Published online Feb 7, 2016. doi: 10.3748/wjg.v22.i5.1779
Peer-review started: July 30, 2015
First decision: August 31, 2015
Revised: October 9, 2015
Accepted: December 8, 2015
Article in press: December 8, 2015
Published online: February 7, 2016
Processing time: 181 Days and 14.2 Hours
Endoscopic ultrasonography (EUS) is a technique with an established role in the diagnosis and staging of gastro-intestinal tumors. In recent years, the spread of new devices dedicated to tissue sampling has improved the diagnostic accuracy of EUS fine-needle aspiration. The development of EUS-guided drainage of the bilio-pancreatic region and abdominal fluid collections has allowed EUS to evolve into an interventional tool that can replace more invasive procedures. Emerging techniques applying EUS in pancreatic cancer treatment and in celiac neurolysis have been described. Recently, confocal laser endomicroscopy has been applied to EUS as a promising technique for the in vivo histological diagnosis of gastro-intestinal, bilio-pancreatic and lymph node lesions. In this state-of-the-art review, we report the most recent data from the literature regarding EUS devices, interventional EUS, EUS-guided confocal laser endomicroscopy and EUS pancreatic cancer treatment, and we also provide an overview of their principles, clinical applications and limitations.
Core tip: The aim of this review is to report the most up-to-date advances and cutting-edge technologies in the field of interventional endoscopic ultrasonography (EUS) and EUS-guided confocal laser endomicroscopy.
- Citation: De Lisi S, Giovannini M. Endoscopic ultrasonography: Transition towards the future of gastro-intestinal diseases. World J Gastroenterol 2016; 22(5): 1779-1786
- URL: https://www.wjgnet.com/1007-9327/full/v22/i5/1779.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i5.1779
Data published in the literature regarding endoscopic ultrasonography (EUS) has described the high accuracy of the detection and staging of malignancies of the gastro-intestinal tract. In the setting of pancreatic lesions, pancreatic cancer exhibits high mortality due to its late diagnosis. EUS has been identified as the most sensitive technique for the early detection of pancreatic cancer compared with traditional imaging methods such as magnetic resonance imaging and computed tomography[1].
The possibility of performing tissue sampling has improved the diagnostic capability of EUS, former known as cytological diagnosis. In recent years, new needles have been developed to enable histological diagnosis. More recently, EUS-elastography and EUS contrast enhancement have emerged; these techniques provide a real-time evaluation of tissue stiffness and enhancement patterns to enable differential diagnoses between benign and malignant lesions, thus improving the targeting of tissue sampling. A recent review discussed their role in the diagnosis of gastro-intestinal lesions[2], although this report is beyond the scope of the present paper.
The role of EUS has progressively changed from a purely diagnostic technique to an interventional tool, especially in the setting of bilio-pancreatic drainage after failure of standard endoscopic retrograde cholangiopancreatography (ERCP), where EUS-guided biliary drainage represents a viable alternative to surgical or radiological procedures.
Confocal endomicroscopy offers the possibility of making in vivo histological diagnoses, and its application in EUS enhances the performance of the technique mainly in the setting of pancreatic and lymph node lesions.
The aim of this review is to report the most up-to-date innovations in the setting of interventional EUS, EUS-guided confocal laser endomicroscopy (CLE) and EUS-pancreatic cancer treatment. We performed a computerized bibliographic search on MEDLINE for studies published between January 2013 and April 2015. The primary search terms were as follows: “EUS”, “EUS AND confocal microscopy”, “EUS AND fine needle aspiration”, “EUS-guided biliary drainage”, and “pancreatic cancer treatment”. Because the purpose of our study is to describe the latest innovations and cutting-edge technologies, all relevant articles were included regardless of their design or sample size. Additionally, pertinent abstracts from major gastroenterology meetings were included.
EUS-guided confocal laser endomicroscopy (CLE) is a promising novel technique that allows for real-time optical biopsy during EUS examination. A confocal miniprobe (Cellvizio AQ Flex™ 19 nCLE probe-Mauna Kea Techn., Paris, France) compatible with 19-G needles has been combined with EUS-FNA for the evaluation of cystic and solid lesions in a technique termed “needle-based CLE” (nCLE).
The principles and techniques of CLE and nCLE have been well described elsewhere[3-6]. Because confocal images depend on fluorescence, a fluorescent dye is required to make objects visible. For nCLE, intravenous fluorescein sodium (10%) is primarily used.
In the setting of pancreatic cystic neoplasms (PCNs), nCLE provides real-time microscopic images of the cyst wall. In a multicenter study, the INSPECT trial, a preliminary unblinded consensus review for the definition of nCLE images and case revision with a gastrointestinal pathologist were performed. Subsequently, a blinded consensus review assessed whether the criteria for nCLE images could identify PCNs or adenocarcinoma[7]. nCLE was performed in 65 patients and exhibited a diagnostic yield of 41.9%, which is greater than either a carcinoembryonic antigen (CEA) level > 192 ng/mL (28.6%) or cytology results consistent with PCN (29.6%). The presence of epithelial villous structures was significantly associated with PCN with a specificity of 100%, although sensitivity was low (59%), and the negative predictive value was 50%[7]. In the recent prospective DETECT study, nCLE was combined with cystoscopy using the SpyGlass fiberoptic probe (Boston Scientific, Natick, Mass) for the diagnosis of PCNs[8].
In a sub-group of 18 patients with high probability of PCNs (“high-certainty patients”), cystoscopy and nCLE reported sensitivities of 90% and 80%, respectively; the combination of the two methods reached a sensitivity of 100% in the clinical diagnosis of mucinous cysts. In all 30 patients enrolled in the study, the sensitivities of cystoscopy (71%) and nCLE (77%) were not as high as in high-certainty patients, whereas the accuracy of their combination was 93%. However, both cystoscopy and nCLE exhibited higher sensitivity and accuracy than CEA levels of 33% and 61%, respectively, in the entire study population.
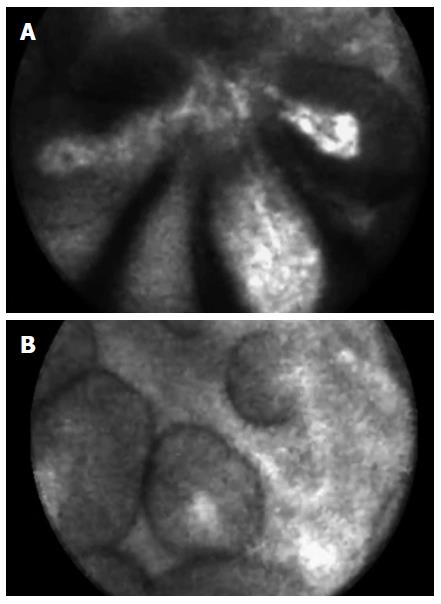
The detection of papillary, finger-like projections and, when imaged in cross section, dark rings with a central core on nCLE, both represent the villous pattern suggestive for gastric and intestinal-type intraductal papillary mucinous neoplasm (IPMN), as previously reported[9] (Figure 1).
The final diagnosis was established by pathology, but only two patients underwent surgery in this cohort, limiting the diagnostic value of the study. In the remaining patients, a final clinical diagnosis was established by blinded review between two independent endosonographers. nCLE was associated with a higher complication rate (7%-9%)[7,8] than that reported for EUS-FNA of pancreatic cysts[10,11].
The larger caliber of the needle employed (19 G vs 22 G), the longer duration of examination and the greater number of needle movements within the cysts compared with standard EUS-FNA can explain these findings. Limiting both the needle dwelling time and the number of needle movements can reduce the risk of pancreatitis and intracystic bleeding. An nCLE diagnostic criterion for the diagnosis of serous cystadenoma (SCA) was recently proposed in a multicenter prospective study of 31 patients with a single pancreatic cyst[12].
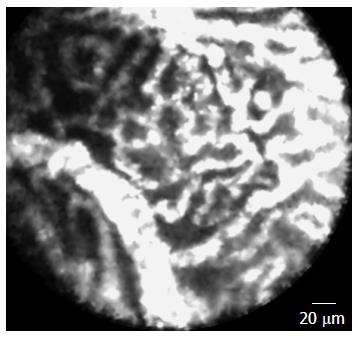
The criterion standard for the final diagnosis was based on either a surgical specimen and/or positive cytopathology or consensus among blinded investigators. A typical finding of nCLE in the clinical diagnosis of SCA was a superficial vascular network (Figure 2) that corresponded to subepithelial capillary vascularization on a pathological specimen that was only observed in SCA.
This feature exhibited a sensitivity of 69%, whereas the specificity and the positive predictive value reached 100 % for the diagnosis of SCA. Good inter-observer agreement (κ = 0.77) was reported.
From a procedural standpoint, no technical failures were reported, even for SCA located in the pancreatic head, which exhibited a mean nCLE procedure duration of 7 min (range 3-10 min).
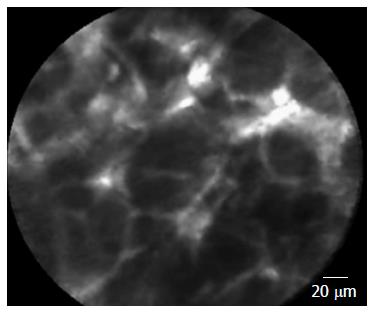
If confirmed in a larger setting, this nCLE criterion could improve the EUS diagnosis of SCA in a rapid real-time manner that avoids the burden and costs of unnecessary follow-up and surgery, which was required in 60% of the 2622 patients with serous cystic neoplasm in a large multicenter study due to doubtful preoperative diagnosis[13]. Available data regarding nCLE of solid pancreatic lesions revealed irregular vessels with vascular leakage of fluorescein into the tumor and large dark clumps, which correspond to lumps of malignant cells in pancreatic adenocarcinomas[6,7] (Figure 3).
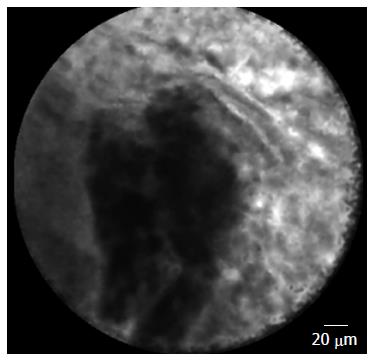
Conversely, the visualization of normal pancreatic parenchyma by nCLE demonstrated a “coffee bean” pattern with regular vessels[6] (Figure 4). nCLE imaging of inflammatory lymph nodes revealed diffuse small cells in a homogeneous stroma with normal vascularization in the lymph node; conversely, malignant lymph nodes were characterized by glandular structures with dark cells, large dark clumps and considerable neo-vascularization with huge leakage of fluorescein[6,9] (Figure 5).
Although the results reported regarding solid lesions are based on case series, they are encouraging, and it is expected that these studies will be confirmed by an ongoing international multicenter study (CONTACT study) on nCLE of cystic and solid pancreatic lesions and lymph nodes.
EUS fine-needle aspiration (EUS-FNA) is a safe technique for the tissue acquisition of gastro-intestinal lesions and exhibits diagnostic accuracy exceeding 80%[14-16]. The primary limitation of EUS-FNA is the provision of cytologic samples, which can affect its accuracy, especially for the diagnosis of pancreatic cancer or the differential diagnosis of benign lesions that mimic other conditions (e.g., chronic or autoimmune pancreatitis). To overcome this limitation, an EUS Tru-Cut biopsy 19-gauge needle has been developed, although its expected results have not been achieved due to its stiffness, which precludes trans-duodenal sampling[17].
In recent years, new reverse-bevel biopsy needles for core tissue have been made available in multiple gauges (ProCore; Cook Medical, Winston-Salem, NC) with diagnostic accuracy > 85% reported in two multicenter prospective studies[18,19]; moreover, EUS-FNB was demonstrated to be technically feasible in 98% of patients with a solid pancreatic mass, thus demonstrating the good performance of this needle when using a trans-duodenal approach[19].
In the setting of pancreatic cystic lesions, a new EUS-guided fine-needle biopsy equipped with a side fenestration (EchoTip ProCore High Definition Ultrasound Biopsy Needle, Cook Endoscopy Inc., Limerick, Ireland) was employed in a recent study. The results described a safe technique with high diagnostic yield (over 90%), even if the lack of a control group affected these findings[20].
A novel method for EUS-guided tissue acquisition has been recently reported. As in standard endoscopy, a biopsy forceps, fit for passing through a standard 19-gauge FNA needle, has been developed. The preliminary reports indicate that this new device is able to safely acquire adequate tissue samples for histologic assessment in both the animal and human settings[21,22].
A similar technique for histopathological diagnosis of submucosal lesions has been reported in an abstract by Wang et al[23]. A definite histological diagnosis was reached using an EUS-guided “deep tunneling” forceps biopsy technique in all 11 cases and with no complications.
In the setting of pancreatic solid lesions, Mohammad Alizadeh et al[24] compared EUS-FNA performed with negative pressure, applied with a 10 mL syringe, to EUS-FNA without suctioning and stylet in a total of 100 patients. Although no statistically significant differences were found between the two methods, EUS-FNA without negative pressure and stylet technique was related with less blood contamination (20% vs 50%) and higher diagnostic yield (14% vs 6%) than FNA performed with suction[24].
To improve the quality of the EUS-FNA of solid lesions, Attam et al[25] recently proposed the wet suction technique (WEST) as follows: a 22-gauge needle was filled with small amount of saline before puncturing the lesion with high suction. In their randomized controlled trial, the WEST technique yielded significantly higher cellularity and specimen adequacy in cell blocks compared with the conventional FNA technique with no difference in the amount of blood contamination.
The refinements in EUS-assisted devices, in conjunction with the emerging techniques in tissue acquisition, have improved EUS diagnostic capabilities.
