Published online Dec 14, 2016. doi: 10.3748/wjg.v22.i46.10219
Peer-review started: August 29, 2016
First decision: September 20, 2016
Revised: October 4, 2016
Accepted: November 14, 2016
Article in press: November 16, 2016
Published online: December 14, 2016
Processing time: 108 Days and 15.6 Hours
To determine the incidence of hepatocellular carcinoma (HCC) and the impact of HCC surveillance on early diagnosis and survival of cirrhotic outpatients.
In this retrospective cohort study, cirrhotic outpatients undergoing HCC surveillance between March 2005 and March 2014 were analyzed. Exclusion criteria were HIV coinfection; previous organ transplantation; diagnosis of HCC at first consultation; missing data in the medical chart; and less than 1 year of follow-up. Surveillance was carried out every six months using ultrasound and serum alpha-fetoprotein determination. Ten-year cumulative incidence and survival were estimated through Kaplan-Meier analysis.
Four hundred and fifty-three patients were enrolled, of which 57.6% were male. Mean age was 55 years. Hepatitis C virus and heavy use of alcohol were the main etiologic agents of cirrhosis. HCC was diagnosed in 75 patients (16.6%), with an estimated cumulative incidence of 2.6% in the 1st year, 15.4% in the 5th year, and 28.8% in the 10th year. Median survival was estimated at 17.6 mo in HCC patients compared to 234 mo in non-HCC patients (P < 0.001). Early-stage HCC was more often detected in patients who underwent surveillance every 6 mo or less (P = 0.05). However, survival was not different between patients with early stage vs non-early stage tumors [HR = 0.54 (0.15-1.89), P = 0.33].
HCC is a frequent complication in patients with cirrhosis and adherence to surveillance programs favors early diagnosis.
Core tip: This retrospective cohort chart review study provides novel data regarding the incidence of hepatocellular carcinoma (HCC) in the South of Brazil. Of 453 patients with cirrhosis attending a specialized reference clinic between March 2005 and March 2014, 75 (16.6%) developed HCC, with a cumulative incidence of 2.6%, 15.4% and 28.8% in the 1st, 5th, and 10th year respectively. Early-stage HCC was more often detected in patients undergoing strict surveillance every 6 mo. Results from this study highlight the need for strict surveillance programs favoring early diagnosis and, probably, a better prognosis.
- Citation: Appel-da-Silva MC, Miozzo SADS, Dossin IA, Tovo CV, Branco F, Mattos AA. Incidence of hepatocellular carcinoma in outpatients with cirrhosis in Brazil: A 10-year retrospective cohort study. World J Gastroenterol 2016; 22(46): 10219-10225
- URL: https://www.wjgnet.com/1007-9327/full/v22/i46/10219.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i46.10219
Liver cancer is the second leading cause of cancer death worldwide; it is also the fifth most common cancer in men and the ninth in women. In 2012, an estimated 782000 new cases of liver cancer occurred in the world, with report of 745000 deaths[1]. Among primary liver malignancies, hepatocellular carcinoma (HCC) accounts for 70%-85% of cases, and is associated with chronic liver disease and/or cirrhosis in 70%-90% of cases[2,3].
The burden of HCC varies with geographic location, especially when associated with cirrhosis[3]. Around 80% of cases occur in developing countries, and 55% in China alone[4]. In highly endemic areas, such as sub-Saharan countries and Asia, the annual incidence rate is around 30/100000 population[4-6]. Mediterranean countries (Italy, Spain and Greece) report intermediate incidence rates, with 10-20 cases/100000/year. An increase in the burden of HCC in low-incidence areas (Australia, North America, South America, and United Kingdom), with fewer than 5 cases/100000/ year, has also been recently noted. In these areas, the growing prevalence of hepatitis C virus (HCV) infection, alcohol consumption, and nonalcoholic fatty liver disease (NAFLD) are the main causes underlying the increasing number of HCC cases[2,3,7-11].
In Latin America, limited data are available on the incidence and population characteristics of patients with HCC[12]. In Brazil, a national epidemiological survey sponsored by the Brazilian Society for Hepatology[13] evaluated 1405 patients with HCC in 29 centers across the country. Using the Barcelona Clinic Liver Cancer (BCLC) staging classification[14], 43% of the individuals were diagnosed with early stage tumors; 35% with intermediate stage tumors; and 22% with advanced stage tumors. Also, 98% had cirrhosis, which was caused by HCV in 39% and heavy use of alcohol in 14%. In the South of Brazil, HCV has been identified as the main etiologic factor of cirrhotic outpatients[15].
Screening and surveillance of HCC using abdominal ultrasound have been shown to detect tumors at an earlier stage, increasing the odds of treatment and the adherence of health care services to current practice guidelines[16-19]. Nevertheless, epidemiological studies in the United States have shown that only 12% to 78.8% of patients receive routine surveillance[20,21]; possible barriers to screening and surveillance include socioeconomic factors and the lack of specific health policies for HCC[22].
The objective of the present study was to determine the incidence of HCC and the impact of HCC surveillance on early diagnosis and survival of cirrhotic outpatients attending a tertiary hospital clinic in the South of Brazil.
We carried out a retrospective cohort chart review study including all patients aged 18 years or older diagnosed with cirrhosis attending a specialized reference clinic (Complexo Hospitalar Santa Casa, Porto Alegre, Brazil) between March 2005 and March 2014. Exclusion criteria were HIV coinfection, previous organ transplantation, diagnosis of HCC at the first clinic appointment, incomplete medical records, or follow-up of less than 1 year. The diagnosis of cirrhosis was based on clinical, laboratory, and on ultrasonographic and/or upper GI endoscopic features. Those patients whose diagnosis remained inconclusive, percutaneous liver biopsy were carried out.
All patients underwent screening and surveillance for HCC, with abdominal ultrasound and serum alpha-fetoprotein (AFP) determination every 6 mo. Computed tomography (CT) or abdominal magnetic resonance imaging (MRI) with contrast were performed in all patients with evidence of nodular lesion measuring ≥ 1 cm in diameter on ultrasound[23].
HCC diagnosis was based on typical findings on contrast-enhanced CT or abdominal MRI - early arterial phase enhancement followed by rapid washout at the late portal/venous phase. Inconclusive cases were referred for biopsy and histological evaluation[23]. Patients with a diagnosis of HCC were classified according to BCLC criteria[14].
All charts were reviewed for selection of study variables and outcomes during the study period, considering the data available for the first and the last consultations. The following variables were analyzed: age, sex, etiology of liver disease, Child-Turcotte-Pugh[24] score, Model for End-Stage Liver Disease score[25], use of statins, and serum levels of AFP.
The establishment of alcohol consumption was made through self-report of regular drinking, in a daily basis. Heavy use of alcohol was considered when alcohol consumption was greater than 40 g per day for men and women.
All patients received specialized treatment according to the etiology of liver disease and risk factors identified. Obese and/or NAFLD patients were referred to a Clinical Nutrition outpatient clinic. Those with alcohol dependency were headed for a public specialized psychiatric service and encouraged to attend support groups to stop drinking.
The patients were divided into two groups: with or without HCC. To compare the groups in terms of continuous variables with normal distribution, Student’s t test was used. Mann-Whitney’s test was used for comparison of variables with non-Gaussian distribution. For the comparison of categorical variables, the χ2 test and Fisher’s exact test were used. To evaluate the performance of AFP as a diagnostic tool, in patients with HCC, sensitivity, specificity, post-test probability, and likelihood ratio were calculated for different serum level ranges. These data were also represented as ROC curves and box plots generated with log-transformed values. Kaplan-Meier analysis was performed to examine cumulative incidence and survival in the 10-year follow-up period, with statistical significance calculated using the log-rank test. HR with 95%CI was calculated using a Cox regression model. Significance level was set at α = 5%.
Microsoft® Office Excel 2010 was used to store data, and the Statistical Package for the Social Sciences v. 22.0 (IBM® SPSS) was used for analysis of results. The normality of data distribution was determined using the Kolmogorov-Smirnov test. Quantitative variables with normal distribution were expressed as mean and standard deviation; variables with non-normal distribution were expressed as median and interquartile range. Simple and relative frequencies were used for categorical variables.
The research protocol was approved by the Research Ethics Committee at Universidade Federal de Ciências da Saúde de Porto Alegre (protocol 367511/2011, approval report 14/2014).
Of 738 eligible patients, the following were excluded: 105 with incomplete medical records, 88 with non-cirrhotic portal hypertension, 54 who were lost to follow-up, and 14 with HIV co-infection. Of the remaining 477 cirrhotic patients, 24 were diagnosed with HCC at the first clinic appointment and were thus excluded from the study. Thus, the final sample included 453 patients.
During follow-up, 75 patients (16.6%) were diagnosed with HCC. Median follow-up for this group was 15.7 mo. Among the 378 patients who did not develop HCC, median follow-up was 58.4 mo. Table 1 shows demographic and clinical data of the groups with and without HCC.
| Characteristic | HCC | Without HCC | P value |
| n = 75 | n = 378 | ||
| Age (yr) | 54.9 ± 10.7 | 53.2 ± 12.2 | 0.23 |
| Male sex | 44 (58.7) | 217 (57.4) | 0.90 |
| Cirrhosis etiology | 0.27 | ||
| HCV | 35 (46.7) | 132 (34.9) | |
| Alcohol | 16 (21.3) | 93 (24.6) | |
| HCV + alcohol | 15 (20.0) | 74 (19.6) | |
| HBV | 2 (2.7) | 3 (0.8) | |
| HBV + alcohol | 0 (0.0) | 5 (1.3) | |
| NAFLD | 1 (1.3) | 7 (1.8) | |
| Cryptogenic | 1 (1.3) | 12 (3.2) | |
| Other | 5 (6.7) | 52 (13.8) | |
| Baseline Child-Pugh | n = 74 | n = 377 | 0.81 |
| A | 45 (60.8) | 229 (60.7) | |
| B | 22 (29.7) | 119 (31.6) | |
| C | 7 (9.5) | 29 (7.7) | |
| End-of-study Child-Pugh | n = 75 | n = 367 | 0.38 |
| A | 30 (40.0) | 168 (45.8) | |
| B | 25 (33.3) | 127 (34.6) | |
| C | 20 (26.7) | 72 (19.6) | |
| Baseline MELD | n = 60 | n = 292 | |
| 11.2 (6; 25) | 12.0 (6 ;27) | 0.12 | |
| End-of-study MELD | n = 71 | n = 330 | |
| 13.4 (6; 31) | 13.1 (6; 45) | 0.65 | |
| Baseline AFP, ng/mL | n = 69 | n = 261 | |
| 6.1 (3.7; 19.0) | 4.0 (1.5; 8.0) | 0.01 | |
| End-of-study AFP, ng/mL | n = 57 | n = 286 | |
| 16 (4.9; 187.0) | 4.0 (2.5; 7.8) | < 0.001 |
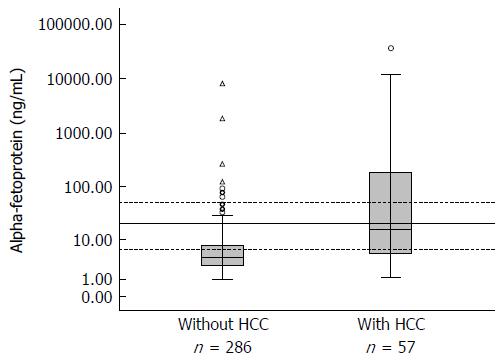
AFP levels were available for 343 patients, of which 57 had a diagnosis of HCC (16.7%). Baseline and end-of-study AFP levels were significantly different between patients with and without HCC. Stratification of serum AFP levels into four ranges (Figure 1 and Table 2) revealed a trend for AFP > 20 ng/mL to predict HCC. The highest diagnostic probability was observed for AFP levels ≥ 50 ng/mL (Table 2). Accuracy of AFP was measured by the area under the ROC curve, whose value was 0.769 (95%CI: 0.70-0.84).
| AFP level (ng/mL) | Pre-test probability | LR+ | Post-test probability | Sensitivity | Specificity |
| < 6.0 | 16.60% | 0.50 | 9.1% | 66.7% | 66.3% |
| 6-19.9 | 16.60% | 1.00 | 16.6% | 45.6% | 89.3% |
| 20-50 | 16.60% | 1.31 | 20.8% | 35.1% | 96.1% |
| > 50 | 16.60% | 10.03 | 66.8% | 35.1% | 96.1% |
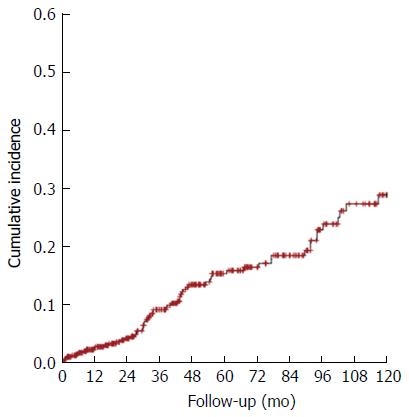
The 10-year cumulative incidence of HCC was analyzed using a Kaplan-Meier curve (Figure 2). During this 10-year period, 453 patients were followed-up. The estimated incidence of HCC was 2.6% in the 1st year, 15.4% in the 5th year, and 28.8% in the 10th year.
Among 419 patients who reported not using statins, 73 (17.4%) had HCC, vs only 1 patient among 34 using statins (2.9%), P = 0.028.
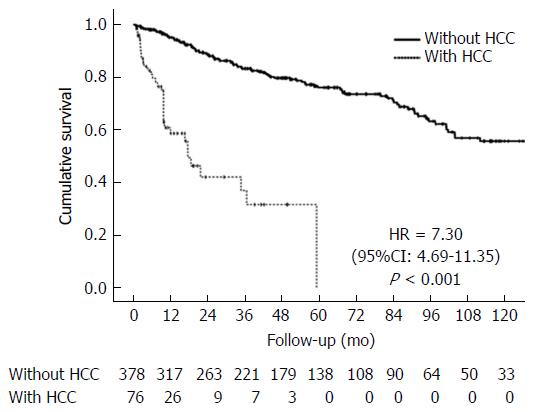
Survival analysis showed median survival of 234 mo (19.5 years) for the group without HCC and 17.6 mo (1.5 year) for patients with HCC. At the end of 10 years, none of the HCC patients were alive, whereas 55.8% of the patients without HCC were still living (P < 0.001, Figure 3).
BCLC staging of HCC at the time of diagnosis showed early stage tumors in 40 (53.3%) patients, intermediate stage tumors in 26 (34.6%) patients, and advanced tumors in 9 (12%) patients. Only 50.7% of individuals with HCC had undergone ultrasound surveillance every 6 mo. The analysis of tumor staging (early vs non-early) according to frequency of ultrasound surveillance showed a higher number of cases diagnosed with early stage tumors in patients with surveillance every 6 mo or less (P = 0.05). However, survival was not different between patients with early stage vs non-early stage tumors [HR = 0.54 (0.15-1.89), P = 0.33].
Given the impact of HCC incidence on patients with cirrhosis, as well as the scarcity of data regarding this population in Latin America, we set out to determine the incidence of HCC and the role of a surveillance program in a cohort of cirrhotic patients attending an outpatient clinic in the South of Brazil, region predominantly composed by European descendants.
In this study, 75 of 453 (16.6%) patients developed HCC over 10 years - a higher incidence than the 8.1% observed in a cohort followed-up in the Southeast of Brazil[26]. Data from other countries also reveal higher incidences in various populations, such as 17.5% in the United States[27] and 27% in an Italian cohort[28]. Because Brazil is a country of continental proportions, the higher incidence detected in the South may be explained by geographic and/or racial heterogeneity, as well as specificities related to risk factors and access to health care services for screening, diagnosis, and follow-up. The predominance of the male sex and the mean age at diagnosis were similar to those described in other national[13,29,30] and international[12,31,32] studies.
In the present study, the etiology of liver disease was similar in patients with or without HCC, with HCV and alcohol being the main etiologic agents. In Brazil, chronic HCV infection and alcohol consumption are a major public health problem[33,34]; nevertheless, in some regions HBV is still an important cause of cirrhosis and HCC[35]. Llovet et al[36] have shown that in Europe and North America, HCV and alcohol are more frequently associated with HCC than HBV, differently than what occurs in Asia and Africa.
The establishment of surveillance programs for patients with chronic liver disease gained momentum after the study by Zhang et al[37], which showed that performing abdominal ultrasound and AFP testing every 6 mo was capable of identifying patients in earlier stages of the disease, increasing survival in up to 37% of cases.
A major objective of follow-up of patients with cirrhosis is the screening and surveillance of HCC according to various consensus statements and guidelines[31,38-40]. Brazilian Society for Hepatology[41] has recently recommended the performance of abdominal ultrasounds every 6 mo, with measurement of AFP strictly in sites where physicians who are experienced in ultrasound are not available.
AFP was recognized in the 1970s as a tumor marker for diagnosis of HCC. This biomarker lost ground after many studies showed low sensitivity and specificity for detection of early stage tumors, leading to the exclusion of AFP dosing from the main consensus statements[31,38,40]. Despite the debate, the Asian Pacific Association for the Study of the Liver and the Japan Society of Hepatology kept the recommendation for serial AFP measurement, based on the understanding that this information could complement ultrasound surveillance[39,42]. In any case, it is well recognized that AFP may play an important prognostic role in the follow-up of these patients, since high AFP levels may signal more aggressive, multifocal tumors associated with venous portal thrombosis and/or metastases[43].
In the present study, serum AFP levels were higher in patients with HCC than in those without HCC. Nevertheless, the absence of a cutoff point with satisfactory sensitivity and specificity to detect HCC compromises the usefulness of this test. We believe that AFP dosing is more valuable to establish HCC prognosis than HCC diagnosis [44].
The incidence of HCC has been increasing globally, especially in the West, as a consequence of the obesity epidemic and of the growing number of patients with chronic liver disease[45]. In our cohort, cumulative HCC incidence was 2.6%, 15.4%, and 28.8% in the 1st, 5th, and 10th year respectively, which is similar to the data reported for other cirrhotic cohorts[27,46].
We observed that more patients were diagnosed with early stage HCC, as determined by BCLC criteria, in the presence of ultrasound monitoring at 6-mo intervals, even if survival was similar in this group, as compared to the group submitted to surveillance ultrasound at broader intervals. The difficulty in demonstrating increased survival associated with surveillance programs involves ethical issues relating to the performance of randomized, controlled trials. In this cohort, despite the lower survival of HCC patients vs those with cirrhosis and without HCC, there was no difference between those who underwent strict surveillance and those who did not. Sangiovanni et al[28] successfully demonstrated increased survival in cirrhotic patients with HCC undergoing surveillance between 1985 and 2011.
Interestingly, we observed a negative association between use of statins and development of HCC. Even though this might be a chance finding, given the low number of patients using this medication, previous studies have reported an effect of statins on patients with chronic liver disease[47-54]. All these previous works have described a protective effect. In fact, Chiu et al[48] described a reduction of 38% in the risk of HCC in patients from a surveillance program.
In conclusion, the findings of the present study underscore the high incidence of HCC in individuals with cirrhosis, highlighting the importance of stimulating the adherence of health care services and patients to surveillance programs.
Liver cancer is the second leading cause of cancer death worldwide and, among primary liver malignancies, hepatocellular carcinoma (HCC) accounts for 70%-85% of cases, and is associated with chronic liver disease and/or cirrhosis in 70%-90% of cases.
All patients with chronic liver diseases are advised and guided to programmed screening and surveillance for HCC in order to allow early detection of nodular lesion.
This study presents the incidence and impact of HCC in patients with cirrhosis in the South of Brazil and demonstrates that the adherence to surveillance programs are indeed effective for early diagnosis.
The present study underscore the high incidence of HCC in individuals with cirrhosis, highlighting the importance of stimulating the adherence of health care services and patients to surveillance programs.
Screening and surveillance programs are usually done through periodic abdominal ultrasound every 6 mo and may be associated with serum alpha-fetoprotein. Computed tomography or abdominal magnetic resonance imaging with contrast were performed in all patients with evidence of nodular lesion measuring ≥ 1 cm in diameter on ultrasound.
This retrospective cohort chart review study does a good job regarding the incidence of HCC in the South of Brazil and displays the need for strict surveillance programs favoring early diagnosis and prognosis. It is very well-written and the Discussion interprets the findings in view of the results obtained in this and in past studies on this topic. The study gives significant information and it may possibly help clinicians to develop further studies.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lachenmeier DW, Zhang ZM, Zhu X S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20491] [Article Influence: 2049.1] [Reference Citation Analysis (20)] |
| 2. | Cabibbo G, Craxì A. Epidemiology, risk factors and surveillance of hepatocellular carcinoma. Eur Rev Med Pharmacol Sci. 2010;14:352-355. [PubMed] |
| 3. | Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47 Suppl:S2-S6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 770] [Cited by in RCA: 878] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 4. | Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1799] [Cited by in RCA: 1816] [Article Influence: 86.5] [Reference Citation Analysis (0)] |
| 5. | Sherman M. Hepatocellular carcinoma: epidemiology, risk factors, and screening. Semin Liver Dis. 2005;25:143-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 310] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 6. | Kew MC. Epidemiology of chronic hepatitis B virus infection, hepatocellular carcinoma, and hepatitis B virus-induced hepatocellular carcinoma. Pathol Biol (Paris). 2010;58:273-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 7. | Hassan MM, Hwang LY, Hatten CJ, Swaim M, Li D, Abbruzzese JL, Beasley P, Patt YZ. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36:1206-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 534] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 8. | Kemp W, Pianko S, Nguyen S, Bailey MJ, Roberts SK. Survival in hepatocellular carcinoma: impact of screening and etiology of liver disease. J Gastroenterol Hepatol. 2005;20:873-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1036] [Cited by in RCA: 1018] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 10. | El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139:817-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 684] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 11. | Mittal S, Sada YH, El-Serag HB, Kanwal F, Duan Z, Temple S, May SB, Kramer JR, Richardson PA, Davila JA. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the veteran affairs population. Clin Gastroenterol Hepatol. 2015;13:594-601.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 209] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 12. | Fassio E, Míguez C, Soria S, Palazzo F, Gadano A, Adrover R, Landeira G, Fernández N, García D, Barbero R. Etiology of hepatocellular carcinoma in Argentina: results of a multicenter retrospective study. Acta Gastroenterol Latinoam. 2009;39:47-52. [PubMed] |
| 13. | Carrilho FJ, Kikuchi L, Branco F, Goncalves CS, Mattos AA. Clinical and epidemiological aspects of hepatocellular carcinoma in Brazil. Clinics (Sao Paulo). 2010;65:1285-1290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2866] [Article Influence: 110.2] [Reference Citation Analysis (1)] |
| 15. | John JA, de Mattos AA, da Silva Miozzo SA, Comerlato PH, Porto M, Contiero P, da Silva RR. Survival and risk factors related to death in outpatients with cirrhosis treated in a clinic in Southern Brazil. Eur J Gastroenterol Hepatol. 2015;27:1372-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. 2014;11:e1001624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 516] [Cited by in RCA: 600] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 17. | Stravitz RT, Heuman DM, Chand N, Sterling RK, Shiffman ML, Luketic VA, Sanyal AJ, Habib A, Mihas AA, Giles HC. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am J Med. 2008;121:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Abe R, Okano J, Imamoto R, Fujise Y, Koda M, Murawaki Y. [Evaluation of the surveillance program for hepatocellular carcinoma]. Nihon Shokakibyo Gakkai Zasshi. 2012;109:741-750. [PubMed] |
| 19. | Kuo YH, Lu SN, Chen CL, Cheng YF, Lin CY, Hung CH, Chen CH, Changchien CS, Hsu HC, Hu TH. Hepatocellular carcinoma surveillance and appropriate treatment options improve survival for patients with liver cirrhosis. Eur J Cancer. 2010;46:744-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Davila JA, Henderson L, Kramer JR, Kanwal F, Richardson PA, Duan Z, El-Serag HB. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 234] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 21. | Tong MJ, Chavalitdhamrong D, Lu DS, Raman SS, Gomes A, Duffy JP, Hong JC, Busuttil RW. Survival in Asian Americans after treatments for hepatocellular carcinoma: a seven-year experience at UCLA. J Clin Gastroenterol. 2010;44:e63-e70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Zhao C, Nguyen MH. Hepatocellular Carcinoma Screening and Surveillance: Practice Guidelines and Real-Life Practice. J Clin Gastroenterol. 2016;50:120-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Bruix J, Sherman M; Practice Guidelines Committee American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4504] [Article Influence: 225.2] [Reference Citation Analysis (0)] |
| 24. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5726] [Article Influence: 110.1] [Reference Citation Analysis (2)] |
| 25. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2063] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 26. | Paranaguá-Vezozzo DC, Ono SK, Alvarado-Mora MV, Farias AQ, Cunha-Silva M, França JI, Alves VA, Sherman M, Carrilho FJ. Epidemiology of HCC in Brazil: incidence and risk factors in a ten-year cohort. Ann Hepatol. 2014;13:386-393. [PubMed] |
| 27. | Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 960] [Article Influence: 64.0] [Reference Citation Analysis (1)] |
| 28. | Sangiovanni A, Del Ninno E, Fasani P, De Fazio C, Ronchi G, Romeo R, Morabito A, De Franchis R, Colombo M. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology. 2004;126:1005-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 429] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 29. | Gonçalves CS, Pereira FE, Gayotto LC. Hepatocellular carcinoma in Brazil: report of a national survey (Florianópolis, SC, 1995). Rev Inst Med Trop Sao Paulo. 1997;39:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Teixeira A, Mentea E, Cantao C, Sankarankutty A, Souza F, Motta T, Monsignore L, Elias Junior J, Muglia VF, Abud DG. Clinical Characteristics of 130 Patients With Hepatocellular Carcinoma Followed at a Tertiary Hospital From Brazil. World Journal of Oncol. 2012;3:165-172. |
| 31. | Méndez-Sánchez N, Ridruejo E, Alves de Mattos A, Chávez-Tapia NC, Zapata R, Paraná R, Mastai R, Strauss E, Guevara-Casallas LG, Daruich J. Latin American Association for the Study of the Liver (LAASL) clinical practice guidelines: management of hepatocellular carcinoma. Ann Hepatol. 2014;13 Suppl 1:S4-40. [PubMed] |
| 32. | Méndez-Sánchez N, Villa AR, Vázquez-Elizondo G, Ponciano-Rodríguez G, Uribe M. Mortality trends for liver cancer in Mexico from 2000 to 2006. Ann Hepatol. 2008;7:226-229. [PubMed] |
| 33. | Ferreira PR, Brandão-Mello CE, Estes C, Gonçales Júnior FL, Coelho HS, Razavi H, Cheinquer H, Wolff FH, Ferraz ML, Pessoa MG. Disease burden of chronic hepatitis C in Brazil. Braz J Infect Dis. 2015;19:363-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Portugal FB, Campos MR, de Carvalho JR, Flor LS, Schramm JM, Costa Mde F. Disease burden in Brazil: an investigation into alcohol and non-viral cirrhosis. Cien Saude Colet. 2015;20:491-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Gonçalves PL, Zago-Gomes Mda P, Gonçalves CS, Pereira FE. Hepatitis virus and hepatocellular carcinoma in Brazil: a report from the State of Espírito Santo. Rev Soc Bras Med Trop. 2014;47:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3241] [Cited by in RCA: 3281] [Article Influence: 149.1] [Reference Citation Analysis (0)] |
| 37. | Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 960] [Cited by in RCA: 939] [Article Influence: 44.7] [Reference Citation Analysis (1)] |
| 38. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4515] [Article Influence: 347.3] [Reference Citation Analysis (2)] |
| 39. | Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 797] [Cited by in RCA: 840] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 40. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6562] [Article Influence: 468.7] [Reference Citation Analysis (1)] |
| 41. | Carrillo FJ, Mattos AA, Vianey AF, Vezozzo DC, Marinho F, Souto FJ, Cotrim HP, Coelho HS, Silva I, Garcia JH. Brazilian society of hepatology recommendations for the diagnosis and treatment of hepatocellular carcinoma. Arq Gastroenterol. 2015;52 Suppl 1:2-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Kokudo N, Hasegawa K, Akahane M, Igaki H, Izumi N, Ichida T, Uemoto S, Kaneko S, Kawasaki S, Ku Y. Evidence-based Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines). Hepatol Res. 2015;45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 320] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 43. | Chan SL, Chan AT, Yeo W. Role of alpha-fetoprotein in hepatocellular carcinoma: prognostication, treatment monitoring or both? Future Oncol. 2009;5:889-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 44. | Schraiber Ldos S, de Mattos AA, Zanotelli ML, Cantisani GP, Brandão AB, Marroni CA, Kiss G, Ernani L, Marcon Pdos S. Alpha-fetoprotein Level Predicts Recurrence After Transplantation in Hepatocellular Carcinoma. Medicine (Baltimore). 2016;95:e2478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 45. | Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15 Suppl 4:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 736] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 46. | Chiaramonte M, Stroffolini T, Vian A, Stazi MA, Floreani A, Lorenzoni U, Lobello S, Farinati F, Naccarato R. Rate of incidence of hepatocellular carcinoma in patients with compensated viral cirrhosis. Cancer. 1999;85:2132-2137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 47. | El-Serag HB, Johnson ML, Hachem C, Morgana RO. Statins are associated with a reduced risk of hepatocellular carcinoma in a large cohort of patients with diabetes. Gastroenterology. 2009;136:1601-1608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 225] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 48. | Chiu HF, Ho SC, Chen CC, Yang CY. Statin use and the risk of liver cancer: a population-based case-control study. Am J Gastroenterol. 2011;106:894-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 49. | Tsan YT, Lee CH, Ho WC, Lin MH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis C virus infection. J Clin Oncol. 2013;31:1514-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 171] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 50. | Singh S, Singh PP, Roberts LR, Sanchez W. Chemopreventive strategies in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2014;11:45-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 220] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 51. | Singh S, Singh PP. Statins for prevention of hepatocellular cancer: one step closer? Hepatology. 2014;59:724-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Lai SW, Liao KF, Lai HC, Muo CH, Sung FC, Chen PC. Statin use and risk of hepatocellular carcinoma. Eur J Epidemiol. 2013;28:485-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 53. | Björkhem-Bergman L, Backheden M, Söderberg Löfdal K. Statin treatment reduces the risk of hepatocellular carcinoma but not colon cancer-results from a nationwide case-control study in Sweden. Pharmacoepidemiol Drug Saf. 2014;23:1101-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 54. | McGlynn KA, Divine GW, Sahasrabuddhe VV, Engel LS, VanSlooten A, Wells K, Yood MU, Alford SH. Statin use and risk of hepatocellular carcinoma in a U.S. population. Cancer Epidemiol. 2014;38:523-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |