Published online Nov 28, 2016. doi: 10.3748/wjg.v22.i44.9865
Peer-review started: May 30, 2016
First decision: July 29, 2016
Revised: August 30, 2016
Accepted: September 28, 2016
Article in press: September 28, 2016
Published online: November 28, 2016
Processing time: 182 Days and 2.7 Hours
This is the first report of living donor liver transplantation (LDLT) for congenital hepatic fibrosis (CHF) using a mother’s graft with von Meyenburg complex. A 6-year-old girl with CHF, who suffered from recurrent gastrointestinal bleeding, was referred to our hospital for liver transplantation. Her 38-year-old mother was investigated as a living donor and multiple biliary hamartoma were seen on her computed tomography and magnetic resonance imaging scan. The mother’s liver function tests were normal and she did not have any organ abnormality, including polycystic kidney disease. LDLT using the left lateral segment (LLS) graft from the donor was performed. The donor LLS graft weighed 250 g; the graft recipient weight ratio was 1.19%. The operation and post-operative course of the donor were uneventful and she was discharged on post-operative day (POD) 8. The graft liver function was good, and the recipient was discharged on POD 31. LDLT using a graft with von Meyenburg complex is safe and useful. Long-term follow-up is needed with respect to graft liver function and screening malignant tumors.
Core tip: Multiple biliary hamartoma is a rare, benign lesion known as von Meyenburg complex. This is the first report of living donor liver transplantation (LDLT) using a liver graft with von Meyenburg complex. A 6-year-old girl with congenital hepatic fibrosis, who suffered from recurrent gastrointestinal bleeding, was transplanted her mother’s liver graft with von Meyenburg complex. Successful LDLT was performed, and the liver and renal function after LDLT were good also in recipient and donor. LDLT using a graft with von Meyenburg complex is safe and useful for a further expansion of living donor pool. Long-term follow-up is needed with respect to graft liver function and screening malignant tumors.
- Citation: Yamada N, Sanada Y, Katano T, Tashiro M, Hirata Y, Okada N, Ihara Y, Miki A, Sasanuma H, Urahashi T, Sakuma Y, Mizuta K. Pediatric living donor liver transplantation for congenital hepatic fibrosis using a mother’s graft with von Meyenburg complex: A case report. World J Gastroenterol 2016; 22(44): 9865-9870
- URL: https://www.wjgnet.com/1007-9327/full/v22/i44/9865.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i44.9865
Multiple biliary hamartoma is a rare, benign lesion known as von Meyenburg complex[1]. The frequency is unknown, but Lin et al[2] have reported that it was seen in 0.35% of patients undergoing liver biopsy. Multiple biliary hamartoma is a benign tumor and does not affect liver function. However, there exist only two case reports of liver transplantation (LT) with a donor having von Meyenburg complex, both of which were orthotropic liver transplantation LT[3,4]. Due to the lack of information, graft liver function with von Meyenburg complex and the risk of malignancy in the graft liver are unknown.
To the best of our knowledge, there have been no reports of living donor liver transplantation (LDLT) from a donor with von Meyenburg complex. We herein report a case of pediatric LDLT for congenital hepatic fibrosis (CHF) with a graft taken from the mother having von Meyenburg complex, and considered the possibility of a liver with von Meyenburg complex for a further expansion of living donor pool.
Interestingly, the recipient (the donor’s daughter) had CHF, which is considered to be associated with von Meyenburg complex. Both of these conditions are considered as type of hepatobiliary fibropolycystic disease[5]. The case especially focuses on the preoperative imaging findings and liver function of the donor and the recipient.
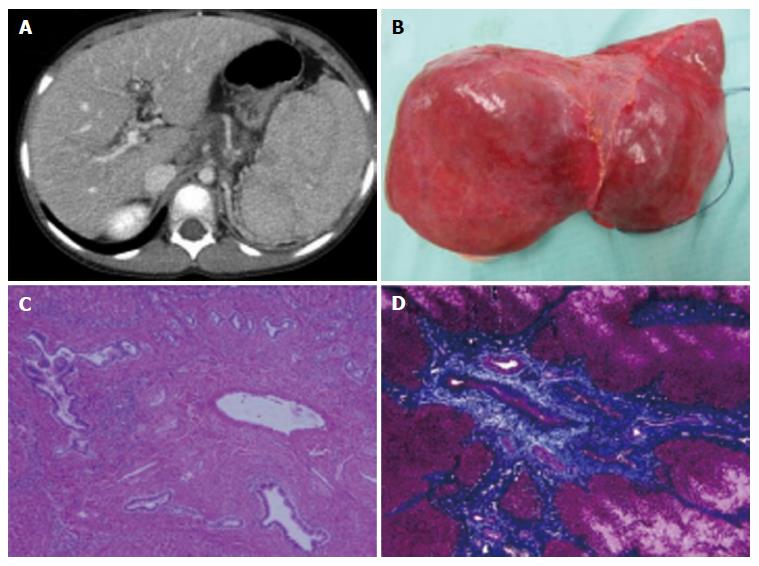
A 6-year-old female patient with CHF was referred to our hospital for LT. She developed sudden hematemesis due to esophagogastric varices and had been diagnosed with CHF based on histological findings from a liver biopsy that was done when she was 4 years old. She had been suffering from recurrent gastrointestinal bleeding for 2 years and was considered competent for LT (Figure 1A, Table 1).
| Items | Results | Units |
| WBC | 3.6 × 104 | /μL |
| RBC | 372 | /μL |
| Hb | 7.9 × 104 | g/dL |
| Ht | 26.4 | % |
| Plt | 6.9 | /μL |
| TP | 6.4 | g/dL |
| Alb | 4.2 | g/dL |
| BUN | 10 | mg/dL |
| Cre | 0.24 | mg/dL |
| T-Bil | 0.67 | mg/dL |
| AST | 43 | U/L |
| ALT | 39 | U/L |
| LDH | 258 | U/L |
| γ-GTP | 41 | U/L |
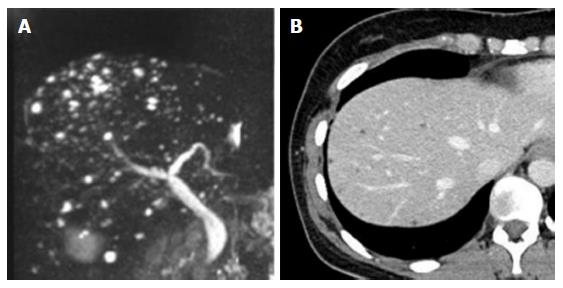
The only living donor candidate was the patient’s 38-year-old mother. The mother had undergone clinical examinations required for evaluation as a potential donor. Her magnetic resonance imaging (MRI) scan showed multiple 3-5 mm nodules with high intensity in T2 weighted imaging (Figure 2A). The nodules were dominant in the right lobe of her liver, although they were seen in the left lobe to some extent. Her computed tomography (CT) scan also revealed multiple low-density areas that were 3-5 mm in diameter, and had higher density in CT scan than the water level (Figure 2B). Therefore, rather than multiple liver cyst, multiple biliary hamartoma, also known as von Meyenburg complex was suspected. Her standard liver function tests were normal (T-Bil; 0.33 mg/dL, AST; 12 U/L, ALT; 11 U/L), and she did not have cystic disease of any other organ, especially polycystic kidney disease. Although her daughter developed repetitive severe gastrointestinal bleeding, no other living donor was available, and therefore the mother was selected as the living donor.
During LDLT, hepatectomy of the donor’s left-lateral segment (LLS) was performed. Multiple cystic lesions were observed in the mother’s liver during surgery, more so in the right lobe of the liver. The LLS graft from the donor weighed 250 g, and it was estimated to be 22.7% of the donor’s total liver volume of the donor. The operation time and amount bleeding were 4 h 24 min and 430 mL, respectively. The operation and post-operative course of the donor were uneventful and she was discharged on post-operative day (POD) 8.
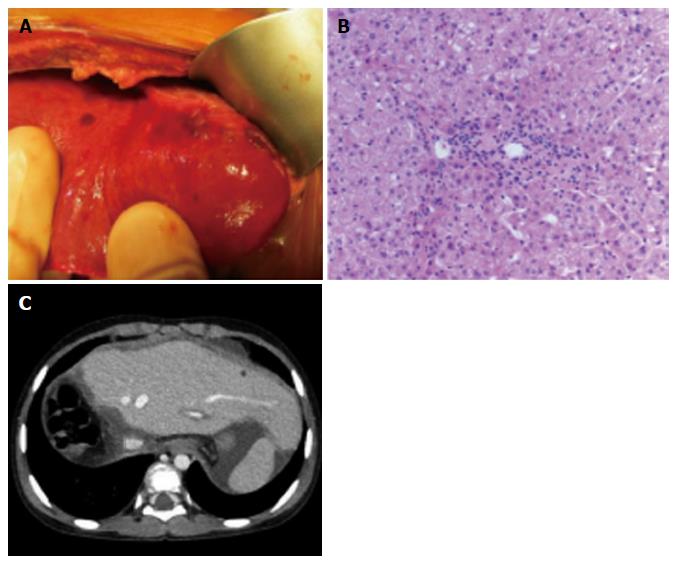
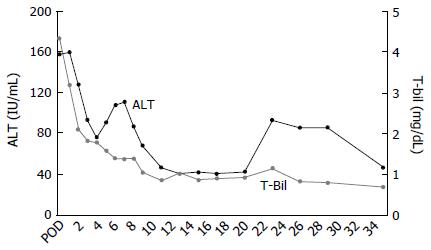
During the recipient’s surgery, the graft liver was transplanted after total hepatectomy. Her native liver showed fibrotic changes in the portal area, with proliferation of the pseudocholangiolar ducts, and it was consistent with CHF (Figure 1B-D). The graft-recipient weight ratio and graft volume/standard liver volume were 1.19% and 43.2%, respectively. The vascular reconstructions were anastomosed from the donor’s left hepatic vein to the recipient’s middle and left hepatic vein, the donor’s left portal vein to the recipient’s main portal vein, and the donor’s left hepatic artery to the recipient’s right hepatic artery. After reperfusion of the graft liver, we observed multiple small lesions suspected to be biliary hamartoma (Figure 3A). Time zero biopsy of the graft liver revealed the slight fibrosis around portal area, with a Metavir fibrosis score F0 (Figure 3B). The operation time and estimated bleeding volume were 10 h 20 min and 380 mL, respectively. Tacrolimus and Methylprednisolone were used as postoperative immunosuppressive therapy. After LDLT, the recipient developed catheter-related bloodstream infection on POD 7 and obstruction of external biliary drainage tube on the POD 22, which was accompanied by slight temporary elevation of alanine transaminase (Figure 4). However, the graft liver function was good, and gastrointestinal bleeding did not occur after LDLT. She was therefore discharged on POD 31. Her routine CT scan on POD 28 revealed some small nodules suspected to biliary hamartoma (Figure 3C). One year have passed after LDLT, and her graft liver and renal function were normal. Her oncogenic surveillance was also with no abnormal findings.
Kerr et al[6] first reported in 1961 that CHF is a hereditary autosomal recessive fibropolycystic disease of the liver. It is caused by ductal plate malformations and is sometimes accompanied by polycystic kidney disease[7,8]. The typical pathological findings of a liver with CHF are progressive fibrosis around the portal veins and cystic changes of the peripheral small-sized bile ducts in the thick fibrotic band. Survival depends mainly on complications of portal hypertension, such as variceal bleeding and hypersplenism, and some cases are complicated with cholangiocarcinoma or hepatocellular carcinoma in the long term. There are some patients with CHF who progress to liver failure or severe gastrointestinal bleeding, and require LT.
Hepatobiliary fibropolycystic disease, including Caroli disease, von Meyenburg complex (multiple biliary hamartoma), CHF, and polycystic liver disease are considered to arise from the same etiology, with different levels of the biliary tract being affected. von Meyenburg complex is now considered to not be a pure liver disease, but rather a multi-organ disorder involving the brain, portal vein, kidneys, and bile ducts; some genetic linked disorders have been identified[9-11]. Interestingly, this is the first case report wherein a parent has von Meyenburg complex and the child has CHF, but neither had multi-organ disorder.
Only two case reports exist of LT from patients with von Meyenburg complex; both of these cases are orthotropic LT. They were diagnosed at procurement of LT, and the recipient’s graft liver function was good after LT. Our case is likely the first case report of LDLT from a donor having von Meyenburg complex. We could not obtain the histopathological diagnosis of the biliary hamartoma; however, the MRI and CT findings of von Meyenburg complex are characteristic and reportedly enable a highly accurate diagnosis[12,13]. Although some lesions of von Meyenburg complex were seen in the donor’s left lobe, they were dominant in the right lobe, and preoperative laboratory liver function tests in the donor were normal. There was no other living donor available, due to which the LLS graft with some lesions of von Meyenburg complex had to be retrieved. Although time zero biopsy of the graft revealed slight fibrosis in the portal area, the graft function and clinical course of the recipient were good. To our experience and according to previous reports, graft liver with von Meyenburg complex shows no problem regarding graft function in the early post-LT phase.
The von Meyenburg complex usually consists of benign tumors, but there have been several case reports of cholangiocarcinoma or hepatocellular carcinoma arising from the liver with the multiple biliary hamartoma[14-17]. To date, the frequency of cholangiocarcinoma arising from a liver with von Meyenburg complex is unknown; whether these cholangiocarcinomas were derived from the biliary hamartoma is also unknown. However, under the circumstances, oncologic surveillance of the graft liver in the recipient should be performed over time. In addition, hepatobiliary fibropolycystic disease, including CHF, is sometimes accompanied by polycystic kidney disease, which can result in renal failure[18]. When following up the LT recipient for CHF in the outpatient clinic, physicians should check renal function as well as for the appearance of cysts in the kidney.
In our institute, we perform the routine blood test (including standard liver function test and renal function test) and echo check per 3 mo for first 5 years and per 6 mo after that. We also examine the CT scan at 1 mo, 6 mo, and 1 year after LT. From 1 year to 5 years after LT, CT scan were performed annually; thereafter they were examined every 2 years[18]. We will perform same follow up for the recipient. In addition, we will add the tumor marker test (carcinoembryonic antigen, carbohydrate antigen 19-9), when blood examination will be performed.
In conclusion, we successfully treated a patient with CHF by performing LDLT using the mother’s graft that had von Meyenburg complex. Long-term follow-up regarding graft liver function, screening for malignant tumors, checking renal function, and watching for the appearance of polycystic kidney disease is needed.
A 6-year-old girl with congenital hepatic fibrosis (CHF), who suffered from recurrent gastrointestinal bleeding, was transplanted her mother’s liver graft with von Meyenburg complex.
The recipient was suffered from hematemesis. The donor had no symptoms; the von Meyenburg complex was detected after donor’s examination.
Hepatobiliary fibropolycystic disease, including Caroli disease, von Meyenburg complex (multiple biliary hamartoma), CHF, and polycystic liver disease.
The donor with von Meyenburg complex was normal liver function.
The donor’s magnetic resonance imaging scan showed multiple 3-5 mm nodules with high intensity in T2 weighted imaging.
The recipient was diagnosed CHF.
Living donor liver transplantation (LDLT).
There exist only two case reports of liver transplantation with a donor having von Meyenburg complex, both of which were orthotropic liver transplantation.
Multiple biliary hamartoma is a rare, benign lesion known as von Meyenburg complex, which is included in the hepatobiliary fibropolycystic disease.
LDLT using a graft with von Meyenburg complex is safe and useful for a further expansion of living donor pool. Long-term follow-up is needed with respect to graft liver function and screening malignant tumors.
This paper is informative and is suitable for publishing.
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Manuscript source: Unsolicited manuscript
P- Reviewer: Arni D, Ma JQ S- Editor: Yu J L- Editor: A E- Editor: Zhang FF
| 1. | Mortelé B, Mortelé K, Seynaeve P, Vandevelde D, Kunnen M, Ros PR. Hepatic bile duct hamartomas (von Meyenburg Complexes): MR and MR cholangiography findings. J Comput Assist Tomogr. 2002;26:438-443. [PubMed] |
| 2. | Lin S, Weng Z, Xu J, Wang MF, Zhu YY, Jiang JJ. A study of multiple biliary hamartomas based on 1697 liver biopsies. Eur J Gastroenterol Hepatol. 2013;25:948-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Saidi RF, Yoon V, Jabbour N, Shah SA, Bozorgzadeh A. Liver transplantation from a donor with multiple biliary hamartomata. Int J Organ Transplant Med. 2013;4:35-37. [PubMed] |
| 4. | Perkins JD. Are we reporting the same thing? Liver Transpl. 2007;13:465-466. [PubMed] |
| 5. | Summerfield JA, Nagafuchi Y, Sherlock S, Cadafalch J, Scheuer PJ. Hepatobiliary fibropolycystic diseases. A clinical and histological review of 51 patients. J Hepatol. 1986;2:141-156. [PubMed] |
| 6. | Kerr DN, Harrison CV, Sherlock S, Walker RM. Congenital hepatic fibrosis. Q J Med. 1961;30:91-117. [PubMed] |
| 7. | Landing BH, Wells TR, Lipsey AI, Oyemade OA. Morphometric studies of cystic and tubulointerstitial kidney diseases with hepatic fibrosis in children. Pediatr Pathol. 1990;10:959-972. [PubMed] |
| 8. | Desmet VJ. What is congenital hepatic fibrosis? Histopathology. 1992;20:465-477. [PubMed] |
| 9. | Yönem O, Ozkayar N, Balkanci F, Harmanci O, Sökmensüer C, Ersoy O, Bayraktar Y. Is congenital hepatic fibrosis a pure liver disease? Am J Gastroenterol. 2006;101:1253-1259. [PubMed] |
| 10. | Gunay-Aygun M, Font-Montgomery E, Lukose L, Tuchman Gerstein M, Piwnica-Worms K, Choyke P, Daryanani KT, Turkbey B, Fischer R, Bernardini I. Characteristics of congenital hepatic fibrosis in a large cohort of patients with autosomal recessive polycystic kidney disease. Gastroenterology. 2013;144:112-121.e2. [PubMed] |
| 11. | Cnossen WR, Drenth JP. Polycystic liver disease: an overview of pathogenesis, clinical manifestations and management. Orphanet J Rare Dis. 2014;9:69. [PubMed] |
| 12. | Madhusudhan KS, Das CJ. von Meyenburg’s complex in a patient with primary malignancy: role of MRI. Indian J Surg. 2009;71:98-100. [PubMed] |
| 13. | Zheng RQ, Zhang B, Kudo M, Onda H, Inoue T. Imaging findings of biliary hamartomas. World J Gastroenterol. 2005;11:6354-6359. [PubMed] |
| 14. | Röcken C, Pross M, Brucks U, Ridwelski K, Roessner A. Cholangiocarcinoma occurring in a liver with multiple bile duct hamartomas (von Meyenburg complexes). Arch Pathol Lab Med. 2000;124:1704-1706. [PubMed] |
| 15. | Dekker A, Ten Kate FJ, Terpstra OT. Cholangiocarcinoma associated with multiple bile-duct hamartomas of the liver. Dig Dis Sci. 1989;34:952-958. [PubMed] |
| 16. | Burns CD, Kuhns JG, Wieman TJ. Cholangiocarcinoma in association with multiple biliary microhamartomas. Arch Pathol Lab Med. 1990;114:1287-1289. [PubMed] |
| 17. | Jain D, Nayak NC, Saigal S. Hepatocellular carcinoma arising in association with von-Meyenburg’s complexes: an incidental finding or precursor lesions? A clinicopatholigic study of 4 cases. Ann Diagn Pathol. 2010;14:317-320. [PubMed] |
| 18. | Hirata Y, Sanada Y, Urahashi T, Ihara Y, Yamada N, Okada N, Tashiro M, Katano T, Otomo S, Ushijima K. Relationship Between Graft Liver Function and the Change of Graft Liver and Spleen Volumes After Technical Variant Liver Transplantation. Transplant Proc. 2016;48:1105-1109. [PubMed] |