Published online Feb 14, 2016. doi: 10.3748/wjg.v22.i6.2126
Peer-review started: June 11, 2015
First decision: August 26, 2015
Revised: September 11, 2015
Accepted: November 19, 2015
Article in press: November 19, 2015
Published online: February 14, 2016
Processing time: 228 Days and 3.7 Hours
AIM: To evaluate the feasibility and survival outcomes of a liver-first approach.
METHODS: Between January 2009 and April 2013, 18 synchronous colorectal liver metastases (sCRLMs) patients with a planned liver-first approach in the Hepatopancreatobiliary Surgery Department I of the Beijing Cancer Hospital were enrolled in this study. Clinical data, surgical outcomes, morbidity and mortality rates were collected. The feasibility and long-term outcomes of the approach were retrospectively analyzed.
RESULTS: Sixteen patients (88.9%) completed the treatment protocol for primary and liver tumors. The main reason for treatment failure was liver disease recurrence. The 1 and 3 year overall survival rates were 94.4% and 44.8%, respectively. The median survival time was 30 mo. The postoperative morbidity and mortality were 22.2% and 0%, respectively, following a hepatic resection, and were 18.8% and 0%, respectively, after a colorectal surgery.
CONCLUSION: The liver-first approach appeared to be feasible and safe. It can be performed with a comparable mortality and morbidity to the traditional treatment paradigm. This approach might offer a curative opportunity for sCRLM patients with a high liver disease burden.
Core tip: This is a retrospective study to investigate the feasibility and survival outcome of the liver-first approach for synchronous colorectal liver metastases. The postoperative morbidity and mortality were acceptable. The 1 and 3 year overall survival rates were 94.4% and 44.8%, respectively. The approach should be performed in patients with synchronous colorectal liver metastases with a high liver disease burden.
- Citation: Wang K, Liu W, Yan XL, Xing BC. Role of a liver-first approach for synchronous colorectal liver metastases. World J Gastroenterol 2016; 22(6): 2126-2132
- URL: https://www.wjgnet.com/1007-9327/full/v22/i6/2126.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i6.2126
The liver is the most common organ for distant metastases from colorectal cancer[1]. Up to 15%-42% of patients present with synchronous colorectal liver metastases at the time of diagnosis of their primary cancer[2,3]. The synchronous presentation has been associated with poor survival outcomes[4,5]. Nevertheless, surgical resection of all tumor sites is considered the only curative therapy for long-term survival from colorectal liver metastases (CRLMs)[4,6]. Several large case series from tertiary centers have reported 5 year survival rates of 21%-58% and 10 year survival rates of 22%-26%[4,7,8].
The traditional surgical strategy for resectable synchronous colorectal liver metastases (sCRLMs) is a two-stage approach that includes colorectal cancer resection followed by chemotherapy and a delayed hepatic resection of a CRLM. This approach might result in liver disease progression between the time of colorectal and hepatic resection and render the CRLM unresectable[9]. This is a particular concern in patients who develop postoperative complications after colorectal cancer resection before the administration of chemotherapy and the hepatic resection of CRLMs[10].
Upon the realization that liver metastases define the prognosis of a patient, the concept of a liver-first approach in patients with locally advanced rectal cancer and synchronous liver metastases was proposed[11]. However, there has been limited data published on the feasibility and safety of the liver-first approach for sCRLMs. Therefore, the present study aims to describe the experience with the liver-first approach in a tertiary referral center. The feasibility, security and long-term outcomes of the liver-first approach were also investigated.
Between January 2009 and April 2013, 168 CRLM patients underwent hepatic resection in the Hepatopancreatobiliary Surgery Department I of Beijing Cancer Hospital. All of the sCRLM patients were identified. Eighteen of these patients with a planned liver-first approach were included in the present study.
All the patients underwent a complete colonoscopy for colorectal cancer, abdominal and thoracic computed tomography scan and liver and pelvic (only rectal cancer patients) magnetic resonance imaging. The Response Evaluation Criteria for Solid Tumors were applied to the serial imaging studies obtained during a preoperative therapy to determine a chemotherapy response[12]. The definition of advanced metastatic disease was based on a clinical risk score (CRS) described by Fong et al[13]. A CRS of 3 or higher has been validated as defining more severe disease.
Preoperative chemotherapy was considered in patients with initially unresectable disease or a high liver disease burden. Patients received oxaliplatin or irinotecan-based chemotherapy. In some recent cases, they also received cetuximab or bevacizumab. The response to chemotherapy was assessed after two or three cycles (more than four cycles for conversion chemotherapy) by MRI and carcinoembryonic antigen levels. When the liver metastases were resectable, a laparotomy was planned more than three weeks after the last course of systemic chemotherapy. Bevacizumab had to be excluded from the last course of chemotherapy to ensure an interval of at least six weeks.
All the patients underwent a hepatic resection with curative intent to achieve R0 and preserve as much normal functional liver parenchyma (with adequate vascular inflow, outflow and biliary drainage) as possible. A resection of three or more segments was considered a major hepatectomy[14]. The normal liver parenchyma remnant volume was more than 40% if a patient received preoperative chemotherapy.
Preoperative chemoradiation was used in only two situations: (1) mid-to-low rectal cancer, defined as ≤ 10 cm distance from the lower edge of the tumor to the anal verge; and (2) a pre-treatment staging by MRI was T3/T4, or any T category, and N positive[15]. Radiation therapy consisted of either a long course (total dose of 50 Gy) therapy or a modified short course (total dose of 30 Gy) therapy with capecitabine 825 mg/m2 twice per day only on radiotherapy days. A total mesorectal/complete mesocolic excision was performed in all patients.
All the patients had a follow-up visit every 3 mo for the first 2 years, with a physical examination, CEA and CA19-9 serum measurement and abdominal ultrasonography. The patients had a computed tomography scan and colonoscopy every 6 mo. No patients were lost to follow-up.
Continuous variables were summarized as a mean. Categorical variables were summarized as a frequency and percentage. A Kaplan-Meier survival was calculated from the date of initial treatment. Statistical analyses were performed using SPSS 17.0 (SPSS, Inc., Chicago, IL, United States).
Between January 2009 and April 2013, 48 sCRLM patients were identified. The liver-first approach was planned for 18 of them (37.5%). There were 10 male and 8 female patients. The median age was 54 years (range: 21-74; mean: 51.9). At the time of presentation, 13 (72.2%) patients had clinical symptoms. The median size of the liver metastases was 4 cm (range: 2-16; mean: 5.33). The median number of metastases was 4 (range: 1-12; mean: 4.06). The median preoperative CEA blood level was 26.3 ng/mL (range: 1-861; mean: 87.37). The median CRS was 3 (range: 2-4; mean: 3.17). The most common site of the primary tumor was the rectum (n = 16; 88.9%). The characteristics of these patients are detailed in Table 1.
| Variable | No. of patients, n = 18 |
| Patient characteristics | |
| Age (yr), median (range) | 54 (21-74) |
| Sex (male) | 10 |
| Pre-operative CEA level (μg/L), median (range) | 26.3 (1-860) |
| Primary tumor site | |
| Colon | 2 |
| Rectum | 16 |
| Symptoms caused by the primary tumor | 13 |
| Symptoms at the time of presentation | |
| None | 5 |
| Rectal blood loss | 7 |
| Changes in bowel habits | 6 |
| AJCC T-stage on pathology | |
| ypT1/ypT2 | 1 |
| ypT3/ypT4 | 15 |
| Lymph node status on pathology | |
| ypN1/ypN2 | 14 |
| ypN0 | 2 |
| Hepatic metastasis | |
| Size of largest metastasis (cm), median (range) | 4 (2-16) |
| No. of metastasis, median (range) | 4 (1-12) |
| Location (unilobular) | 11 |
| CRS score | |
| < 3 | 2 |
| ≥ 3 | 16 |
| Preoperative chemotherapy | 14 |
| Cycles, median (range) | 3 (0-5) |
| Indication | |
| Conversion | 4 |
| Locally advanced liver metastases | 10 |
| Regimens of preoperative chemotherapy | |
| Oxaliplatin | 10 |
| Irinotecan | 4 |
| Cetuximab | 4 |
| Bevacizumab | 2 |
| Response of preoperative chemotherapy | |
| PR | 9 |
| SD | 4 |
| PD | 1 |
Of the 18 patients in whom a liver-first approach was planned, a major hepatectomy was performed in 14 patients (77.8%). Due to liver recurrence after the hepatectomy, only 2 patients did not undergo surgery for the primary tumor. The operative characteristics of primary and liver metastases are detailed in Table 2. The complication rates after the hepatic and primary resections were 22.2% (n = 4) and 18.8% (n = 3), respectively. According to the Clavien-Dindo classification system[16], all the complications were minor (Clavien grade < 3). Importantly, there was no postoperative mortality after the liver or primary surgeries. The specifics are detailed in Table 2.
| Variable | No. of patients, n = 18 |
| Type of hepatic resection | |
| Major | 14 |
| Minor | 4 |
| Extent of hepatic resection | |
| Partial | 10 |
| Hemihepatectomy | 3 |
| Extended hepatectomy | 5 |
| Type of colorectal resection | |
| Low anterior resection | 12 |
| Abdominoperineal resection | 2 |
| Left hemicolectomy | 2 |
| Resected lymph nodes, median (range) | 11 (6-20) |
| Complications | |
| Hepatectomy-related | |
| hydrothorax | 3 |
| Abdominal abscess | 1 |
| Minor (Clavien grade < 3) | 4 |
| Major (Clavien grade ≥ 3) | 0 |
| Post-operative mortality (within 90 d) | 0 |
| Surgery on primary cancer | |
| Anastomotic leakage | 1 |
| Abdominal abscess | 2 |
| Minor (Clavien grade < 3) | 3 |
| Major (Clavien grade ≥ 3) | 0 |
| Post-operative mortality (within 90 d) | 0 |
A flow diagram of the treatment overview of all 18 patients is shown in Figure 1. At the time of the initial presentation, 4 patients had unresectable CRLMs and received conversion chemotherapy. Ten patients had locally advanced liver metastases and received neoadjuvant chemotherapy. Two patients refused any neoadjuvant therapy and the other 2 patients had a CRS of less than 3. All of them immediately underwent a hepatic resection. The median preoperative chemotherapy cycle was 3 (range: 0-5; mean: 2.5). It included an oxaliplatin-based chemotherapy in 10 patients and an irinotecan-based chemotherapy in 4 patients. During the first courses of the preoperative chemotherapy, cetuximab was added to 4 patients and bevacizumab was added to 2 patients. Between the window of the hepatic and colorectal surgeries, six patients received radiation therapy, 3 patients a short course of radiation therapy and 3 patients a long course of radiation therapy. The specifics are detailed in Table 3.
| Patient | Largest size (cm) | CEA level (μg/L) | cTN | No. of mets | CTx | Responseon CTx | Liver surgery | RTx | Primary surgery |
| 1 | 16 | 861.4 | cT3N1 | 1 | Xelox | PD | Hemihep | None | Left Hemicol |
| 2 | 2 | 1 | cT4N2 | 4 | Folfox | PR | Extended hemihep | None | LAR |
| 3 | 3.5 | 13.4 | cT4N1 | 1 | None | None | Partial | 30 Gy/Xeloda | LAR |
| 4 | 6 | 113.4 | cT3N1 | 5 | Folfox | SD | Partial | None | Left Hemicol |
| 5 | 2.5 | 16.8 | cT3N1 | 6 | Xelox | PR | Extended hemihep | 30 Gy/Xeloda | None |
| 6 | 7.9 | 30.4 | cT4N1 | 4 | Xelox | PR | Extended hemihep | None | LAR |
| 7 | 4 | 3.2 | cT2N1 | 3 | Folifiri + Cet | PR | Hemihep | None | LAR |
| 8 | 2.4 | 2.4 | cT3N2 | 3 | Folifiri + Cet | PR | Partial | 50 Gy/Xeloda | LAR |
| 9 | 5 | 78.1 | cT4N2 | 1 | None | None | Partial | 50 Gy/Xeloda | APR |
| 10 | 3.8 | 160.9 | cT3N2 | 1 | Folfox | SD | Partial | None | APR |
| 11 | 4 | 3.7 | cT3N0 | 6 | Folifiri + Cet | PR | Partial | 30 Gy/Xeloda | LAR |
| 12 | 1.5 | 19.7 | cT3N1 | 4 | Folfox + Bev | PR | Partial | None | LAR |
| 13 | 8.5 | 87.2 | cT3N1 | 5 | None | None | Partial | None | LAR |
| 14 | 12 | 22.1 | cT4N1 | 6 | Folfox + Cet | PR | Hemihep | None | LAR |
| 15 | 6 | 79.4 | cT3N1 | 3 | Folfoxiri | SD | Extended hemihep | None | LAR |
| 16 | 4.3 | 6.1 | cT3N0 | 12 | Xelox + Bev | PR | Partial | None | LAR |
| 17 | 3 | 34.7 | cT3N1 | 2 | None | None | Partial | None | LAR |
| 18 | 3.5 | 39.0 | cT3N2 | 6 | Xelox | SD | Extended hemihep | 50 Gy/Xeloda | None |
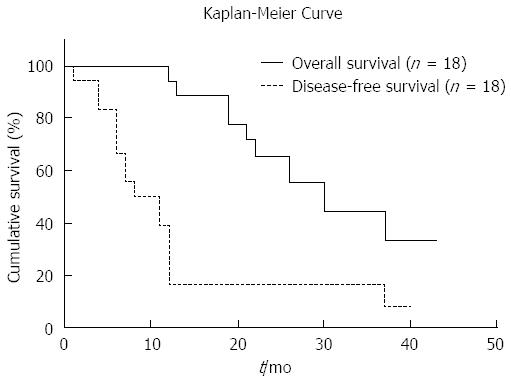
At the time of the last follow-up, 16 (88.9%) patients completed a curative paradigm. The median follow-up was 30 mo (range: 12-43; mean: 30.54). The 1 and 3 year overall survival rates were 94.4% and 44.8%, respectively (Figure 2). The median disease-free survival after surgery was 11 mo (range: 1-40; mean: 13.4). After the hepatic resection, 16 patients had a recurrence during the follow-up. Nine died of disease recurrence. The patterns of recurrence were intrahepatic only (10, 62.5%) and combined intra and extrahepatic (6, 37.5%).
In the current series, 18 patients who were scheduled to undergo the liver-first approach were included in this study. Sixteen (88.9%) of them completed the treatment protocol for liver and primary tumors. The percentage of feasibility is in concordance with those reported in assorted cohorts of sCRLM[17,18]. The remaining two patients deviated from the protocol as a result of recurrence of liver metastasis after resection. For the patients who underwent the liver-first approach, the 1 and 3 year overall survival rates were 94.4% and 44.8%, respectively. The median disease free survival time after surgery was 10 mo (range: 1-40; mean: 13.3). The complication rate after hepatic resection and primary resection was 22.2% (n = 4) and 18.8% (n = 3), respectively. These surgical outcomes were comparable with other results associated with the liver-first approach[19,20]. In addition, our results may need to be confirmed in a prospective, randomized clinical trial with a larger sample size.
Numerous surgical series have demonstrated that a hepatic resection for CRLM may offer the possibility of long-term survival[5,6]. Additionally, except for the hepatic resection, no other treatment has shown a survival plateau. These results support that a hepatic resection is the standard practice and only curative treatment for CRLM. Apparently, metastatic disease, rather than primary colorectal cancer, has been proposed to be the main determinant of patient survival. Thus, treating a CRLM should be the first priority[11,17]. It has been suggested that liver disease burden rather than the primary cancer leads to subsequent systemic metastatic disease[6,21].
The optimal timing and sequence of surgical resection for sCRLM has been a topic of much debate. The timing of when to undergo a “classic”, “simultaneous” or “liver-first” approach remains controversial[22]. Following the EORTC trial[23], many centers still favor the classical approach. The rationale for this approach was that the colorectal primary tumor was the usual source of symptoms and thus should be removed first[24]. Recent studies have demonstrated that a primary resection in patients with metastatic colorectal cancer significantly increased the 30 d mortality by 10% when compared with a non-metastatic setting[25]. Therefore, a CRLM might progress beyond resectability during the primary tumor resection (especially in patients with postoperative complications after the colorectal resection).
In the past decade, a simultaneous resection for sCRLMs has been performed more often. The strategy for the simultaneous resection was to avoid missing the surgical opportunity[26]. Equivalent perioperative morbidity and mortality and survival outcomes were achieved if the colorectal resection was combined with a minor hepatic resection[19,27]. Compared with a staged resection, a simultaneous resection in patients was accompanied with much milder complications[28]. Thus, a simultaneous resection was preferred in highly selected patients[29,30].
The alternative paradigm for the management of sCRLMs is the reverse, or so-called liver-first approach. This modern procedure has evolved as a result of the increasing complexity of care of primary colorectal cancer with the development of preoperative chemoradiotherapy and colonic stenting[31]. It allows the ability to first control the CRLM and optimizes the chance of a potentially curative hepatic resection, which improves the long-term survival in these patients[32]. The approach also evaluates the biological behavior of the neoplasm, treats the occult disease and avoids an operation in patients with rapidly progressing tumors[33].
De Rosa et al[34] summarized the indications for the liver-first approach or patients with a high or low liver disease burden with a locally advanced primary tumor. In fact, the ideal patient is likely to be someone who has advanced synchronous liver metastatic disease and rectal cancer[35]. In our study, 12 patients had locally advanced liver metastases and 4 patients had initially unresectable liver tumors. All of them had a high liver disease burden, which was largely in accordance with the attitude of van der Pool et al[27] who reported that the appropriate patients for the liver-first approach had a heavier tumor size, diameter and distribution for liver disease burden.
Knowledge of the natural history and pattern of metastatic dissemination in patients with colorectal cancer has revolutionized the understanding and management of this disease. It may be more appropriate to first use chemotherapy to provide early systemic treatment[18]. Current evidence indicates that colorectal cancer is a chemosensitive disease. Thus, it is logical to start early systemic treatment[31,36]. Additionally, in patients with a high liver tumor burden, it is crucial to control the disease with down-staging chemotherapy[37].
Generally, candidates for the liver-first approach include those with a heavy liver disease burden and/or required down-staging therapy with a hepatic resection containing more than three segments.
The liver is the most common organ for distant metastases from colorectal cancer. Up to 15%-42% of patients present with synchronous colorectal liver metastases (sCRLMs) at the time of a primary cancer diagnosis. However, a standard surgical approach for sCRLM remains undetermined. There were three surgical strategies, including the traditional or classic resection, liver-first resection and simultaneous resection. In this study, the authors retrospectively analyzed the feasibility and survival outcome of the liver-first approach.
In fact, liver metastases define the prognosis of CRLM patients. The liver-first approach was performed and compared with patient outcomes in the last decades. There were only 4 studies that analyzed the feasibility and survival outcomes of the approach.
Based on present results and daily work experience, the liver-first approach is an appropriate surgical strategy for sCRLM patients with a high liver disease burden. We proposed exact indications for the liver-first approach.
The candidates for the liver-first approach included those with a heavy liver disease burden and/or who required down-staging therapy with a hepatic resection containing more than three segments.
The classic approach is a primary cancer resection followed by a hepatic resection. The liver-first approach is a colorectal liver metastases hepatic resection followed by a primary cancer resection. The simultaneous approach is a resection for a primary cancer and liver metastasis that is performed simultaneously.
This is an interesting study about the liver first approach for synchronous colorectal liver metastases.
P- Reviewer: Sharma Ricky A S- Editor: Yu J L- Editor: Roemmele A E- Editor: Wang CH
| 1. | Thelen A, Jonas S, Benckert C, Schumacher G, Lopez-Hänninen E, Rudolph B, Neumann U, Neuhaus P. Repeat liver resection for recurrent liver metastases from colorectal cancer. Eur J Surg Oncol. 2007;33:324-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Norstein J, Silen W. Natural history of liver metastases from colorectal carcinoma. J Gastrointest Surg. 1997;1:398-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Blumgart LH, Allison DJ. Resection and embolization in the management of secondary hepatic tumors. World J Surg. 1982;6:32-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Chua TC, Saxena A, Chu F, Zhao J, Morris DL. Predictors of cure after hepatic resection of colorectal liver metastases: an analysis of actual 5- and 10-year survivors. J Surg Oncol. 2011;103:796-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Pulitanò C, Castillo F, Aldrighetti L, Bodingbauer M, Parks RW, Ferla G, Wigmore SJ, Garden OJ. What defines ‘cure’ after liver resection for colorectal metastases? Results after 10 years of follow-up. HPB (Oxford). 2010;12:244-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 6. | Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, Kemeny N, Brennan MF, Blumgart LH, D’Angelica M. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575-4580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 894] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 7. | Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, Lillemoe KD, Yeo CJ, Cameron JL. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1105] [Cited by in RCA: 1118] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 8. | Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, Hess K, Curley SA. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818-825; discussion 825-827. [PubMed] |
| 9. | Law WL, Choi HK, Lee YM, Ho JW. The impact of postoperative complications on long-term outcomes following curative resection for colorectal cancer. Ann Surg Oncol. 2007;14:2559-2566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 186] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 10. | Reddy SK, Pawlik TM, Zorzi D, Gleisner AL, Ribero D, Assumpcao L, Barbas AS, Abdalla EK, Choti MA, Vauthey JN. Simultaneous resections of colorectal cancer and synchronous liver metastases: a multi-institutional analysis. Ann Surg Oncol. 2007;14:3481-3491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 297] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 11. | Mentha G, Majno PE, Andres A, Rubbia-Brandt L, Morel P, Roth AD. Neoadjuvant chemotherapy and resection of advanced synchronous liver metastases before treatment of the colorectal primary. Br J Surg. 2006;93:872-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 236] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 12. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12751] [Cited by in RCA: 13076] [Article Influence: 523.0] [Reference Citation Analysis (0)] |
| 13. | Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309-318; discussion 318-321. [PubMed] |
| 14. | Bismuth H. Surgical anatomy and anatomical surgery of the liver. World J Surg. 1982;6:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 465] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 15. | Zhan T, Gu J, Li M, Du C. Intermediate-fraction neoadjuvant radiotherapy for rectal cancer. Dis Colon Rectum. 2013;56:422-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24779] [Article Influence: 1180.0] [Reference Citation Analysis (0)] |
| 17. | Verhoef C, van der Pool AE, Nuyttens JJ, Planting AS, Eggermont AM, de Wilt JH. The “liver-first approach” for patients with locally advanced rectal cancer and synchronous liver metastases. Dis Colon Rectum. 2009;52:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Mentha G, Roth AD, Terraz S, Giostra E, Gervaz P, Andres A, Morel P, Rubbia-Brandt L, Majno PE. ‘Liver first’ approach in the treatment of colorectal cancer with synchronous liver metastases. Dig Surg. 2008;25:430-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Brouquet A, Mortenson MM, Vauthey JN, Rodriguez-Bigas MA, Overman MJ, Chang GJ, Kopetz S, Garrett C, Curley SA, Abdalla EK. Surgical strategies for synchronous colorectal liver metastases in 156 consecutive patients: classic, combined or reverse strategy? J Am Coll Surg. 2010;210:934-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 20. | Lam VW, Laurence JM, Pang T, Johnston E, Hollands MJ, Pleass HC, Richardson AJ. A systematic review of a liver-first approach in patients with colorectal cancer and synchronous colorectal liver metastases. HPB (Oxford). 2014;16:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | de Jong MC, van Dam RM, Maas M, Bemelmans MH, Olde Damink SW, Beets GL, Dejong CH. The liver-first approach for synchronous colorectal liver metastasis: a 5-year single-centre experience. HPB (Oxford). 2011;13:745-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Brouquet A, Nordlinger B. Surgical strategies to synchronous colorectal liver metastases. Dig Dis. 2012;30 Suppl 2:132-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1478] [Cited by in RCA: 1441] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 24. | Bismuth H, Castaing D, Traynor O. Surgery for synchronous hepatic metastases of colorectal cancer. Scand J Gastroenterol Suppl. 1988;149:144-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Stillwell AP, Buettner PG, Siu SK, Stitz RW, Stevenson AR, Ho YH. Predictors of postoperative mortality, morbidity, and long-term survival after palliative resection in patients with colorectal cancer. Dis Colon Rectum. 2011;54:535-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Ihnát P, Vávra P, Zonča P. Treatment strategies for colorectal carcinoma with synchronous liver metastases: Which way to go? World J Gastroenterol. 2015;21:7014-7021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | van der Pool AE, de Wilt JH, Lalmahomed ZS, Eggermont AM, Ijzermans JN, Verhoef C. Optimizing the outcome of surgery in patients with rectal cancer and synchronous liver metastases. Br J Surg. 2010;97:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Feng Q, Wei Y, Zhu D, Ye L, Lin Q, Li W, Qin X, Lyu M, Xu J. Timing of hepatectomy for resectable synchronous colorectal liver metastases: for whom simultaneous resection is more suitable--a meta-analysis. PLoS One. 2014;9:e104348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Martin RC, Augenstein V, Reuter NP, Scoggins CR, McMasters KM. Simultaneous versus staged resection for synchronous colorectal cancer liver metastases. J Am Coll Surg. 2009;208:842-850; discussion 850-852. [PubMed] |
| 30. | Hillingsø JG, Wille-Jørgensen P. Staged or simultaneous resection of synchronous liver metastases from colorectal cancer--a systematic review. Colorectal Dis. 2009;11:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Rödel C. Radiotherapy: Preoperative chemoradiotherapy for rectal cancer. Nat Rev Clin Oncol. 2010;7:129-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Pawlik TM, Schulick RD, Choti MA. Expanding criteria for resectability of colorectal liver metastases. Oncologist. 2008;13:51-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 326] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 33. | Lambert LA, Colacchio TA, Barth RJ. Interval hepatic resection of colorectal metastases improves patient selection. Arch Surg. 2000;135:473-479; discussion 479-480. [PubMed] |
| 34. | De Rosa A, Gomez D, Brooks A, Cameron IC. “Liver-first” approach for synchronous colorectal liver metastases: is this a justifiable approach? J Hepatobiliary Pancreat Sci. 2013;20:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Punt CJ. New options and old dilemmas in the treatment of patients with advanced colorectal cancer. Ann Oncol. 2004;15:1453-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 36. | Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pintér T, Lim R, Bodoky G. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2901] [Cited by in RCA: 3122] [Article Influence: 195.1] [Reference Citation Analysis (1)] |
| 37. | Castellanos JA, Merchant NB. Strategies for Management of Synchronous Colorectal Metastases. Curr Surg Rep. 2014;2:62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |