected or the hepatic artery is anastomosed, the risks of damaging the posterior portal vein and causing bleeding remain unknown. We believe that opening the portal vein sheath to completely expose the portal vein is an important measure to avoid injuries. However, in the three-tube structure of the liver ligament, the portal vein was located distally; as such, this vein cannot be easily revealed via the conventional method. Therefore, an appropriate approach should be determined. In this study, when the probing confirmed that the tumour could be removed, we firstly dissected the proximal jejunum and then transected the pancreatic uncinate process neck to expose the whole SMV and the portal vein. We anastomosed and dissected the hepatoduodenal ligament in the final stages of resection. At this time, the small branches that assemble into the portal vein system were processed when the uncinate process was freed; therefore, the clearance of the hepatoduodenal ligament could be completed only after anastomising along the constant common hepatic artery. Such bottom-to-top anatomy would not only benefit the endoscopic operation but also “simplify” the complex skeletonisation of the hepatoduodenal ligament. As a result, operation efficiency could be improved and operation time could be reduced. This approach is safe and effective for patients with a history of cholangitis, which does not show a clear anatomy of the hepatoduodenal ligament; this approach is also beneficial for patients with liver tumours located in deep regions that cause difficulty in exposing the hepatoduodenal ligament.
In summary, this approach fully combined the duodenal anatomical characteristics and laparoscopic advantages; thus, probing, separation and sample resection could be finished in one step. With this approach, probing can be performed in early stages, surgical procedures can be optimised and operation time can be shortened. However, the number of cases in this study was small, and the advantages of the proposed procedure should be further confirmed through comparative studies.
Laparoscopic pancreaticoduodenectomy (LPD) is one of surgeries with the most difficulty in endoscopic surgery. This procedure is in the exploratory stage, and numerous problems, such as surgical approaches, remain unresolved. At present, laparotomy approach is often used for LPD, but this approach is not entirely suitable for laparoscopic operation. Studies should further discuss the mechanisms by which the characteristics and advantages of laparoscopy can be maximised and determine a suitable surgical approach.
In 1994, LPD was completed for the first time. After 20 years of efforts, LPD has been gradually performed, and some surgical procedures, including pancreatic anastomosis and hook excision, have been improved. However, studies on surgical approaches of LPD are very rare.
On the basis of the duodenum anatomical characteristics and advantages of laparoscopy, we proposed the inferoposterior duodenal approach (IPDA). Compared with traditional anterior approaches, IPDA can be performed not only to probe in early stages but also to optimize the operation process and shorten the operation time.
IPDA is suitable for LPD. This procedure can optimise the surgical procedure, shorten the operation time and promote further applications of LPD.
LPD is pancreaticoduodenectomy completed through laparoscopy.
This study investigated the advantages of the inferoposterior duodenal approach for laparoscopic pancreaticoduodenectomy. The results are significant and applicable to clinical practices and studies.
P- Reviewer: Lynch HT, Syngal S S- Editor: Yu J L- Editor: Wang TQ E- Editor: Ma S
| 1. | Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc. 1994;8:408-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 659] [Article Influence: 21.3] [Reference Citation Analysis (1)] |
| 2. | Asbun HJ, Stauffer JA. Laparoscopic vs open pancreaticoduodenectomy: overall outcomes and severity of complications using the Accordion Severity Grading System. J Am Coll Surg. 2012;215:810-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 295] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 3. | Kim SC, Song KB, Jung YS, Kim YH, Park do H, Lee SS, Seo DW, Lee SK, Kim MH, Park KM. Short-term clinical outcomes for 100 consecutive cases of laparoscopic pylorus-preserving pancreatoduodenectomy: improvement with surgical experience. Surg Endosc. 2013;27:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 4. | Nakamura M, Nakashima H. Laparoscopic distal pancreatectomy and pancreatoduodenectomy: is it worthwhile? A meta-analysis of laparoscopic pancreatectomy. J Hepatobiliary Pancreat Sci. 2013;20:421-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | Correa-Gallego C, Dinkelspiel HE, Sulimanoff I, Fisher S, Viñuela EF, Kingham TP, Fong Y, DeMatteo RP, D’Angelica MI, Jarnagin WR. Minimally-invasive vs open pancreaticoduodenectomy: systematic review and meta-analysis. J Am Coll Surg. 2014;218:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 6. | Subar D, Gobardhan PD, Gayet B. Laparoscopic pancreatic surgery: An overview of the literature and experiences of a single center. Best Pract Res Clin Gastroenterol. 2014;28:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Dulucq JL, Wintringer P, Mahajna A. Laparoscopic pancreaticoduodenectomy for benign and malignant diseases. Surg Endosc. 