Since the first report of EUS-guided biliary drainage (EUS-BD) for biliary decompression after the failure of conventional drainage[26], the technique has evolved rapidly. The consensus guidelines for the management of biliary obstruction using the various technical approaches of EUS-BD have recently been published following a consortium meeting held in 2012[27].
In a large multicenter retrospective cohort, EUS-guided extrahepatic (EH) and intrahepatic (IH) approaches were compared in benign and malignant disease[28].
No significant differences in the success rates of the EH and IH methods were found (84.3% vs 90.4%, P = 0.15), whereas superior performance was described for malignant diseases than for benign indications (90.2% vs 77.3%, P = 0.02). The complication rate remained high, although there were no differences between the IH and the EH approaches (32.6% vs 35.6%, P = 0.64). Overall complications were: pneumoperitoneum (5%), bleeding (11%), bile leak/peritonitis (10%), and cholangitis (5%). Similar rates of adverse events were observed in benign and malignant diseases (26.7% vs 37.1%, P = 0.19).
Dhir et al[29] retrospectively compared the outcomes of ERCP and EUS-BD in the stenting of 208 patients with malignant distal biliary obstruction. The main outcome measurement was composite success, defined by the combination of technical success (stent placement) and functional success (a > 50% decrease in serum bilirubin levels at 2 wk post-procedure). Although the overall results of ERCP and EUS-BD were similar, EUS-BD exhibited significantly higher composite success than the standard approach in a group of patients without duodenal stenosis who failed ERCP due to difficult biliary access (93.9% vs 78.3%, P = 0.002). As expected, the difference was dramatically higher in the sub-group of patients with duodenal stenosis (90.5% vs 57.1%, P = 0.0003).
All adverse events recorded in this study were managed conservatively. No pancreatitis was observed in patients who underwent EUS-BD, whereas 15.7% of patients in the ERCP group with difficult biliary access developed this complication (P = 0.024), scored as mild in four patients and as moderate in one patients. No statistically significant differences were observed for the other adverse events between the two treatment methods. Bleeding occurred in three patients (2 in the EUS-BD group; 1 in the ERCP group). Cholangitis was recorded in four patients (1 in the in the EUS-BD group; 3 in the ERCP group). A minor bile leak was observed in three patients in the EUS-BD group. In 2 patients with duodenal stenosis in the EUS-BD group, a perforation occurred after the dilation of choledochoduodenostomy tract by using a precut papillotome[29].
EUS-guided hepaticojejunostomy combined with EUS-guided antegrade stent placement using a fine-gauge delivery system has been recently described in a patient who developed obstruction of the proximal bile duct after gastrectomy with a Roux-en-Y anastomosis. After puncturing the intrahepatic bile duct using a 19-gauge needle from the jejunum, a 7F tapered ERCP catheter was inserted and advanced a guide wire into the intestine across the site of bile duct stenosis. A fine-gauge delivery system (6F) was inserted, followed by antegrade placement of the metal stent (Zilver 635, Cook Medical, Bloomington, IN, USA). Finally, EUS-guided hepaticojejunostomy (Niti-S biliary covered stent) was performed without adverse events. This technique reduces the risk of bile leakage because the small caliber of the delivery system does not require dilation of the fistula, and cases of reintervention for dysfunctional stents can be performed easily through the hepaticojejunostomy stent[30].
The same author reported a similar technique for endoscopic ultrasound-guided hepaticogastrostomy (EUS-HGS). In a pilot study enrolling 12 patients, EUS-HGS was performed with EUS-guided antegrade stenting using a novel uncovered metallic stent with a fine-gauge delivery system. The procedure was feasible in all patients without dilation of the fistula between the stomach and the intrahepatic bile duct, which prevented adverse events. Indeed, no bile peritonitis or stent dysfunction occurred during the follow-up (mean 122 d, range 62 - 210 d)[31].
A novel method of EUS-HGS was proposed in a retrospective series of 20 consecutive patients with obstructive jaundice who underwent EUS-BD after failed ERCP. Thirteen patients underwent EUS-HGS with the locking stent method using end-bare covered metallic stents (EBCMS). No difference was reported in technical and functional success rates (100% in both groups). In two of the seven patients treated with conventional EUS-HGS, stent migration occurred, whereas no stent dysfunction was observed in patients who underwent the procedure with the locking stent method[32]. The locking stent method was recently reported to be feasible and safe in four cases of patients with hepatic hilar obstruction and isolated right intrahepatic bile ducts[33].
The technical success of EUS-BD exceeded 90% in all reported studies, with negligible adverse events. However, the retrospective design, small sample size, patient selection bias and the use of a single operator within tertiary centers all affect the external validity of these new techniques. Prospective, multicenter, well-designed, randomized trials with long-term follow-up are required to validate these techniques for the treatment of intrahepatic and distal biliary obstruction.
EUS-guided drainage of pancreatic fluid collections (PFC) and pseudocysts has been widely described[34,35], demonstrating the technique is effective for surgical drainage while resulting in shorter hospital stays and lower costs in a randomized trial of 40 patients[36].
In the setting of PFC, a prospective, multicenter study employing a novel self-expanding metal stent (SEMS) designed specifically for transmural drainage has recently been published[37]. Sixty-one patients with symptomatic PFCs were enrolled; 46 patients had walled-off necrosis (WON), and 15 had a pancreatic pseudocyst. A large-diameter (10 mm) SEMS with bilateral flanges, the AXIOS stent (Xlumena Inc., Mountain View, California, USA), was placed with technical success in 60 patients (98%). The second study endpoint was clinical success, which was defined as the resolution of clinical symptoms in combination with a decrease in the PFC size to ≤ 2 cm on imaging, without the need for placement of an additional endoscopic or percutaneous stent or drain or surgery. In this study, 81% of patients with WON and 93% of patients with a pancreatic pseudocyst achieved clinical success. Treatment failure occurred in nine patients (16%), including four patients who required surgical intervention. Endoscopic stent removal was easily performed in 47 of 57 patients (82%) after a median of 32 d using a snare or rat-tooth forceps. In 10 patients, stent removal was not performed because of migration of the stent (n = 3), stent dislodgement during necrosectomy (n = 3), removal during surgery (n = 2), and refusal by the patient (n = 2). Stent migration was observed during the follow-up without symptoms. Severe adverse events occurred in five patients (9%); these consisted of PFC infection (n = 4) and perforation (n = 1).
This prospective study represents the largest available study of EUS-guided SEMS placement for the transmural drainage of PFCs, which confirms the feasibility and efficacy of the technique. However, to the study is limited by the lack of a control group, selection bias and no planned long-term follow-up. Finally, improvements in stent design are desirable to improve its anchoring capacity to reduce the risk of stent migration.
The role of EUS in the local treatment of pancreatic cancer and for pain control through the celiac plexus neurolysis (CPN) has been extensively reviewed[38,39].
The available data regarding the topic are promising, although most studies rely on case series and therapeutic procedures that remain experimental.
The first prospective study evaluating EUS-guided pancreatic fiducial placement with a 22-gauge needle was recently published[40]. EUS-guided gold fiducials were successfully inserted in all 23 patients before image-guided radiation therapy (IGRT), and this was possible in 95.2% of patients. Only one adverse event occurred; this consisted of transient bleeding at the site of needle insertion. Although these findings confirm the feasibility of fiducial placement even with a 22-G needle, no data about the patients follow-up and treatment outcomes were reported in this study; only two patients did not receive radiotherapy due to a rapid disease progression[40].
An interesting single center prospective study compared circulating tumor cells (CTCs) in the portal vein to CTCs in the peripheral blood of 18 patients with suspected pancreatico-biliary cancer[41]. The assessment of CTCs in the peripheral blood is limited in early-stage disease.
Portal vein sampling was performed by aspiration of a small amount of blood (7.5 mL) with a 19-G EUS-FNA needle, peripheral blood samples were obtained prior to EUS.
All blood samples were subjected to identical processation to identify cells with morphological and immunocytochemical characteristics consistent with epithelial cells: epithelial cell adhesion molecule positive (EpCAM+), 4′,6-diamidino-2-phenylindole positive (DAPI+), cytokeratin 8/18 and or 19 positive and CD45 negative (CD45-). With flow cytometry CTC isolation, the identified cells were collected as CTCs and underwent genomic (KRAS sequencing) and proteomic (P16, SMAD4 and P53) analyses.
Detection of CTCs in the portal vein sampling was significantly higher than in the peripheral blood in all 18 patients (mean CTCs 111.8 vs 0.7, P < 0.01). No adverse events from EUS-guided portal vein sampling were observed.
This study can be considered to be the first EUS application to translational medicine; if confirmed, this approach can be helpful for the early diagnosis of pancreatic cancer[41].
In the setting of CPN, an evolution of the standard procedure is EUS-guided celiac ganglia neurolysis (EUS-CGN), in which a neurolytic agent is directly injected into an individual celiac ganglion[42].
In a randomized controlled trial, 68 patients with pancreatic cancer underwent EUS-CPN or EUS-CGN. The procedures were both technically successful in all patients. EUS-CGN achieved a postoperative reduction in pain score that was significantly greater (3.9 ± 2.4) than in the EUS–CPN group (2.7 ± 2.4, P = 0.044). Moreover, EUS-CGN was shown to be superior to EUS-CPN in achieving a complete response (pain score ≤ 1): (50.0% vs 18.2%, P = 0.010). No differences between the two groups were observed with respect to adverse events or the duration of pain relief[43].
A recent retrospective case-control study evaluated the impact of celiac neurolysis on survival in patients with pancreatic cancer. EUS-CPN was associated with longer survival compared with non-EUS approaches, and those who underwent EUS-CPN had longer survival than those who underwent EUS-CGN[44]. Due to the retrospective design of the study, these findings should be confirmed by prospective studies.
A mixture (7% phenol and 60% glycerol) was employed as a new neurolytic agent through a 22-G EUS-FNA needle for EUS-CPN. Because of its higher viscosity relative to ethanol, the author hypothesized that the agent would remain localized around the celiac plexus to provide better pain relief. In 8 of the 9 prospectively enrolled patients, a positive response (89%) without severe adverse events was achieved. The median duration of pain relief was 19.1 wk. Although this case series was limited by the small sample size and lacks a control group, these preliminary results are promising with respect to the improvement of the EUS-CPN technique[45].
Although the latest advances in interventional EUS require a high level of skills both in EUS and interventional endoscopy and although their execution remains limited to tertiary centers, these advances portend a future where EUS can replace current invasive techniques, especially in the setting of abdominal drainages. nCLE improves EUS diagnostic performance with the desirable aim of replacing histology in the discrimination of the nature of pancreatic lesions, thus avoiding unnecessary surgery for benign lesions.
Finally, the role of EUS in the treatment and early biological diagnosis of pancreatic cancer could open new research opportunities and impact patient care in a meaningful manner.
P- Reviewer: Sun SY, Tomizawa M, Yamagata M S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Canto MI, Hruban RH, Fishman EK, Kamel IR, Schulick R, Zhang Z, Topazian M, Takahashi N, Fletcher J, Petersen G. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142:796-804; quiz e14-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 487] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 2. | Meng FS, Zhang ZH, Ji F. New endoscopic ultrasound techniques for digestive tract diseases: A comprehensive review. World J Gastroenterol. 2015;21:4809-4816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Kiesslich R, Goetz M, Vieth M, Galle PR, Neurath MF. Confocal laser endomicroscopy. Gastrointest Endosc Clin N Am. 2005;15:715-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Giovannini M. Endoscopic ultrasound-guided confocal microscopy: a new tool for the new year? Endosc Ultrasound. 2013;2:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Peter S, Bang JY, Mönkemuller K, Varardarajulu S, Wilcox CM. Endomicroscopy of the pancreaticobiliary system. Diagn Ther Endosc. 2013;2013:310105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Giovannini M, Caillol F, Poizat F, Bories E, Pesenti C, Monges G, Raoul JL. Feasibility of Intratumoral Confocal Microscopy under Endoscopic Ultrasound Guidance. Endosc Ultrasound. 2012;1:80-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Konda VJ, Meining A, Jamil LH, Giovannini M, Hwang JH, Wallace MB, Chang KJ, Siddiqui UD, Hart J, Lo SK. A pilot study of in vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy under endosonographic guidance. Endoscopy. 2013;45:1006-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 8. | Nakai Y, Iwashita T, Park do H, Samarasena JB, Lee JG, Chang KJ. Diagnosis of pancreatic cysts: EUS-guided, through-the-needle confocal laser-induced endomicroscopy and cystoscopy trial: DETECT study. Gastrointest Endosc. 2015;81:1204-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 9. | Konda VJ, Aslanian HR, Wallace MB, Siddiqui UD, Hart J, Waxman I. First assessment of needle-based confocal laser endomicroscopy during EUS-FNA procedures of the pancreas (with videos). Gastrointest Endosc. 2011;74:1049-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 10. | de Jong K, Poley JW, van Hooft JE, Visser M, Bruno MJ, Fockens P. Endoscopic ultrasound-guided fine-needle aspiration of pancreatic cystic lesions provides inadequate material for cytology and laboratory analysis: initial results from a prospective study. Endoscopy. 2011;43:585-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087-1095. [PubMed] |
| 12. | Napoléon B, Lemaistre AI, Pujol B, Caillol F, Lucidarme D, Bourdariat R, Morellon-Mialhe B, Fumex F, Lefort C, Lepilliez V, Palazzo L, Monges G, Filoche B, Giovannini M. A novel approach to the diagnosis of pancreatic serous cystadenoma: needle-based confocal laser endomicroscopy. Endoscopy. 2015;47:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Jais B, Rebours V, Malleo G, Salvia R, Fontana M, Maggino L, Bassi C, Manfredi R, Moran R, Lennon AM. Serous cystic neoplasm of the pancreas: a multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas). Gut. 2016;65:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 214] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 14. | Puli SR, Bechtold ML, Buxbaum JL, Eloubeidi MA. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass?: A meta-analysis and systematic review. Pancreas. 2013;42:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 279] [Article Influence: 23.3] [Reference Citation Analysis (1)] |
| 15. | Puli SR, Batapati Krishna Reddy J, Bechtold ML, Ibdah JA, Antillon D, Singh S, Olyaee M, Antillon MR. Endoscopic ultrasound: it’s accuracy in evaluating mediastinal lymphadenopathy? A meta-analysis and systematic review. World J Gastroenterol. 2008;14:3028-3037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Polkowski M, Larghi A, Weynand B, Boustière C, Giovannini M, Pujol B, Dumonceau JM. Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline. Endoscopy. 2012;44:190-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 216] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 17. | Varadarajulu S, Fraig M, Schmulewitz N, Roberts S, Wildi S, Hawes RH, Hoffman BJ, Wallace MB. Comparison of EUS-guided 19-gauge Trucut needle biopsy with EUS-guided fine-needle aspiration. Endoscopy. 2004;36:397-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 167] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Iglesias-Garcia J, Poley JW, Larghi A, Giovannini M, Petrone MC, Abdulkader I, Monges G, Costamagna G, Arcidiacono P, Biermann K. Feasibility and yield of a new EUS histology needle: results from a multicenter, pooled, cohort study. Gastrointest Endosc. 2011;73:1189-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 232] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 19. | Larghi A, Iglesias-Garcia J, Poley JW, Monges G, Petrone MC, Rindi G, Abdulkader I, Arcidiacono PG, Costamagna G, Biermann K. Feasibility and yield of a novel 22-gauge histology EUS needle in patients with pancreatic masses: a multicenter prospective cohort study. Surg Endosc. 2013;27:3733-3738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Barresi L, Tarantino I, Fabbri C, Granata A, Curcio G, Azzopardi N, Liotta R, Cennamo V, Traina M. Technique of FNA and biopsy by using a needle with side fenestration in pancreatic cystic lesions. Gastrointest Endosc. 2014;80:897-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Samarasena JB, Nakai Y, Shinoura S, Lee JG, Chang KJ. EUS-guided, through-the-needle forceps biopsy: a novel tissue acquisition technique. Gastrointest Endosc. 2015;81:225-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Nakai Y, Isayama H, Chang KJ, Yamamoto N, Sasaki T, Kogure H. A pilot study of EUS-guided through-the-needle biopsy (EUS-TTNB) of solid masses. Gastrointest Endosc. 2014;79 Suppl: AB175–AB176. [DOI] [Full Text] |
| 23. | Wang D, Xie J, Soon TE, Jin Z, Li Z. The diagnostic value of EUS-guided “Tunneling” forces biopsy technique for gastrointestinal sub-mucosal lesions. Gastrointest Endosc. 2014;79 Suppl:AB432. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Mohammad Alizadeh AH, Hadizadeh M, Padashi M, Shahbaazi S, Molaee M, Shariatpanahi ZV. Comparison of two techniques for endoscopic ultrasonography fine-needle aspiration in solid pancreatic mass. Endosc Ultrasound. 2014;3:174-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Attam R, Arain MA, Bloechl SJ, Trikudanathan G, Munigala S, Bakman Y, Singh M, Wallace T, Henderson JB, Catalano MF. “Wet suction technique (WEST)”: a novel way to enhance the quality of EUS-FNA aspirate. Results of a prospective, single-blind, randomized, controlled trial using a 22-gauge needle for EUS-FNA of solid lesions. Gastrointest Endosc. 2015;81:1401-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 26. | Giovannini M, Dotti M, Bories E, Moutardier V, Pesenti C, Danisi C, Delpero JR. Hepaticogastrostomy by echo-endoscopy as a palliative treatment in a patient with metastatic biliary obstruction. Endoscopy. 2003;35:1076-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Kahaleh M, Artifon EL, Perez-Miranda M, Gaidhane M, Rondon C, Itoi T, Giovannini M. Endoscopic ultrasonography guided drainage: summary of consortium meeting, May 21, 2012, San Diego, California. World J Gastroenterol. 2015;21:726-741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Gupta K, Perez-Miranda M, Kahaleh M, Artifon EL, Itoi T, Freeman ML, de-Serna C, Sauer B, Giovannini M. Endoscopic ultrasound-assisted bile duct access and drainage: multicenter, long-term analysis of approach, outcomes, and complications of a technique in evolution. J Clin Gastroenterol. 2014;48:80-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 29. | Dhir V, Itoi T, Khashab MA, Park do H, Yuen Bun Teoh A, Attam R, Messallam A, Varadarajulu S, Maydeo A. Multicenter comparative evaluation of endoscopic placement of expandable metal stents for malignant distal common bile duct obstruction by ERCP or EUS-guided approach. Gastrointest Endosc. 2015;81:913-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 30. | Ogura T, Edogawa S, Imoto A, Masuda D, Yamamoto K, Takeuchi T, Inoue T, Uchiyama K, Higuchi K. EUS-guided hepaticojejunostomy combined with antegrade stent placement. Gastrointest Endosc. 2015;81:462-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Ogura T, Masuda D, Imoto A, Takeushi T, Kamiyama R, Mohamed M, Umegaki E, Higuchi K. EUS-guided hepaticogastrostomy combined with fine-gauge antegrade stenting: a pilot study. Endoscopy. 2014;46:416-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Ogura T, Kurisu Y, Masuda D, Imoto A, Hayashi M, Malak M, Umegaki E, Uchiyama K, Higuchi K. Novel method of endoscopic ultrasound-guided hepaticogastrostomy to prevent stent dysfunction. J Gastroenterol Hepatol. 2014;29:1815-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Ogura T, Sano T, Onda S, Imoto A, Masuda D, Yamamoto K, Kitano M, Takeuchi T, Inoue T, Higuchi K. Endoscopic ultrasound-guided biliary drainage for right hepatic bile duct obstruction: novel technical tips. Endoscopy. 2015;47:72-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Seewald S, Ang TL, Kida M, Teng KY, Soehendra N. EUS 2008 Working Group document: evaluation of EUS-guided drainage of pancreatic-fluid collections (with video). Gastrointest Endosc. 2009;69:S13-S21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Varadarajulu S, Christein JD, Tamhane A, Drelichman ER, Wilcox CM. Prospective randomized trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts (with videos). Gastrointest Endosc. 2008;68:1102-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 278] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 36. | Varadarajulu S, Bang JY, Sutton BS, Trevino JM, Christein JD, Wilcox CM. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology. 2013;145:583-90.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 332] [Article Influence: 27.7] [Reference Citation Analysis (1)] |
| 37. | Walter D, Will U, Sanchez-Yague A, Brenke D, Hampe J, Wollny H, López-Jamar JM, Jechart G, Vilmann P, Gornals JB. A novel lumen-apposing metal stent for endoscopic ultrasound-guided drainage of pancreatic fluid collections: a prospective cohort study. Endoscopy. 2015;47:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 38. | Luz LP, Al-Haddad MA, Sey MS, DeWitt JM. Applications of endoscopic ultrasound in pancreatic cancer. World J Gastroenterol. 2014;20:7808-7818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Buscarini E, De Lisi S. Endoscopic Ultrasound of the pancreas. Ultrasonography of the Pancreas Imaging and Pathologic Correlations. Berlin: Springer 2012; 31-45. |
| 40. | Dávila Fajardo R, Lekkerkerker SJ, van der Horst A, Lens E, Bergman JJ, Fockens P, Bel A, van Hooft JE. EUS-guided fiducial markers placement with a 22-gauge needle for image-guided radiation therapy in pancreatic cancer. Gastrointest Endosc. 2014;79:851-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 41. | Catenacci DV, Chapman CG, Xu P, Koons A, Konda VJ, Siddiqui UD, Waxman I. Acquisition of Portal Venous Circulating Tumor Cells From Patients With Pancreaticobiliary Cancers by Endoscopic Ultrasound. Gastroenterology. 2015;149:1794-1803.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 42. | Levy MJ, Topazian MD, Wiersema MJ, Clain JE, Rajan E, Wang KK, de la Mora JG, Gleeson FC, Pearson RK, Pelaez MC. Initial evaluation of the efficacy and safety of endoscopic ultrasound-guided direct Ganglia neurolysis and block. Am J Gastroenterol. 2008;103:98-103. [PubMed] |
| 43. | Doi S, Yasuda I, Kawakami H, Hayashi T, Hisai H, Irisawa A, Mukai T, Katanuma A, Kubota K, Ohnishi T. Endoscopic ultrasound-guided celiac ganglia neurolysis vs. celiac plexus neurolysis: a randomized multicenter trial. Endoscopy. 2013;45:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 44. | Fujii-Lau LL, Bamlet WR, Eldrige JS, Chari ST, Gleeson FC, Abu Dayyeh BK, Clain JE, Pearson RK, Petersen BT, Rajan E. Impact of celiac neurolysis on survival in patients with pancreatic cancer. Gastrointest Endosc. 2015;82:46-56.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 45. | Ishiwatari H, Hayashi T, Yoshida M, Ono M, Sato T, Miyanishi K, Sato Y, Takimoto R, Kobune M, Masuko H. EUS-guided celiac plexus neurolysis by using highly viscous phenol-glycerol as a neurolytic agent (with video). Gastrointest Endosc. 2015;81:479-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |