Published online Nov 21, 2016. doi: 10.3748/wjg.v22.i43.9525
Peer-review started: April 14, 2016
First decision: August 29, 2016
Revised: September 7, 2016
Accepted: October 10, 2016
Article in press: October 10, 2016
Published online: November 21, 2016
Processing time: 219 Days and 16.5 Hours
To determine the expression and localization of the electrogenic Na+/HCO3- cotransporter (NBC1) in rat pancreas during development.
The rat pancreas from postnatal and embryos removed from the uterus of pregnant rats that had been sacrificed by CO2 asphyxiation were used. Rat pancreas from embryonic day (E) 15.5 and E18.5 rat embryos was isolated under a stereomicroscope. Rat pancreas from postnatal (P) days 0, 7, 14, 21 and adult was directly isolated by the unaided eye. The RT-PCR analysis of the NBC1 specific region on rat pancreas tissues from different developmental stages. The two antibodies which target the NBC1 common COOH-terminal region and NH2-terminal region detected a clear band of about 145 kDa in the Western blot analysis. The localization of NBC1 was examined by immuno-fluorescence detection.
The results revealed the first peak of NBC1 expression at E18.5 and the second peak at P14. Meanwhile, the low NBC1 expression occurred at P7 and adult stages. Our results demonstrated, for the first time, the presence of NBC1 in the plasma membrane of β and α cells, as well as in the basolateral membrane of acinar cells of the rat pancreas at different stages of development.
The data strongly suggests that NBC1 is diversely expressed in the pancreas at different developmental stages, where it may exert its functions in pancreatic development especially islet cell growth through HCO3- transport and pH regulation.
Core tip: This manuscript demonstrated that Na+/HCO3- cotransporter (NBC1) was present in the plasma membrane of β and α cells as well as in the basolateral membrane of acinar cells in rat developing pancreas by immuno-fluorescence detection. NBC1 mRNA and protein were diversely expressed in the pancreas at different developmental stages by reverse transcription polymerase chain reaction and Western blot analysis. The result suggests that it may exert its functions in pancreatic development especially islet cell growth through HCO3- transport and pH regulation.
- Citation: Cao LH, Xia CC, Shi ZC, Wang N, Gu ZH, Yu LZ, Wan Q, De W. Na+/HCO3- cotransporter is expressed on β and α cells during rat pancreatic development. World J Gastroenterol 2016; 22(43): 9525-9533
- URL: https://www.wjgnet.com/1007-9327/full/v22/i43/9525.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i43.9525
The pancreas is an organ which contains two distinct cell populations; the exocrine cells secrete enzymes into the digestive tract and the endocrine cells secrete hormones into the bloodstream, which are involved in nutrient homeostasis, such as insulin and glucagons[1]. It arises from the endoderm as a dorsal and a ventral bud which fuse together to form the single organ. By embryonic day (E)14.5, the development of exocrine cells into the acini and ducts is recognizable. The islets are not fully formed until shortly before birth on E18.5-E19.0[2-4] and undergo further remodeling and maturation for 2-3 wk after birth[5]. It is well known that the maintenance of cell pH within a normal range is crucial for essential biochemical and metabolic functions. Previous studies have established that Na+/HCO3- cotransporter (NBC1) is one of the major factors which regulate intracellular and extracellular acid-base homeostasis[6-10]. Consistent with this notion, our study suggests that NBC1 mRNA is specifically and significantly expressed in the later embryonic and neonatal stages of the rat pancreas when its architecture and function are progressively being accomplished (unpublished observations).
NBC1 is one of the integral membrane protein families that mediate sodium-bicarbonate cotransport. It plays important roles in systemic and cellular pH homeostasis and trans-epithelial solute transport together with other members of the bicarbonate transporter superfamily[11-13]. Its activity was first identified in the kidney of the salamander Ambystoma tigrinum[14]. To date, NBC1 isoforms have also been found in various tissues, such as pNBC1 from pancreas[15], hNBC1 from heart[16], and rbNBC1 from brain[17]. These isoforms differ at the NH2-terminal or COOH-terminal. The importance of NBC1 in normal organ function is highlighted by the latest evidence revealing that patients with homozygous missense mutations in the NBC1 gene have proximal renal tubular acidosis, short stature, enamel hypoplasia, and bilateral ocular disease[18]. However, even though a detailed knowledge of the abundance of NBC1 has long been exploited, less is known about its expression pattern in the normal developing pancreas. Accordingly, to gain further insight into the molecular mechanisms responsible for NBC1 in the pancreatic cells, we used rabbit polyclonal antibodies which target the common epitopes of pNBC1 and kNBC1 to determine the expression pattern and cellular localization of NBC1 during the development of the rat pancreas.
Sprague Dawley (SD) rats were purchased from Animal Center of Nanjing Medical University (Nanjing, China). Male and female SD rats were mated overnight. At noon of the next day, if the vaginal plug was discovered it was considered as Day 0.5 of gestation (E0.5). The embryos were removed from the uterus of pregnant rats which had been sacrificed by CO2 asphyxiation. Pancreas from E15.5 and E18.5 rat embryos was isolated under a stereomicroscope, as previously described[19]. Rat pancreas from postnatal (P) days 0, 7, 14, 21 and adult were directly isolated by the unaided eye. The harvested tissues were immediately rinsed three times with PBS to remove serum proteins, and then fixed with 4% paraformaldehyde in PBS overnight for histology or frozen in liquid nitrogen for RNA and protein isolation.
Isolated rat pancreas tissue was dissolved in Trizol (Invitrogen, Carlsbad, CA, United States) and total RNA was purified using the RNeasy Mini Kit (QIAGEN,V alencia, CA, United States) following the manufacturer’s instructions. First strand cDNA was synthesized using 2 μg DNA-free total RNA, random 9 primers and reverse transcriptase. PCR amplification was performed in a 25-μL reaction system containing 25 ng of cDNA, 0.2 nmol of each primer set, and 0.3 μL of Taq DNA polymerase (QIAGEN). For detection of NBC1 variants, the following protocol was used: denaturation at 95 °C for 5 min. Twenty-nine cycles of PCR amplification performed under following conditions: denaturation at 95 °C for 30 s, annealing at 53 °C for 30 s, and elongation at 72 °C for 1 min. The final extension step was at 72 °C for 10 min and followed by rapid cooling at 4 °C to terminate the reaction. The control used was the amplification of the 18S rRNA. The primer sets used are as follows: NBC1, forward: 5’-CTACCTCTTCTCCCAGCACGAC-3’, reverse: 5’-TGGTTTCTGTTCCCTTGTTCCT-3’ (total amplified fragment, 402 bp); 18S rRNA, forward: 5’-ACGAACCAGAGCGAAAGC-3’, reverse: 5’-GGACATCTAAGGGCATCACAG-3’ (total amplified fragment, 538 bp). The amplified products were analyzed on 1% agarose gels and visualized by ethidium bromide staining. The data were normalized to the 18S rRNA subunit.
Pancreatic tissue was purified by a combination of differential centrifugation and discontinuous sucrose gradient centrifugation[20,21]. The concentration of the membrane proteins fraction of each sample was analyzed by a modified Bradford assay. An equal amount of protein samples (100 μg protein in 40 μL buffer) from each time point separated by 7.5% SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA, United States). For blocking unspecific binding, membranes were incubated in 5% fat-free milk in Tris-buffered saline plus 0.05% Tween 20 for 2 h at room temperature. The membranes were then incubated with the primary antibodies (ab3208 or ab3212, two affinity purified rabbit polyclonal antibodies raised against a peptide mapping to the COOH-terminal amino acids 928-1035 and NH2-terminal amino acids 338-391 of NBC1 respectively; Chemicon, Temecula, CA, United States), diluted 1:500, overnight at 4 °C. After washing in TBST, the membranes were incubated with the peroxidase-linked goat anti-rabbit IgG conjugates (1858415, diluted 1:10000; Pierce, Rockford, CA, United States) for 1 h at room temperature. At the end, the membranes were washed again in TBST, incubated in chemiluminescence enhancer reagents (ECL, Amersham Life Science, Cleveland, OH, United States) for 5 min, and exposed to VA 711 B Blue Sensitive X-ray films. Loading controls of presumably constitutively expressed proteins, such as β-actin, were used. However, their variability and increase in development precluded their use[22]. Densitometric quantification of the bands at subsaturating levels was performed using the Syngene tool gel analysis software (Syngene, Cambridge, United Kingdom).
The paraffin sections were deparaffinized in xylene, then rehydrated in graded ethanol and distilled water. The nonspecific binding sites were blocked in 1% BSA for 30 min. For NBC1 and amylase, insulin, glucagon double immunofluorescence, the rabbit anti-NBC1 primary polyclonal antibodies were applied and revealed using the FITC-labeled donkey anti-rabbit IgG (AP-182F, diluted 1:200; Chemicon, Inc). Mouse anti-amylase primary polyclonal antibody (sc-45667, diluted 1:500; Santa Cruz Biotechnology, Inc, Santa Cruz, CA, United States), mouse anti-insulin primary monoclonal antibody (ab6995, diluted 1:2000; abcam, Cambridge, United Kingdom) or mouse anti-glucagon primary polyclonal antibody (G-2654,diluted 1:2500; Sigma Co, St. Louis, MO, United States) was then applied and revealed by CY3-labeled donkey anti-mouse IgG (AP192C, diluted 1:400; Chemicon Inc). Sections were placed in Mounting Medium (Gel Mount Aqueous, G0918; Sigma Co.) with a cover glass and were examined under an Olympus BX51 Research Microscope. To rule out cross-reactivity in this staining system, the controls used were: first, single staining with the alternative secondary antibody; and second, staining in the absence of primary antibody. In neither case was staining detectable. Images were acquired at × 400 magnification.
All rats were sacrificed by CO2 asphyxiation.
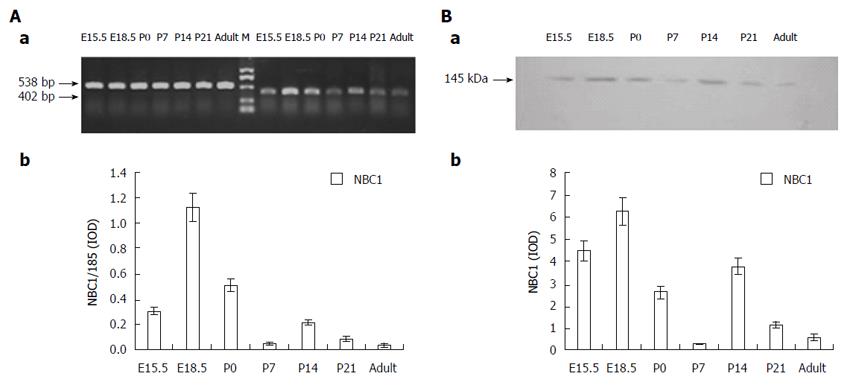
The RT-PCR analysis of the NBC1 specific region on rat pancreas tissues from different developmental stages yielded a band of the expected size (402 bp), as shown in Figure 1A, where it is also obvious that NBC1 mRNA was expressed at all the developmental stages tested. Detectable expression was observed at E15.5, and the high NBC1 mRNA expression was observed at E18.5, followed by P14. Meanwhile, the low NBC1 mRNA expression was observed at P7 and in the adult rat.
The two antibodies which target the NBC1 common COOH-terminal region and NH2-terminal region detected a clear band of about 145 kDa in the Western blot analysis of the membrane protein fraction samples extracted from pancreas of E15.5, E18.5, P0, P7, P14, P21 and adult rats, as illustrated in Figure 1B. Densitometric quantification of each band showed that the expression level of NBC1 was high at E18.5, followed by P14, after which it steadily decreased and was the lowest at P7 and in adult rats. No labeling was obtained by incubation with pre-immune serum (data not shown). The pattern of NBC1 protein expression and abundance tendency revealed by this analysis (Figure 1B) are consistent with the NBC1 mRNA expression (Figure 1A) profile described above.
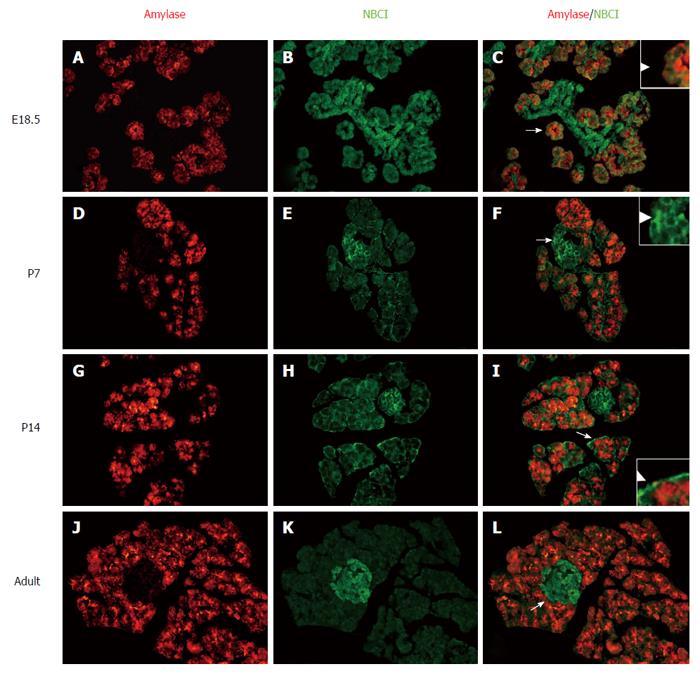
Serial pancreas sections (from E18.5, P7, P14 and adult rat) were double-stained with immunofluorescence labeled antibodies against both the NBC1 and the acinar cell markers amylase. The anti-NBC1 antibody-labeled acinar cells and other cells were mainly observed at the basolateral cell sides. The results additionally showed the exclusive overlapping at the basolateral membrane between NBC1-positive cells and cells labeled with antibodies against amylase at every stage tested (Figure 2C, F, I and L). The NBC1 positive signal was extensive and strong at E18.5 (Figure 2A-C) and P14 (Figure 2G-I), after which the fluorescence signals at the basolateral membrane of the acinar cells declined to a weak but detectable level at P7 (Figure 2D-F) and adult rat (Figure 2J-L). Moreover, along with the developing islet clusters, NBC1 expression tended to be more grouped in the islets cells compared to that in acinar cells especially in adult rats (Figure 2J-L).
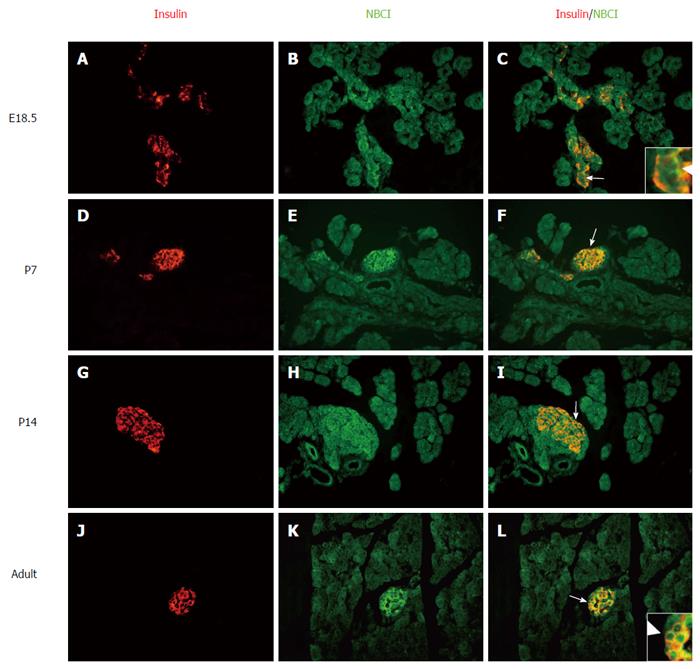
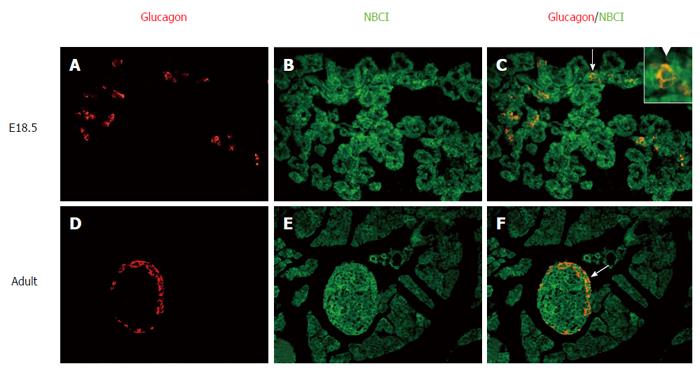
To further identity the other cells (Figure 2) that express NBC1, pancreatic sections from different developmental stages were double-stained with fluorescently labeled antibodies to both NBC1 and the β cell markers insulin (Figure 3) or the α cell markers glucagon (Figure 4). Together, these results showed the exclusive colocalization, at the plasma membrane, of NBC1 and insulin (Figure 3C, F, I and L) or glucagon (Figure 4C and F) at every stage tested. The result of these analyses demonstrated a close association between NBC1 and the developing pancreas.
The pancreas develops from simple budlike structures on the primitive gut tube to a highly branched organ containing many specialized cell types. This is a highly coordinated process. As it is well known, the pH of internal and external body fluids is mainly controlled by the HCO3-/CO2 buffer system. Additionally, both the pNBC1 and kNBC1 proteins were detected in the basolateral sides of the rat pancreas acinar cells and ducts using N-terminal variant specific antibodies[23,24]. NBC1 expressed in pancreas may contribute to HCO3- transport and pH regulation to maintain the internal environment homeostasis[25,26]. From later embryonic stages to adult stages, pancreatic cells undergo dramatic differentiation, proliferation, apoptosis and remodeling. Therefore, the observed change on NBC1 expression level during the later embryonic period and after birth may not be a coincidence.
Studies have shown region-specific expression of different forms of NBC1, with kNBC1 being prominent in the kidney and pNBC1 being dominant in the pancreas. Moreover, reports also suggested that both pNBC1 and kNBC1 were transcribed from the same gene. Indeed, they only differ at the NH2-terminal or COOH-terminal region and might even have identical function[27]. In this study the antibodies used were developed against the common epitope of the two NBC1 isoforms, which could simultaneously identify the two isoforms expressed in the rat pancreatic tissue. These antibodies were successfully certified by Jensen et al[28] on their research on NBC1 distribution with convincing results. Accordingly, it can be asserted that the antibodies used in this study are reliably, sensitively and specifically detecting NBC1. Our results provide a better understanding of the tissue expression and cellular localization of NBC1 in rat pancreas.
Immunoblotting analysis of pancreatic tissues in different developmental stages revealed similar bands with molecular weight of about 145 kDa (Figure 1B). Specifically, the Western blot analysis revealed that NBC1 peptide expression was increased at E18.5 and P14, then was markedly decreased at P7 and the adult stage. Consistent with the results of Western blot analysis, the mRNA level of NBC1 exhibited a similar expression pattern (Figure 1A). Both of these results on protein and mRNA in adult rat pancreas are fully consistent with the study by Roussa et al[23] and Satoh et al[29]. From E18.5 to birth, active cellular organization proceeds to form the classic cellular features of the pancreas. In addition, after birth, a great amount of islet cells undergo apoptosis. From P14 to P21, islet cell proliferation increases dramatically and the islet structure is remodeled[30,31]. Acid-base homeostasis of the internal environment is necessary for the formation of the pancreatic architecture and islet structure remodeling. The specific expression pattern of NBC1 protein and mRNA suggest its potential involvement at different stages of rat pancreatic development.
The anti-NBC1 antibodies labeled acinar cells, shown in Figure 2, shows that the labeling was quite intense at E18.5 and P14, weak but detectable at P7 and adult rat. This result was also in agreement with the mRNA and protein data. Interestingly, at E18.5, acinar cells positive for messenger RNA were weak comparable to other cells. However, in the postnatal period when the endocrine cells migrate to form the mature islet structure, the labeling by the anti-NBC1 antibodies was observed not only in acinar cells but also in islet cells (Figure 2D-L). The signal of the islet cells was obviously stronger than that observed acinar cells in adult rat (Figure 2L). NBC1 is one of the transporters that maintain the intercellular pH stable at approximately 7.13 in acinar cells, where the Na+/HCO3- cotransporter removes excess base from the cytosol. Other potential roles of this transporter include participating in NaCl and fluid secretion by the pancreatic acini and regulating cell growth[25]. Taken together, our results point to a possible role of NBC1 in the development and function of acinar cells. Further study could provide additional insight into the exact mechanism underlying this expression pattern and its precise roles.
The localization of NBC1 in islet cells is shown in Figures 3 and 4. There was a bulk of overlapping between the NBC1 positive cells and cells stained with antibodies against insulin and glucagon which are present in pancreatic α and β cells. The staining of α and β cells was strong but no marked distinction was observed at any of the stages analyzed. Nevertheless, the above results revealed that the low expression was at P7 and adult rat. The endocrine pancreas undergoes major remodeling during neonatal development involving a mechanism whereby the β cell growth or apoptosis is regulated[31,32]. Thus, β cell apoptosis could be the main factor in the refinement of cell mass at P7. The endocrine pancreas makes up only 1.5 percent of the entire pancreas[33], accordingly the total expression level of NBC1 could be low in adult rat. Some studies have shown that an acidic granular pH is important for Ca2+-induced insulin secretion[34]. Given the pivotal role played by NBC1 in cellular acid-base homeostasis, we believe that NBC1 may play a role during islet cell growth and insulin secretion. Overall our results are at odds with the previous report that the anti-pNBC1 and anti-kNBC1 antibodies did not label the islet cells in rat pancreas[29]. In their study they used two polyclonal antibodies against variant-specific regions in the NH2-terminal of the rat kNBC1 (amino acids 4-16) and the rat pNBC1 (amino acids 2-12). On the other hand, in our study two affinity purified rabbit polyclonal antibodies raised against the common region of the two NBC1 isoforms at the COOH-terminal amino acids 928-1035 or NH2-terminal amino acids 338-391 were used in the immunohistochemical analysis. Thus, our results establish for the first time that NBC1 is strongly expressed on the plasma membrane of α and β cells in islets with a distinct spatiotemporal expression pattern.
In conclusion, the present study investigated the expression and cellular distribution of the NBC1 in rat pancreatic development by RT-PCR, immunoblotting and double-labeling immunofluorescence analyses. This study provided the first evidence for NBC1 localization on the plasma membrane of β and α cells and its dynamic expression in rat pancreas from late embryonic stages to the neonatal stage. The expression profile combined with the information on the biology of NBC1 suggests that this transporter play a significant role during the late embryonic and early postnatal stages and could be one of the factors involved in pancreatic cell behavior. Future studies are warranted to verify the exact significance of NBC1 expression and its localization pattern.
Na+/HCO3- cotransporter (NBC1) plays important roles in systemic and cellular pH homeostasis and trans-epithelial solute transport together with other members of the bicarbonate transporter superfamily. However, less is known about its expression pattern in the normal developing pancreas.
To date, NBC1 isoforms have also been found in various tissues, such as pNBC1 from pancreas and rbNBC1 from brain. The importance of NBC1 in normal organ function is highlighted by the latest evidence revealing that patients with homozygous missense mutations in the NBC1 gene have proximal renal tubular acidosis, short stature, enamel hypoplasia, and bilateral ocular disease.
The results establish for the first time that NBC1 is strongly expressed on the plasma membrane of α and β cells in islets with a distinct spatiotemporal expression pattern.
This study provided the first evidence for NBC1 localization on the plasma membrane of β and α cells and its dynamic expression in rat pancreas from late embryonic stages to the neonatal stage. The expression profile suggests that this transporter plays a significant role during the late embryonic and early postnatal stages and could be one of the factors involved in pancreatic cell behavior. Future studies are warranted to verify the exact significance of NBC1 expression and its localization pattern.
NBC1 is one of the integral membrane protein families that mediate sodium-bicarbonate cotransport. It plays important roles in systemic and cellular pH homeostasis and trans-epithelial solute transport together with other members of the bicarbonate transporter superfamily.
Authors demonstrated that the first evidence for NBC1 localization on the plasma membrane of β and α cells and its dynamic expression in rat pancreas from late embryonic stages to the neonatal stage. The expression profile suggests that this transporter plays a significant role during the late embryonic stages and early postnatal stages and could be one of the factors involved in pancreatic cell behavior. These results are interesting. However, as the authors point out, future studies are needed to verify the exact significances of NBC1 expression and its localization pattern.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kaya I S- Editor: Yu J L- Editor: O’Neill M E- Editor: Wang CH
| 1. | Habener JF, Kemp DM, Thomas MK. Minireview: transcriptional regulation in pancreatic development. Endocrinology. 2005;146:1025-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 288] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 2. | Jørgensen MC, Ahnfelt-Rønne J, Hald J, Madsen OD, Serup P, Hecksher-Sørensen J. An illustrated review of early pancreas development in the mouse. Endocr Rev. 2007;28:685-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 265] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 3. | Pictet RL, Clark WR, Williams RH, Rutter WJ. An ultrastructural analysis of the developing embryonic pancreas. Dev Biol. 1972;29:436-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 359] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Spooner BS, Walther BT, Rutter WJ. The development of the dorsal and ventral mammalian pancreas in vivo and in vitro. J Cell Biol. 1970;47:235-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 85] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Jensen J. Gene regulatory factors in pancreatic development. Dev Dyn. 2004;229:176-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 273] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 6. | Seki G, Yamada H, Horita S. Activation and Inactivation Mechanisms of Na-HCO3 Cotransporter NBC1. J Epithel Biol Pharmacol. 2008;1:35-39. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Romero MF, Fulton CM, Boron WF. The SLC4 family of HCO 3 - transporters. Pflugers Arch. 2004;447:495-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 342] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 8. | Lo YF, Yang SS, Seki G, Yamada H, Horita S, Yamazaki O, Fujita T, Usui T, Tsai JD, Yu IS. Severe metabolic acidosis causes early lethality in NBC1 W516X knock-in mice as a model of human isolated proximal renal tubular acidosis. Kidney Int. 2011;79:730-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Endo Y, Yamazaki S, Moriyama N, Li Y, Ariizumi T, Kudo A, Kawakami H, Tanaka Y, Horita S, Yamada H. Localization of NBC1 variants in rat kidney. Nephron Physiol. 2006;104:p87-p94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Soleimani M, Burnham CE. Physiologic and molecular aspects of the Na+: HCO3- cotransporter in health and disease processes. Kidney Int. 2000;57:371-384. [PubMed] |
| 11. | Bae WK, Lee J, Park JW, Bae EH, Ma SK, Kim SH, Kim SW. Decreased Expression of Na/K-ATPase, NHE3, NBC1, AQP1 and OAT in Gentamicin-induced Nephropathy. Korean J Physiol Pharmacol. 2008;12:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Romero MF, Hediger MA, Boulpaep EL, Boron WF. Expression cloning and characterization of a renal electrogenic Na+/HCO3- cotransporter. Nature. 1997;387:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Li HC, Collier JH, Shawki A, Rudra JS, Li EY, Mackenzie B, Soleimani M. Sequence- or position-specific mutations in the carboxyl-terminal FL motif of the kidney sodium bicarbonate cotransporter (NBC1) disrupt its basolateral targeting and alpha-helical structure. J Membr Biol. 2009;228:111-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Boron WF, Boulpaep EL. Intracellular pH regulation in the renal proximal tubule of the salamander. Basolateral HCO3- transport. J Gen Physiol. 1983;81:53-94. [PubMed] |
| 15. | Abuladze N, Lee I, Newman D, Hwang J, Boorer K, Pushkin A, Kurtz I. Molecular cloning, chromosomal localization, tissue distribution, and functional expression of the human pancreatic sodium bicarbonate cotransporter. J Biol Chem. 1998;273:17689-17695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 199] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | Choi I, Romero MF, Khandoudi N, Bril A, Boron WF. Cloning and characterization of a human electrogenic Na+-HCO-3 cotransporter isoform (hhNBC). Am J Physiol. 1999;276:C576-C584. [PubMed] |
| 17. | Bevensee MO, Schmitt BM, Choi I, Romero MF, Boron WF. An electrogenic Na(+)-HCO(-)(3) cotransporter (NBC) with a novel COOH-terminus, cloned from rat brain. Am J Physiol Cell Physiol. 2000;278:C1200-C1211. [PubMed] |
| 18. | Demirci FY, Chang MH, Mah TS, Romero MF, Gorin MB. Proximal renal tubular acidosis and ocular pathology: a novel missense mutation in the gene (SLC4A4) for sodium bicarbonate cotransporter protein (NBCe1). Mol Vis. 2006;12:324-330. [PubMed] |
| 19. | Ni XF, Yuan L, Cheng ZX. Expression and localization of nestin in pancreas of E18.5, newborn and adult rats. Disi Junyi Daxue Xuebao. 2004;25:193-196. |
| 20. | Thévenod F, Dehlinger-Kremer M, Kemmer TP, Christian AL, Potter BV, Schulz I. Characterization of inositol 1,4,5-trisphosphate-sensitive (IsCaP) and -insensitive (IisCaP) nonmitochondrial Ca2+ pools in rat pancreatic acinar cells. J Membr Biol. 1989;109:173-186. [PubMed] |
| 21. | Roussa E, Shmukler BE, Wilhelm S, Casula S, Stuart-Tilley AK, Thévenod F, Alper SL. Immunolocalization of potassium-chloride cotransporter polypeptides in rat exocrine glands. Histochem Cell Biol. 2002;117:335-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Yashpal NK, Li J, Wheeler MB, Wang R. Expression of {beta}1 integrin receptors during rat pancreas development--sites and dynamics. Endocrinology. 2005;146:1798-1807. [PubMed] |
| 23. | Roussa E, Nastainczyk W, Thévenod F. Differential expression of electrogenic NBC1 (SLC4A4) variants in rat kidney and pancreas. Biochem Biophys Res Commun. 2004;314:382-389. [PubMed] |
| 24. | West BJ, Brockman SJ, Scott A. Action research and standards of care. The prevention and treatment of pressure sores in elderly patients. Health Bull (Edinb). 1991;49:356-361. [PubMed] |
| 25. | Muallem S, Loessberg PA. Intracellular pH-regulatory mechanisms in pancreatic acinar cells. II. Regulation of H+ and HCO3- transporters by Ca2(+)-mobilizing agonists. J Biol Chem. 1990;265:12813-12819. [PubMed] |
| 26. | Kurtz I. NBCe1 as a model carrier for understanding the structure-function properties of Na+ -coupled SLC4 transporters in health and disease. Pflugers Arch. 2014;466:1501-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Jensen LJ, Schmitt BM, Berger UV, Nsumu NN, Boron WF, Hediger MA, Brown D, Breton S. Localization of sodium bicarbonate cotransporter (NBC) protein and messenger ribonucleic acid in rat epididymis. Biol Reprod. 1999;60:573-579. [PubMed] |
| 29. | Satoh H, Moriyama N, Hara C, Yamada H, Horita S, Kunimi M, Tsukamoto K, Iso-O N, Inatomi J, Kawakami H. Localization of Na+-HCO-3 cotransporter (NBC-1) variants in rat and human pancreas. Am J Physiol Cell Physiol. 2003;284:C729-C737. [PubMed] |
| 30. | Slack JMW. Developmental biology of the pancreas. Development. 1995;121:1569-1580. |
| 31. | Georgia S, Bhushan A. Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. J Clin Invest. 2004;114:963-968. [PubMed] |
| 32. | Petrik J, Arany E, McDonald TJ, Hill DJ. Apoptosis in the pancreatic islet cells of the neonatal rat is associated with a reduced expression of insulin-like growth factor II that may act as a survival factor. Endocrinology. 1998;139:2994-3004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Zhou GM, Zhong CP. Histology and Embryology. 1st ed. Shanghai: Fu Dan University Press 2005; . |
| 34. | Stiernet P, Guiot Y, Gilon P, Henquin JC. Glucose acutely decreases pH of secretory granules in mouse pancreatic islets. Mechanisms and influence on insulin secretion. J Biol Chem. 2006;281:22142-22151. [PubMed] |