2006;20:1045-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Corcione F, Pirozzi F, Cuccurullo D, Piccolboni D, Caracino V, Galante F, Cusano D, Sciuto A. Laparoscopic pancreaticoduodenectomy: experience of 22 cases. Surg Endosc. 2013;27:2131-2136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Schwarz RE, Smith DD. Extent of lymph node retrieval and pancreatic cancer survival: information from a large US population database. Ann Surg Oncol. 2006;13:1189-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 286] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 10. | Massucco P, Ribero D, Sgotto E, Mellano A, Muratore A, Capussotti L. Prognostic significance of lymph node metastases in pancreatic head cancer treated with extended lymphadenectomy: not just a matter of numbers. Ann Surg Oncol. 2009;16:3323-3332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Kanda M, Fujii T, Nagai S, Kodera Y, Kanzaki A, Sahin TT, Hayashi M, Yamada S, Sugimoto H, Nomoto S. Pattern of lymph node metastasis spread in pancreatic cancer. Pancreas. 2011;40:951-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Shimada K, Sakamoto Y, Sano T, Kosuge T. The role of paraaortic lymph node involvement on early recurrence and survival after macroscopic curative resection with extended lymphadenectomy for pancreatic carcinoma. J Am Coll Surg. 2006;203:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Murakami Y, Uemura K, Sudo T, Hashimoto Y, Yuasa Y, Sueda T. Prognostic impact of para-aortic lymph node metastasis in pancreatic ductal adenocarcinoma. World J Surg. 2010;34:1900-1907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Doi R, Kami K, Ito D, Fujimoto K, Kawaguchi Y, Wada M, Kogire M, Hosotani R, Imamura M, Uemoto S. Prognostic implication of para-aortic lymph node metastasis in resectable pancreatic cancer. World J Surg. 2007;31:147-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Schwarz L, Lupinacci RM, Svrcek M, Lesurtel M, Bubenheim M, Vuarnesson H, Balladur P, Paye F. Para-aortic lymph node sampling in pancreatic head adenocarcinoma. Br J Surg. 2014;101:530-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 16. | Huguet F, André T, Hammel P, Artru P, Balosso J, Selle F, Deniaud-Alexandre E, Ruszniewski P, Touboul E, Labianca R. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 370] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 17. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5640] [Article Influence: 402.9] [Reference Citation Analysis (1)] |
| 18. | Loehrer PJ, Feng Y, Cardenes H, Wagner L, Brell JM, Cella D, Flynn P, Ramanathan RK, Crane CH, Alberts SR. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29:4105-4112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 622] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 19. | Fuhrman GM, Leach SD, Staley CA, Cusack JC, Charnsangavej C, Cleary KR, El-Naggar AK, Fenoglio CJ, Lee JE, Evans DB. Rationale for en bloc vein resection in the treatment of pancreatic adenocarcinoma adherent to the superior mesenteric-portal vein confluence. Pancreatic Tumor Study Group. Ann Surg. 1996;223:154-162. [PubMed] |
| 20. | Fukuda S, Oussoultzoglou E, Bachellier P, Rosso E, Nakano H, Audet M, Jaeck D. Significance of the depth of portal vein wall invasion after curative resection for pancreatic adenocarcinoma. Arch Surg. 2007;142:172-179; discussion 180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Nagakawa T, Konishi I, Ueno K, Ohta T, Akiyama T, Kanno M, Kayahara M, Miyazaki I. The results and problems of extensive radical surgery for carcinoma of the head of the pancreas. Jpn J Surg. 1991;21:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Pessaux P, Varma D, Arnaud JP. Pancreaticoduodenectomy: superior mesenteric artery first approach. J Gastrointest Surg. 2006;10:607-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Dumitrascu T, David L, Popescu I. Posterior versus standard approach in pancreatoduodenectomy: a case-match study. Langenbecks Arch Surg. 2010;395:677-684. [PubMed] |
| 24. | Weitz J, Rahbari N, Koch M, Büchler MW. The “artery first” approach for resection of pancreatic head cancer. J Am Coll Surg. 2010;210:e1-e4. [PubMed] |
| 25. | Shrikhande SV, Barreto SG, Bodhankar YD, Suradkar K, Shetty G, Hawaldar R, Goel M, Shukla PJ. Superior mesenteric artery first combined with uncinate process approach versus uncinate process first approach in pancreatoduodenectomy: a comparative study evaluating perioperative outcomes. Langenbecks Arch Surg. 2011;396:1205-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Kurosaki I, Minagawa M, Takano K, Takizawa K, Hatakeyama K. Left posterior approach to the superior mesenteric vascular pedicle in pancreaticoduodenectomy for cancer of the pancreatic head. JOP. 2011;12:220-229. [PubMed] |
| 27. | Sanjay P, Takaori K, Govil S, Shrikhande SV, Windsor JA. ‘Artery-first’ approaches to pancreatoduodenectomy. Br J Surg. 2012;99:1027-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 243] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 28. | Palanivelu C, Rajan PS, Rangarajan M, Vaithiswaran V, Senthilnathan P, Parthasarathi R, Praveen Raj P. Evolution in techniques of laparoscopic pancreaticoduodenectomy: a decade long experience from a tertiary center. J Hepatobiliary Pancreat Surg. 2009;16:731-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 29. | Zureikat AH, Breaux JA, Steel JL, Hughes SJ. Can laparoscopic pancreaticoduodenectomy be safely implemented? J Gastrointest Surg. 2011;15:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |