Published online Dec 21, 2016. doi: 10.3748/wjg.v22.i47.10424
Peer-review started: August 1, 2016
First decision: September 28, 2016
Revised: October 10, 2016
Accepted: November 13, 2016
Article in press: November 13, 2016
Published online: December 21, 2016
Processing time: 143 Days and 4.2 Hours
To assess the safety and feasibility of laparoscopic and endoscopic co-operative surgery (LECS) for early non-ampullary duodenal tumors.
Twelve patients with a non-ampullary duodenal tumor underwent LECS at our hospital. One patient had two mucosal lesions in the duodenum. The indication for this procedure was the presence of duodenal tumors with a low risk for lymph node metastasis. In particular, the tumors included small (less than 10 mm) submucosal tumors (SMT) and epithelial mucosal tumors, such as mucosal cancers or large mucosal adenomas with malignant suspicion. The LECS procedures, such as full-thickness dissection for SMT and laparoscopic reinforcement after endoscopic submucosal dissection (ESD) for epithelial tumors, were performed for the 13 early duodenal lesions in 12 patients. Here we present the short-term outcomes and evaluate the safety and feasibility of this new technique.
Two SMT-like lesions and eleven superficial epithelial tumor-like lesions were observed. Seven and Six lesions were located in the second and third parts of the duodenum, respectively. All lesions were successfully resected en bloc. The defect in the duodenal wall was manually sutured after resection of the duodenal SMT. For epithelial duodenal tumors, the ulcer bed was laparoscopically reinforced via manual suturing after ESD. Intraoperative perforation occurred in two out of eleven epithelial tumor-like lesions during ESD; however, they were successfully laparoscopically repaired. The median operative time and intraoperative estimated blood loss were 322 min and 0 mL, respectively. Histological examination of the tumors revealed one adenoma with moderate atypia, ten adenocarcinomas, and two neuroendocrine tumors. No severe postoperative complications (Clavien-Dindo classification grade III or higher) were reported in this series, but minor leakage secondary to pancreatic fistula occurred in one patient.
LECS can be a safe and minimally invasive treatment option for non-ampullary early duodenal tumors.
Core tip: We performed laparoscopic and endoscopic co-operative surgery (LECS) procedures, such as full-thickness dissection for submucosal tumors and laparoscopic reinforcement after endoscopic submucosal dissection for epithelial tumors, for 13 early duodenal lesions in 12 patients, and analyzed the safety and feasibility of LECS for early non-ampullary duodenal tumors. All lesions were successfully resected en bloc. No severe postoperative complications (Clavien-Dindo classification grade III or higher) were reported in this series, but minor leakage secondary to pancreatic fistula occurred in one patient. LECS can be a safe and minimally invasive treatment option for non-ampullary early duodenal tumors.
- Citation: Ichikawa D, Komatsu S, Dohi O, Naito Y, Kosuga T, Kamada K, Okamoto K, Itoh Y, Otsuji E. Laparoscopic and endoscopic co-operative surgery for non-ampullary duodenal tumors. World J Gastroenterol 2016; 22(47): 10424-10431
- URL: https://www.wjgnet.com/1007-9327/full/v22/i47/10424.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i47.10424
Pancreatoduodenectomy is considered as the standard approach for the resection of duodenal tumors[1,2]. Partial duodenal resection has also been attempted as an alternative treatment option for duodenal tumors such as mucosal adenocarcinoma and small submucosal tumors (SMT), which do not require lymph node dissection[3-6]. Conventional surgery, however, is still invasive especially for patients with such early duodenal tumors.
Recently, postoperative quality of life has received considerable attention, in addition to the oncological outcomes of patients with malignant tumors. Therefore, endoscopic treatments, such as endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), are being increasingly performed for early epithelial duodenal tumors. However, endoscopic treatments for duodenal tumors are still controversial. The anatomical features of the duodenum, such as the narrow lumen and thin wall, make endoscopic resection of tumors very difficult. In fact, several recent reports have demonstrated that severe complications, such as perforation and bleeding, frequently occur during and after endoscopic treatments[7-11]. Recently, Hiki et al[12,13] reported on laparoscopic and endoscopic co-operative surgery (LECS) for gastrointestinal stromal tumors (GIST). This revolutionary procedure enables the precise assessment of tumor boundaries, thereby facilitating adequate tumor resection.
Taking the abovementioned factors into consideration, we have recently developed an LECS concept for the treatment of early non-ampullary duodenal tumors. In this report, we present the details of our procedure and its short-term outcomes and evaluate the safety and feasibility of this new technique.
Between 2015 and 2016, twelve patients (7 males and 5 females) with a non-ampullary duodenal tumor underwent LECS at Kyoto Prefectural University of Medicine. One patient had two mucosal lesions in the second and third parts of the duodenum. All patients underwent diagnostic endoscopy with biopsy. Indications for endoscopic and/or laparoscopic local resection of the duodenal tumors were those with a low risk for lymph node metastasis. Our therapeutic strategies for the early duodenal epithelial tumors, such as mucosal cancers or large mucosal adenomas with malignant suspicion, were as follows: (1) epithelial mucosal tumors of less than 10 mm diameter in the duodenum were treated with EMR; (2) epithelial mucosal tumors of less than 20 mm diameter in the first part of the duodenum were generally treated with common ESD; and (3) epithelial mucosal tumors of more than 10 mm diameter in the second and third parts of the duodenum and those of more than 20 mm in the first part of the duodenum were treated with LECS under general anesthesia. LECS procedure was also indicated for small SMTs (less than 10 mm diameter) of the duodenum with negligible risk for lymph node metastasis in principle. All subjects gave written informed consent prior to undergoing treatment by LECS.
The clinicopathological features of these patients were retrospectively reviewed from their hospital records. Macroscopic and microscopic classifications of tumors were based on the International Union against Cancer/ Tumor, Node, Metastasis (UICC/TMN) staging system. Postoperative functional evaluation using cine-magnetic resonance imaging examination (cine-MRI) was also performed in patients who one year had passed after operation as previously reported[14]. In brief, patients fasted for 4 h prior to the examination. After intake of a jelly drink, imaging was performed for 30 min with the patients in a supine position. Serial images were obtained in a single slice of the plane along the long axis of the second and third parts of the duodenum including the resected area.
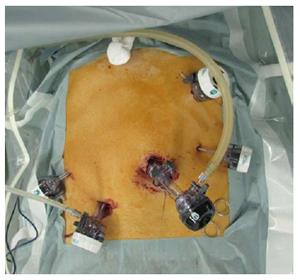
Under general anesthesia, a 12-mm laparoscopy port was inserted through the umbilicus using the open technique. After pneumoperitoneum with carbon dioxide, four additional trocars were inserted as shown in Figure 1. The trocars, except the trocar for the laparoscope, were symmetrically located along the line connecting the location of target tumor and the umbilicus.
From the left side of the patient, the greater omentum was divided at 3 cm from the right gastroepiploic vessels, using laparoscopic coagulating shears (Harmonic Scalpel, Ethicon Endo-Surgery, Cincinnati, OH, United States).
The attachment of the transverse mesocolon was freed from the pancreatic head and retroperitoneal tissues. During exfoliation, the accessory right colic vein was clipped and divided to avoid unnecessary bleeding due to intraoperative tensions during ESD procedures. For tumors located in the third part of the duodenum, the hepatocolic ligament was also dissected, and the right colic flexure was mobilized from the retroperitoneum to achieve a good visual field for the horizontal part of duodenum. Then, the duodenum was mobilized along with the pancreatic head from the retroperitoneum.
Before the endoscopic procedure, the jejunum was clamped using laparoscopic removal forceps. The tumor location was confirmed using both endoscopy and laparoscopy, and the periphery of the tumor was carefully marked using the endoscope. Using ESD technique, a circumferential mucosal incision was made around the tumor with a flush knife and/or a clutch cutter (Fujifilm Co., Tokyo, Japan) after the injection of sodium hyaluronate solution (MucoUp; Johnson and Johnson K.K., Tokyo, Japan). Full-thickness circumferential dissection was subsequently performed endoscopically and laparoscopically for SMT. On the other hand, the common ESD procedure was performed for epithelial tumors such as early duodenal cancers and large adenomas.
Thereafter, the edge of the duodenal wall defect was closed using a laparoscopic hand-sewn suturing technique for cases with SMT and for perforation during ESD. Even after successful ESD, we reinforced the ESD ulcer bed of the duodenum using the laparoscopic hand-sewn suturing technique. After tumor resection, intraluminal endoscopic lavage was performed with a copious amount of saline. Then, the precise location of the ulcer bed was confirmed based on the transmitted light of the endoscope and the pressure exerted from the serosal surface using laparoscopy forceps. Interrupted full-thickness and sero-muscular sutures were added to this site, with taken care to avoid any stenosis or deformity in this series.
After completing the procedure, the endoscope was inserted and passed over the resected location to confirm that there was neither stenosis nor leakage.
Patient and tumor characteristics are shown in Table 1. All patients had no symptoms related to the duodenal lesions, and all the lesions were detected on endoscopy performed as a part of a routine physical examination.
| Parameters | Statistics |
| No. of patients | 12 |
| No. of lesions | 13 |
| Age (yr) | 70 (63-79)1 |
| Sex (male/female) | 7/5 |
| Location | |
| 2nd | 7 |
| 3rd | 6 |
| Macroscopic types | |
| Submucosal tumor | 2 |
| Epithelial (elevated) | 11 |
| Size (mm) | 22 (11-38)1 |
| Operation time (min) | 322 (220-570)1 |
| ESD time (min) | 105 (20-210)1 |
| Blood loss (mL) | 0 (0-150)1 |
| Postoperative complications2 | |
| Minor ( ≤ grade II) | 2 |
| Major (≥ grade III) | 0 |
| Pathological diagnosis | |
| NET | 2 |
| Adenoma | 1 |
| Adenocarcinoma | 10 |
| Depth of tumor | |
| Mucosal | 10 |
| Submucosal | 3 |
| Postoperative hospital stay (d) | 9 (7-49)1 |
| Follow-up period (mo) | 14 (3-19)1 |
During the study period, EMR was indicated for 30 cases of epithelial mucosal tumors of less than 10 mm diameter in the several parts of the duodenum, and common ESD was indicated for three cases of epithelial mucosal tumors of less than 20 mm diameter in the first part of the duodenum. On the other hand, a total of 12 patients with 13 duodenal lesions were treated by the LECS procedures. Two SMT-like lesions and eleven superficial epithelial tumor-like lesions were observed. Seven and six lesions were located in the second and third parts of the duodenum, respectively. Five lesions were located on the oral side of ampulla of Vater, while eight lesions were on the anal side.
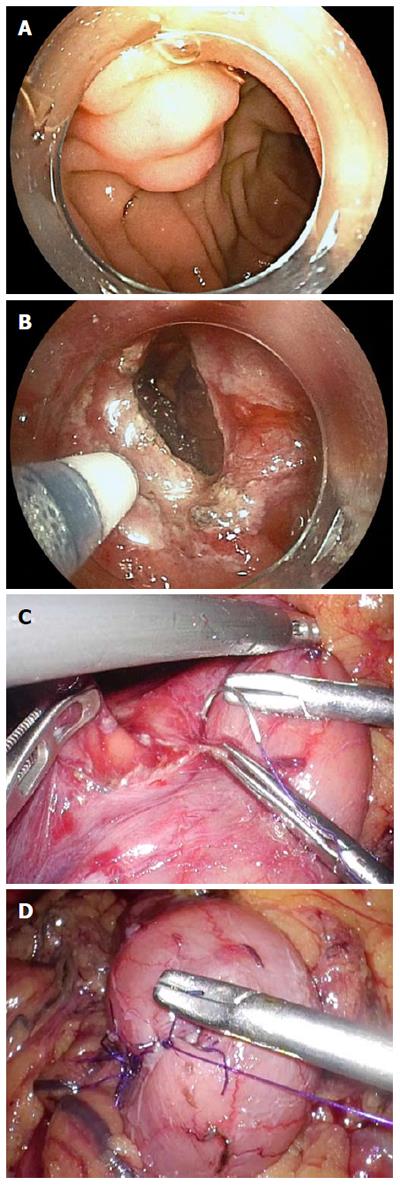
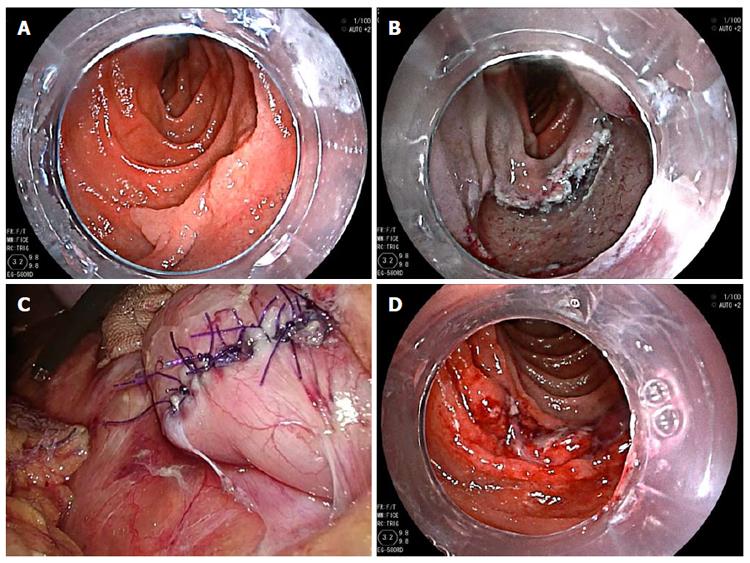
Figure 2 shows intraoperative views of the LECS procedure for duodenal SMT. After tumor resection, the defect in the duodenal wall was closed by a hand-sewn suturing procedure. Figure 3 shows intraoperative views of the LECS procedure for epithelial duodenal tumors, in which the resulting ulcer after ESD was laparoscopically reinforced. The ulcer bed was very thin and easily recognized by the transmitted light of the endoscope, even after successful ESD. Intraoperative perforation was found in two out of the eleven epithelial tumor-like lesions of ESD. These perforations were successfully repaired laparoscopically by hand-sewn sutures. All lesions were successfully resected en bloc. The median operative time and intraoperative estimated blood loss were 322 min and 0 mL, respectively. Conversion to open surgery was not required in this series.
The median time for restarting oral intake was 1 d. The median postoperative hospital stay was 9 d. No severe postoperative complications (Clavien-Dindo classification grade III or higher) were reported in this series, but minor leakage secondary to a pancreatic fistula occurred in one patient. The leakage was minor and asymptomatic and was conservatively managed. One patient experienced obstruction of the food passage after discharge. Endoscopic examination revealed near circumferential duodenal wall edema, which resolved over a week with conservative management.
Histological examination of the resected tumors revealed one adenoma with moderate atypia, ten adenocarcinomas, and two neuroendocrine tumors. The median follow-up periods of these patients were 14 mo (range 3-19 mo). No local or distant recurrence was detected in any patient during the short postoperative follow-up period.
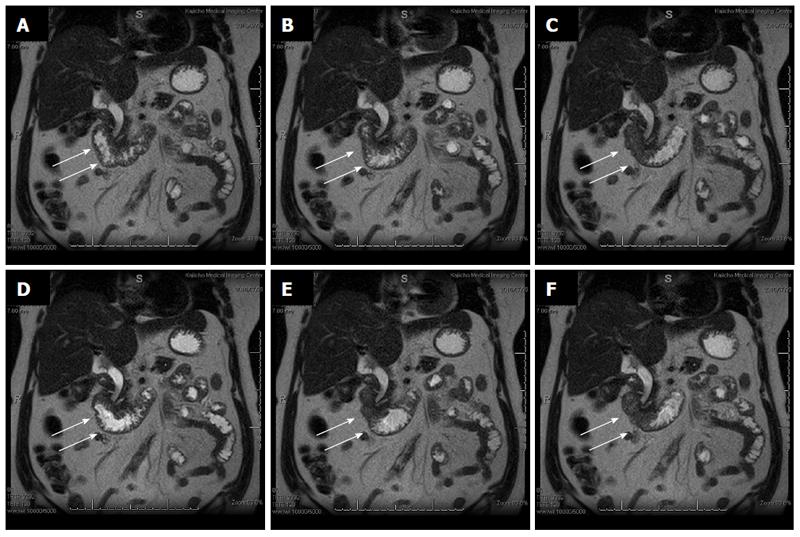
Cine-MRI showed the presence of periodic active peristalsis in the entire duodenum, which suggested the usefulness of LECS as a functional preservation treatment option (Figure 4).
Although the incidence of early duodenal tumors is rare[15,16], the recent widespread use of endoscopy in regular medical check-ups may increase the detection rates in near future. Previously, aggressive surgical procedures, such as pancreatoduodenectomy or segmental duodenectomy with regional lymph node dissection, were indicated for duodenal tumors regardless of their stage[1,2]. However, these conventional surgical procedures may be excessively invasive for early duodenal tumors and are associated with high rates of severe postoperative complications and a decrease in the patients’ quality of life[1,17].
Recently, various less invasive treatment options have been attempted for the resection of early-stage duodenal tumors with a negligible risk for lymph node metastasis[3-11,18-23]. One is the endoscopic tumor resection which is widely accepted for early gastrointestinal tumors[7-11]. Among the endoscopic treatment procedures, ESD has been recognized to have advantages over EMR in achievement of en bloc and complete (R0; no residual tumor) resection[9,11]. However, ESD for duodenal tumors is considered to be more difficult when compared with that for tumors in other parts of the gastrointestinal tract, such as the esophagus, stomach, and colon[7-11]. Maneuvering of a flexible endoscope in the narrow duodenal lumen is technically difficult, and the thin wall increases the perforation risk of ESD for superficial duodenal tumors. Several studies reported a high rate of severe complications such as bleeding and perforation[7-11,15]. The incidence of perforation was reported to be around 30%, and delayed perforation was reported in some cases even after successful ESD[10,11]. Duodenal perforation results in the spillage of duodenal fluids into the abdominal cavity, causing severe peritonitis and sometimes retroperitonitis; this leads to increased hospitalization periods and medical care costs.
Other less invasive treatment option for early duodenal tumors is the laparoscopic local resection[18-23]. However, in intraluminal tumors, it is difficult to determine an adequate resection line from the serosal surface on laparoscopic view. Massive resection with excessive margins may cause deformity and/or stenosis, whereas insufficient surgical margins may result in local recurrence.
These findings prompted us to refine less invasive treatments and utilize the advantages of the endoscopic and laparoscopic approaches for early duodenal tumors. LECS was first reported for GIST by Hiki et al[12]. In the original LECS procedure, endoscopists determined the resection line with adequate margins, and consequently, laparoscopic surgeons resected the tumors along the line determined by endoscopists.
In the present study, we applied LECS techniques for the local resection of early-stage non-ampullary duodenal tumors. Local resection without destroying surrounding tissues was not only a less invasive but also function-preserving treatment, as shown in the results of the duodenal motility assay.
The conventional LECS procedure was applied to SMT with a low potential for lymph node metastasis. The indication includes GIST of less than 20 mm, carcinoid tumors of less than 10 mm, and other small SMT (less than 20 mm) with a low malignant potential[13,24]. An endoscopic circumferential incision for duodenal SMT was difficult and time-consuming due to a narrow duodenal lumen, and therefore, laparoscopic resection was finally performed with endoscopic-marking guidance. In this study, the original LECS procedure enabled the resection of submucosal tumors with minimal margins and less postoperative duodenal deformity. On the other hand, we also applied the LECS technique for mucosal cancers and large adenomas with a suspected focal malignancy. These superficial epithelial tumors generally have no risk for lymph node metastasis and are considered an indication for ESD. However, bleeding and perforation during and after ESD occurred at a considerable frequency in our hospital; this outcome was similar to that of other reports, and the procedure was sometimes associated with severe complications[7-11]. Therefore, we applied the LECS technique for the superficial tumors as a safety-sensitive strategy. Our method involved ESD and subsequent reinforcement of the thinned ulcer bed by hand-sewn stiches. Firstly, we used single-layer seromuscular sutures for the reinforcement of the ESD ulcer. However, reinforcement by seromuscular sutures alone required strict recognition of the ESD ulcer boundaries and resulted in intraluminal protrusion of the ulcer bed between the sutured mucosa. This kind of reinforcement was assumed to be effective for perforation but not for hemorrhagic complications. In fact, Irino et al[23] have reported that postoperative bleeding occurred after this kind of LECS procedure. Therefore, we sutured the ESD ulcer bed with two layers of stitches, full-thickness and seromuscular, using a laparo-endoscopic view to decrease the rate of postoperative complications, with care to avoid any stenosis or deformity in this series. The LECS procedure achieved a good short-term outcome, except for 1 case with minor leakage secondary to a pancreatic fistula, which resolved after a week of conservative management. The fistula was suspected to be due to damage to the pancreatic tissues caused by the sutures. From our experience, preoperative diagnostic endoscopic ultrasonography is advocated for the circumferential localization of tumors. If the tumor is found to extend to the pancreatic side of duodenum, endoscopic closure using clips should be considered.
Our LECS procedure had another issue to consider. A full-thickness suture may possibly cause the intra-abdominal dissemination of tumor cells detached from the tumor. However, previous studies have reported that no recurrence was recorded in 90 cases of perforation during ESD treatment[25]. Therefore, we assumed that the small amount of tumor cells or tumor debris from superficial tumors was unlikely to adhere to the peritoneum and develop into peritoneal recurrences. Nevertheless, in order to decrease intraluminal free tumor cells, we performed a lavage of the duodenal lumen with an adequate amount of saline (more than 1000 mL) after ESD and thereafter stitched the ulcer bed. Another advantage of the LECS procedure is that it enabled the immediate closure of perforation occurring during the ESD technique. In fact, perforation during ESD occurred in three cases in this series. Among them, one case developed a micro-perforation that was not recognized by the endoscopist during ESD, yet was detected by careful observation of the serosal surface. This kind of latent micro-perforation may be the cause of delayed perforation that sometimes results in lethal complications after ESD.
We should give further consideration to the indications of laparoscopic reinforcement after duodenal ESD. Some reports demonstrated that most delayed perforations after ESD develop in tumors on the anal side of the ampulla of Vater because of the direct exposure of the ESD ulcer bed to pancreatic juice and biliary enzymes[8,10,11]. Therefore, the additional reinforcement by laparoscopic sutures might be required only after ESD for tumors on the anal side of the ampulla of Vater. Although future large trials are needed for confirmation, the LECS procedure could be safe, minimally invasive, and represent an adequate treatment option for non-ampullary early duodenal tumors with an ignorable risk of lymph node metastasis.
Conventional surgery for early duodenal tumors is excessively invasive. Endoscopic submucosal dissection has been increasingly performed for early duodenal tumors. However, severe complications, such as bleeding and perforation, frequently occur during the perioperative period of endoscopic submucosal dissection (ESD).
Various less invasive treatment options have been attempted for the resection of early duodenal tumors with a negligible risk for lymph node metastasis. One of the less invasive approaches is the laparoscopic local resection. Recently, laparoscopic and endoscopic co-operative surgery (LECS) has been attempted for the treatments of early duodenal tumors. However, laparoscopy-based local resection can expose the epithelial tumor to the abdominal cavity, which may cause an opportunity for peritoneal metastasis.
In this study, the authors applied the LECS technique for non-ampullary early duodenal tumors. The common LECS procedure - full-thickness dissection - was performed for small submucosal tumors (SMT), whereas a modified LECS procedure - laparoscopic reinforcement after ESD - was performed for mucosal epithelial tumors. LECS procedures could be a safe, minimally invasive and function preserving treatment option for non-ampullary early duodenal tumors with a negligible risk of lymph node metastasis.
This study suggested that LECS can be a safe and minimally invasive treatment option for non-ampullary early duodenal tumors with a negligible risk of lymph node metastasis although future large trials are needed for confirmation.
Endoscopic mucosal resection (EMR) and ESD are well-established techniques of endoscopic resection for the treatment of gastrointestinal epithelial lesions. LECS, which stands for laparoscopy and endoscopy co-operative surgery, was developed by Hiki et al. A SMT is a tumor derived from the submucosal or deeper layer and includes gastrointestinal stromal tumor and neuroendocrine tumor.
This paper is an intriguing manuscript showing clinical results and usefulness of LECS procedures for non-ampullary early duodenal tumors. The objective is very curious and important. Few centers would have such an opportunity and this unusual experience is worth recording.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Hiki N, Shibata T, Slomiany BL, Tovey FI S- Editor: Gong ZM L- Editor: A E- Editor: Liu WX
| 1. | Chok AY, Koh YX, Ow MY, Allen JC, Goh BK. A systematic review and meta-analysis comparing pancreaticoduodenectomy versus limited resection for duodenal gastrointestinal stromal tumors. Ann Surg Oncol. 2014;21:3429-3438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Cloyd JM, George E, Visser BC. Duodenal adenocarcinoma: Advances in diagnosis and surgical management. World J Gastrointest Surg. 2016;8:212-221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 3. | Stauffer JA, Raimondo M, Woodward TA, Goldberg RF, Bowers SP, Asbun HJ. Laparoscopic partial sleeve duodenectomy (PSD) for nonampullary duodenal neoplasms: avoiding a whipple by separating the duodenum from the pancreatic head. Pancreas. 2013;42:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Kanaji S, Nakamura T, Nishi M, Yamamoto M, Kanemitu K, Yamashiita K, Imanishi T, Sumi Y, Suzuki S, Tanaka K. Laparoscopic partial resection for hemangioma in the third portion of the duodenum. World J Gastroenterol. 2014;20:12341-12345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Tanaka E, Kim M, Lim JS, Choi YY, Saklani A, Noh SH, Hyung WJ. Usefulness of laparoscopic side-to-side duodenojejunostomy for gastrointestinal stromal tumors located at the duodenojejunal junction. J Gastrointest Surg. 2015;19:313-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Chung JC, Kim HC, Hur SM. Limited resections for duodenal gastrointestinal stromal tumors and their oncologic outcomes. Surg Today. 2016;46:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Takimoto K, Imai Y, Matsuyama K. Endoscopic tissue shielding method with polyglycolic acid sheets and fibrin glue to prevent delayed perforation after duodenal endoscopic submucosal dissection. Dig Endosc. 2014;26 Suppl 2:46-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Inoue T, Uedo N, Yamashina T, Yamamoto S, Hanaoka N, Takeuchi Y, Higashino K, Ishihara R, Iishi H, Tatsuta M. Delayed perforation: a hazardous complication of endoscopic resection for non-ampullary duodenal neoplasm. Dig Endosc. 2014;26:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 136] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 9. | Matsumoto S, Yoshida Y. Selection of appropriate endoscopic therapies for duodenal tumors: an open-label study, single-center experience. World J Gastroenterol. 2014;20:8624-8630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Kakushima N, Kanemoto H, Tanaka M, Takizawa K, Ono H. Treatment for superficial non-ampullary duodenal epithelial tumors. World J Gastroenterol. 2014;20:12501-12508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Marques J, Baldaque-Silva F, Pereira P, Arnelo U, Yahagi N, Macedo G. Endoscopic mucosal resection and endoscopic submucosal dissection in the treatment of sporadic nonampullary duodenal adenomatous polyps. World J Gastrointest Endosc. 2015;7:720-727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Hiki N, Yamamoto Y, Fukunaga T, Yamaguchi T, Nunobe S, Tokunaga M, Miki A, Ohyama S, Seto Y. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc. 2008;22:1729-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 340] [Article Influence: 18.9] [Reference Citation Analysis (2)] |
| 13. | Hiki N, Nunobe S, Matsuda T, Hirasawa T, Yamamoto Y, Yamaguchi T. Laparoscopic endoscopic cooperative surgery. Dig Endosc. 2015;27:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 14. | Ichikawa D, Komatsu S, Okamoto K, Shiozaki A, Fujiwara H, Otsuji E. Esophagogastrostomy using a circular stapler in laparoscopy-assisted proximal gastrectomy with an incision in the left abdomen. Langenbecks Arch Surg. 2012;397:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Honda T, Yamamoto H, Osawa H, Yoshizawa M, Nakano H, Sunada K, Hanatsuka K, Sugano K. Endoscopic submucosal dissection for superficial duodenal neoplasms. Dig Endosc. 2009;21:270-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Buchbjerg T, Fristrup C, Mortensen MB. The incidence and prognosis of true duodenal carcinomas. Surg Oncol. 2015;24:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Müller MW, Dahmen R, Köninger J, Michalski CW, Hinz U, Hartel M, Kadmon M, Kleeff J, Büchler MW, Friess H. Is there an advantage in performing a pancreas-preserving total duodenectomy in duodenal adenomatosis? Am J Surg. 2008;195:741-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Sakon M, Takata M, Seki H, Hayashi K, Munakata Y, Tateiwa N. A novel combined laparoscopic-endoscopic cooperative approach for duodenal lesions. J Laparoendosc Adv Surg Tech A. 2010;20:555-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Ohata K, Murakami M, Yamazaki K, Nonaka K, Misumi N, Tashima T, Minato Y, Shozushima M, Mitsui T, Matsuhashi N. Feasibility of endoscopy-assisted laparoscopic full-thickness resection for superficial duodenal neoplasms. ScientificWorldJournal. 2014;2014:239627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Tsushimi T, Mori H, Harada T, Nagase T, Iked Y, Ohnishi H. Laparoscopic and endoscopic cooperative surgery for duodenal neuroendocrine tumor (NET) G1: Report of a case. Int J Surg Case Rep. 2014;5:1021-1024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Tamaki I, Obama K, Matsuo K, Kami K, Uemoto Y, Sato T, Ito T, Tamaki N, Kubota K, Inoue H. A case of primary adenocarcinoma of the third portion of the duodenum resected by laparoscopic and endoscopic cooperating surgery. Int J Surg Case Rep. 2015;9:34-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Kyuno D, Ohno K, Katsuki S, Fujita T, Konno A, Murakami T, Waga E, Takanashi K, Kitaoka K, Komatsu Y. Laparoscopic-endoscopic cooperative surgery is a safe and effective treatment for superficial nonampullary duodenal tumors. Asian J Endosc Surg. 2015;8:461-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Irino T, Nunobe S, Hiki N, Yamamoto Y, Hirasawa T, Ohashi M, Fujisaki J, Sano T, Yamaguchi T. Laparoscopic-endoscopic cooperative surgery for duodenal tumors: a unique procedure that helps ensure the safety of endoscopic submucosal dissection. Endoscopy. 2015;47:349-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Kachare SD, Liner KR, Vohra NA, Zervos EE, Fitzgerald TL. A modified duodenal neuroendocrine tumor staging schema better defines the risk of lymph node metastasis and disease-free survival. Am Surg. 2014;80:821-826. [PubMed] |
| 25. | Ikehara H, Gotoda T, Ono H, Oda I, Saito D. Gastric perforation during endoscopic resection for gastric carcinoma and the risk of peritoneal dissemination. Br J Surg. 2007;94:992-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |