Published online Nov 14, 2016. doi: 10.3748/wjg.v22.i42.9333
Peer-review started: June 29, 2016
First decision: August 22, 2016
Revised: September 16, 2016
Accepted: October 19, 2016
Article in press: October 19, 2016
Published online: November 14, 2016
Processing time: 140 Days and 19.6 Hours
To investigate the mechanisms underlying the potential contribution of the heme oxygenase/carbon monoxide (HO/CO) pathway in the constipating effects of granisetron.
For in vivo studies, gastrointestinal motility was evaluated in male rats acutely treated with granisetron [25, 50, 75 μg/kg/subcutaneous (sc)], zinc protoporphyrin IX [ZnPPIX, 50 μg/kg/intraperitoneal (ip)] and hemin (50 μmol/L/kg/ip), alone or in combination. For in vitro studies, the contractile neurogenic response to electrical field stimulation (EFS, 3, 5, 10 Hz, 14 V, 1 ms, pulse trains lasting 10 s), as well as the contractile myogenic response to acetylcholine (ACh, 0.1-100 μmol/L) were evaluated on colon specimens incubated with granisetron (3 μmol/L, 15 min), ZnPPIX (10 μmol/L, 60 min) or CO-releasing molecule-3 (CORM-3, 100, 200, 400 μmol/L) alone or in combination. These experiments were performed under co-treatment with or without atropine (3 μmol/L, a muscarinic receptor antagonist) or NG-nitro-L-Arginine (L-NNA, 100 μmol/L, a nitric oxide synthase inhibitor).
Administration of granisetron (50, 75 μg/kg) in vivo significantly increased the time to first defecation (P = 0.045 vs vehicle-treated rats), clearly suggesting a constipating effect of this drug. Although administration of ZnPPIX or hemin alone had no effect on this gastrointestinal motility parameter, ZnPPIX co-administered with granisetron abolished the granisetron-induced constipation. On the other hand, co-administration of hemin and granisetron did not modify the increased constipation observed under granisetron alone. When administered in vitro, granisetron alone (3 μmol/L) did not significantly modify the colon’s contractile response to either EFS or ACh. Incubation with ZnPPIX alone (10 μmol/L) significantly reduced the colon’s contractile response to EFS (P = 0.016) but had no effect on contractile response to ACh. Co-administration of ZnPPIX and atropine (3 μmol/L) abolished the ZnPPIX-mediated decrease in contractile response to EFS. Conversely, incubation with CORM-3 (400 μmol/L) alone increased both the contractile response to EFS at 10 Hz (10 Hz: 71.02 ± 19.16 vs 116.25 ± 53.70, P = 0.01) and the contractile response to ACh (100 μmol/L) (P = 0.012). Co-administration of atropine abolished the CORM-3-mediated effects on the EFS-mediated response. When granisetron was co-incubated in vitro with ZnPPIX, the ZnPPIX-mediated decrease in colon contractile response to EFS was lost. On the other hand, co-incubation of granisetron and CORM-3 (400 μmol/L) further increased the colon’s contractile response to EFS (at 5 Hz: P = 0.007; at 10 Hz: P = 0.001) and to ACh (ACh 10 μmol/L: P = 0.001; ACh 100 μmol/L: P = 0.001) elicited by CORM-3 alone. L-NNA co-administered with granisetron and CORM-3 abolished the potentiating effect of CORM-3 on granisetron on both the EFS-induced and ACh-induced contractile response.
Taken together, findings from in vivo and in vitro studies suggest that the HO/CO pathway is involved in the constipating effects of granisetron.
Core tip: We studied whether in vivo and in vitro effects of granisetron might be influenced, at least in part, by the heme oxygenase/carbon monoxide (HO/CO) pathway. Our findings demonstrate for the first time that the HO/CO pathway takes part in the contractile colon activity in rats. Interestingly, the constipating effects of granisetron are positively correlated with levels of carbon monoxide, thus suggesting that treatments able to modulate carbon monoxide levels may potentially reduce the constipation mediated by granisetron.
- Citation: Nacci C, Fanelli M, Potenza MA, Leo V, Montagnani M, De Salvia MA. Carbon monoxide contributes to the constipating effects of granisetron in rat colon. World J Gastroenterol 2016; 22(42): 9333-9345
- URL: https://www.wjgnet.com/1007-9327/full/v22/i42/9333.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i42.9333
In recent decades, the role played by carbon monoxide (CO) in several biochemical processes has been increasingly recognized[1-3]. Once considered only for its lethal effects, the therapeutic use of CO has been proposed after the discovery of its potential “positive” functions (http:/clinicaltrials.gov/ct2/search, “carbon monoxide”).
CO is a gas that is produced, together with iron and biliverdin, from the catalysis of heme by the microsomal heme oxygenase (HO) enzyme. Of the two HO isoforms, HO-2 is the constitutive one, whereas HO-1 is a highly inducible isoform whose activity is intended to provide protection against oxidative stress, injury and inflammation[1,2].
The first physiological role suggested for CO was in non-adrenergic non-cholinergic (NANC) neurotransmission at the gastrointestinal level[4]. The hypothesis of CO as a neurotransmitter is strongly supported by the wide expression of HO-2 throughout the gastrointestinal tract in the enteric nerves, as well as in the non-neuronal cells of the mucosal epithelium, smooth muscle cells, endothelium of blood vessels and interstitial cells of Cajal[3-5]. Moreover, HO-1 is upregulated in several gastrointestinal pathologies such as colitis, inflammatory bowel disease and gastric ulcers (see[3] for references). Because endogenously produced CO diffuses to blood where it binds to hemoglobin, increased HO-1 expression may result in augmented blood levels of carboxyhemoglobin (normal levels 0.8%). However, high levels of carboxyhemoglobin are more typically the consequence of smoking habits or environmental pollution[2]. Either from endogenous or exogenous sources, altered CO levels may affect physiological processes or modulate pathological conditions via several distinct mechanisms[6]. Ion channels have been shown to be, among others, the target of CO; thus, it is possible that CO may modulate the effects of other signals by acting directly on the same target or indirectly on the shared pool of second messengers[6-8]. A similar modulating activity of CO might also be plausible toward specific drugs; indeed, in a previous report, we observed the involvement of the HO/CO pathway in granisetron-mediated effects on rat duodenal motility[9].
Granisetron is a highly selective competitive antagonist of the 5-HT3 receptor, the only serotonin-gated ion channel that, if activated, allows an influx of cations[10]. Granisetron is currently used for the chemotherapy-induced nausea and vomiting[11], and constipation is reported among its side effects[12]. On the other hand, constipation is the desired effect for 5-HT3 receptor antagonists such as alosetron and cilansetron in the treatment of irritable bowel syndrome with diarrhea[13] in which the delayed transit in the large bowel may reduce pain and discomfort in those patients[14]. Unfortunately, despite their clinical efficacy, the potential use of these drugs has been restricted due to reports of severe ischemic colitis (see[15] for review). Nevertheless, these observations support the ability of 5-HT3 receptor antagonists to induce constipation.
To explore potential mechanisms linking the activity of the HO/CO pathway to granisetron-induced constipation, we investigated whether the constipating effects of granisetron administered in vivo may be modulated by agents that induce (such as hemin) or inhibit (such as zinc protoporphyrin, ZnPPIX) the endogenous HO activity. A 3 μmol/L concentration of granisetron was chosen for the present investigation based on dose-response curves previously obtained[9]. Moreover, because constipation has been ascribed to abnormalities of various contractile activities of the colon[16-19], parallel in vitro studies on isolated colon preparations were performed to evaluate (1) the neurogenic contractile responses to electrical field stimulation indicative of cholinergic and non-cholinergic transmitter release from enteric neurons[20,21] in the absence and in the presence of the muscarinic antagonist atropine as well as the nitric oxide synthase inhibitor L-NNA; and (2) the myogenic contractile response to ACh, one of the major contractile neurotransmitters at the gastrointestinal level in the absence and in the presence of L-NNA.
All experimental procedures were performed in accordance with the Guidelines and Authorization for the Use of Laboratory Animals (Italian Government, Ministry of Health) and according to the European Community Guidelines for Animal Care (DL 116/92, application of the European Communities Council Directive of 24 November 1986 - 86/609/EEC).
Ten-week-old male Sprague-Dawley rats weighing 220-250 g at arrival (Envigo, San Pietro al Natisone, Udine, Italy) were used. The animal protocol was designed to minimize pain or discomfort to the animals.
Rats were housed in an animal facility with monitored temperature and light (12-h cycle and 21 ± 2 °C). All cages were floored with sawdust, and bedding was replaced on a regular basis. The animals were allowed to acclimate to the environment for at least 7 d. Rats undergoing in vivo treatments were randomly chosen and allocated into individual cages before initiating the study, with the remaining rats caged together (4 rats/cage) in close proximity to allow experimental animals to see and smell their companions. Rats had free access to water and food when they were not under testing. All animals were handled and trained for at least 1 wk to minimize the possible stress of the drug administration procedure.
A repeated measures protocol was designed for in vivo study, so that each rat, at one-week intervals, received the following treatments either subcutaneously (sc) or intraperitoneally (ip): vehicle (1 mL/kg), granisetron (25, 50, 75 μg/kg/sc soon before testing), ZnPPIX (50 μg/kg/ip, 60 min before testing), hemin (50 μmol/L/kg/ip 24 h before testing), ZnPPIX (50 μg/kg/ip, 60 min before granisetron) with granisetron (25, 50, 75 μg/kg/sc), or hemin (50 μmol/L/kg/ip 24 h before granisetron) with granisetron (25, 50, 75 μg/kg/sc). The timing and dosing for ZnPPIX and hemin were carefully chosen to obtain the greatest level of HO inhibition or induction, respectively[9,22,23]. In a pilot study, we observed that the average time to first defecation in vehicle-treated rats was between 80-110 min (median 105 min; interquartile range 90-110; full range 80-180). Based on these preliminary findings, the observation cut-off time was set at 180 min. In the late afternoon preceding the test day, rats were fasted with free access to water. On the test day, animals were weighed and then allowed to free feed for 20 min. The amount of food eaten and the weight of the fed rats were calculated.
Following drug administration, each rat was monitored every 10 min for 180 min, and the time to first defecation was assumed as an index of whole-gut transit[24,25].
After induction of general anesthesia (pentobarbital 80 mg/kg ip), rats were killed by cervical dislocation. A 3-cm section of proximal colon (1 cm from the ileocecal sphincter), obtained through a midline incision of the abdomen, was immediately placed in a cooled modified Krebs’ solution (pH = 7.4) of the following composition (mmol/L): NaCl 113, KCl 4.8, MgSO4 1.2, CaCl2 (H2O) 2.2, NaH2PO4 1.2, NaHCO3 25, glucose 5.5, and ascorbic acid 5.5. The specimen was then cleaned and rinsed, and a circular ring (0.5-cm length) was mounted in an organ bath (20 mL) filled with modified Krebs’ solution, maintained at 37 °C and gassed with a mixture of 95% O2 and 5% CO2. One end of the circular ring was connected to a metal rod, while the other end was attached to a strain gauge transducer (FORT 25, WPI, Sarasota, FL, United States). Isometric tension was measured by the PowerLab data acquisition system and recorded using Chart 5.5.5 (ADInstruments, Castle Hill, Australia). The colon ring was allowed to equilibrate for at least 30 min prior to the experiment. An initial load of 0.5 g tension was applied to the preparation.
The neurogenic contractile response was measured by applying a transmural stimulation (Electrical Field Stimulation, EFS) at frequencies of 3, 5, and 10 Hz (14 V, 1 ms pulse, trains lasting 10 s) through two parallel platinum electrodes connected to a stimulator (Digital Stimulator, LE 12106, Letica, Ugo Basile, Italy). The EFS results in an immediate relaxation, followed at the end of EFS by a so-called off-contraction. This contractile response is indicative of a nervous reflex that is abolished by tetrodotoxin and reduced by atropine and tackykinin antagonists[26]. Activation of enteric nerves by EFS mimics the in vivo conditions in which neurotransmitters are released by motor neurons to the neuroeffector apparatus; the interaction between the interstitial cells of Cajal, neurons, glial cells and smooth muscle cells generates contraction[27,28].
The myogenic contractile response was explored by calculating the extent of contraction induced by acetylcholine (ACh, 0.1-100 μmol/L).
Both neurogenic and myogenic contractile responses were measured after incubation with the following agents alone or in combination: granisetron hydrochloride (3 μmol/L, 15 min), ZnPPIX (10 μmol/L, 60 min), L-NNA (100 μmol/L, 20 min), and CORM-3 (100, 200, 400 μmol/L). For the last compound, CORM-3, a water-soluble Ru-containing compound releasing one mole of CO per mole[29], the effect was evaluated within 10 min from administration to avoid its spontaneous breakdown.
The neurogenic contractile responses were expressed as a percentage of three consecutive contractile responses to EFS (10 Hz, 14 V, 1 ms pulse, trains lasting 10 s) recorded and averaged before drug administration.
The myogenic contractile responses to ACh (0.1-100 μmol/L) were expressed as a percentage of tension values elicited by the highest ACh concentration (100 μmol/L) before drug administration.
The activity of ZnPPIX and CORM-3 (indicative of a specific CO-dependent effect) on neurogenic contractile response was measured in the absence and in the presence of atropine (3 μmol/L).
The following drugs were used: atropine sulphate and granisetron hydrochloride dissolved in saline (Sigma Chemical Co., St. Louis, Missouri, United States). Zinc protoporphyrin IX and hemin were dissolved in 0.1 N NaOH and equilibrated to a pH of 7.4 with HCl (Sigma Chemical Co., St. Louis, Missouri, United States). Tricarbonyl Chloro(glycinato)ruthenium (II) (CORM-3) and NG-nitro-L-Arginine (L-NNA) were dissolved in distilled water (Sigma Chemical Co., St. Louis, Missouri, United States). In in vivo studies, vehicle-treated rats received the same amount of vehicle as did drug-treated animals. In in vitro experiments, vehicle-treated preparations were exposed to the same amount of vehicle as drug-treated preparations.
For in vivo study, Friedman’s ANOVA for repeated measures followed by a post hoc test was performed. For in vitro study, two-way ANOVA for repeated measures (treatment effect, frequencies or concentrations effect and interaction effect, with frequency or concentrations as repeated measure) was performed. When the interaction effect was significant, a one-way ANOVA at each frequency or concentration was performed with pre-planned multiple comparison tests for each treatment vs vehicle.
The results are presented as individual observations (n = 8) for each in vivo treatment; results are expressed as the mean ± SD of 6-8 preparations for each in vitro treatment. Statistical analysis was performed by the biomedical statistician Dr. Margherita Fanelli (coauthor) using SPSS software (version 20.0). A P value < 0.05 was considered to indicate statistical significance.
Effect of granisetron, ZnPPIX and hemin on the time to first defecation: The average amount of food eaten before drug administration was 5 g. After 20 min of free access to food, the body weight increased by approximately 8 g in all animals.
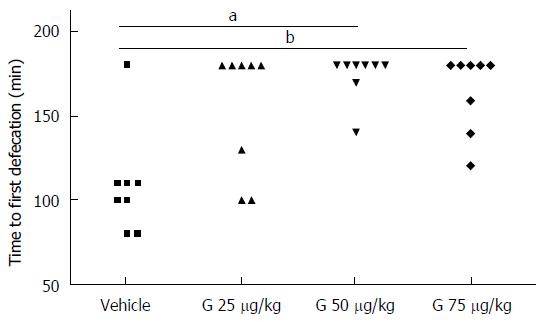
Consistent with results obtained in our previous study[9], acute administration of granisetron increased the time to first defecation. Interestingly, the delay to first defecation was dose-dependent, with no significant effect measured for the lowest dose of granisetron used (25 μg/kg) and with a substantial increase in the time to first defecation observed in animals administered higher doses of granisetron; in this respect, both 50 and 75 μg/kg of granisetron were equally effective (Friedman’s test = 13, P = 0.005, post hoc: granisetron 25 μg/kg vs vehicle, P = 0.132; granisetron 50 μg/kg vs vehicle, P = 0.045; granisetron 75 μg/kg vs vehicle: P = 0.045) (Figure 1). A preliminary comparison of the amount of food eaten before vehicle or drug administration showed no statistically significant differences among treatments (Friedman’s test = 0.958, P = 0.811).
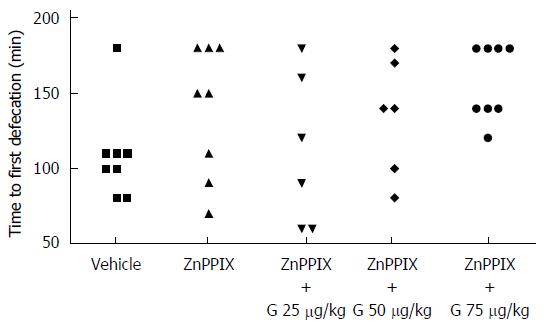
Although ZnPPIX (50 μg/kg) alone did not modify the time to first defecation, co-administration of ZnPPIX (50 μg/kg) with granisetron (25, 50, 75 μg/kg) was able to counteract the constipating effect of granisetron: Friedman’s test = 10.486, P = 0.033; post hoc comparisons: ZnPPIX vs vehicle: P = 1; granisetron 25 μg/kg with ZnPPIX vs vehicle: P = 1; granisetron 50 μg/kg with ZnPPIX vs vehicle: P = 1; granisetron 75 μg/kg with ZnPPIX vs vehicle: P = 0.132 (Figure 2). Similar to the previous case, a preliminary comparison of the amount of food eaten before vehicle or drug administration showed no statistically significant differences among treatments (Friedman’s test = 1.077, P = 0.898).
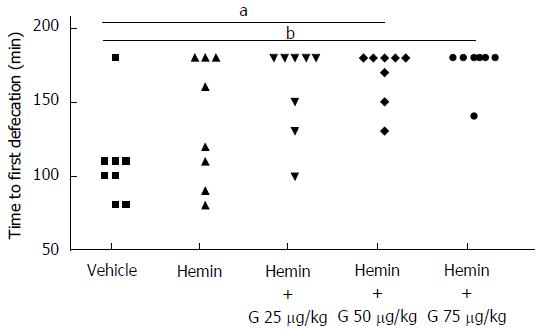
On the other hand, hemin (50 μmol/L/kg) alone or co-administered with granisetron (25, 50, 75 μg/kg) showed the following results: Friedman’s test = 20.364, P = 0.000; post hoc comparisons: hemin vs vehicle: P = 1.000; granisetron 25 μg/kg with hemin vs vehicle: P = 0.108; granisetron 50 μg/kg with hemin vs vehicle: P = 0.028; granisetron 75 μg/kg with hemin vs vehicle: P = 0.004), thus suggesting that hemin does not alter the time to first defecation when administered alone and does not modify the constipating effect of granisetron when administered in combination (Figure 3). Similar to the previous case, a preliminary comparison of the amount of food eaten before vehicle or drug administration showed no statistically significant differences among treatments (Friedman’s test = 2.205, P = 0.698).
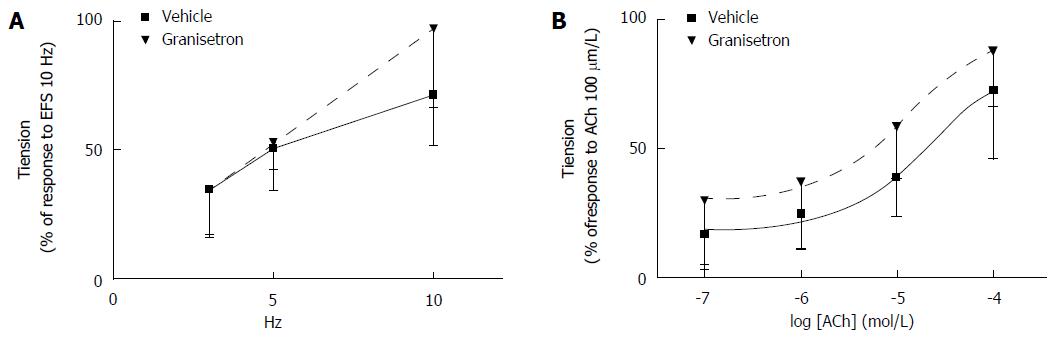
Effects of granisetron on EFS-induced and ACh-induced contractile response of colon preparations: Incubation of colon specimens with granisetron did not significantly modify the contractile response to EFS obtained in vehicle-treated samples (Ftreatments = 1.26, df = 1/9, P = 0.29; Ffrequencies = 22.50, df = 2/18, P = 0.001; Ftreatments x frequencies = 1.79, df = 2/18, P = 0.21) (Figure 4A). Interestingly, a trend to increase the contractile effect induced by ACh (0.1-100 μmol/L) was measured in samples incubated with granisetron, although no statistical significance was measured with respect to vehicle-treated samples (Ftreatments = 3.48, df = 1/9, P = 0.09; Fconcentrations = 21.35, df = 3/27, P < 0.0001; Ftreatments x concentrations = 0.08, df = 3/27, P = 0.85) (Figure 4B).
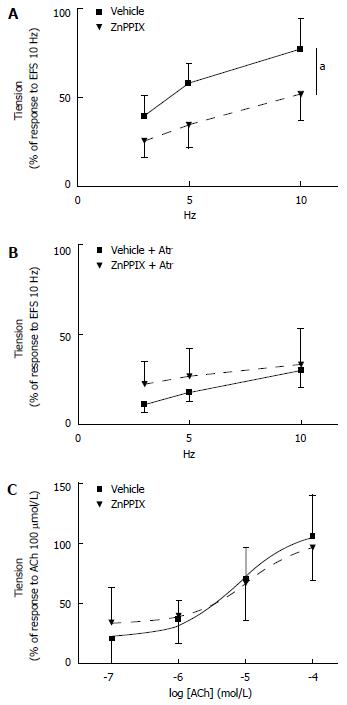
Effects of ZnPPIX on EFS-induced and ACh-induced contractile response of colon preparations: When compared to vehicle-treated preparations, a significant decrease in the contractile response to EFS was observed in specimens incubated with ZnPPIX (10 μmol/L, 60 min) (Ftreatments = 8.78, df = 1/9, P = 0.016; Ffrequencies = 50.33, df = 2/18, P < 0.0001; Ftreatments x frequencies = 1.79, df = 2/18, P = 0.21) (Figure 5A). Interestingly, the ZnPPIX-mediated effect on EFS was abolished by concomitant incubation with atropine (3 μmol/L, 20 min) (Ftreatments = 1.44, df = 1/11, P = 0.25; Ffrequencies = 37.66, df = 2/22, P < 0.0001; Ftreatments x frequencies = 2.74, df = 2/22, P = 0.09), therefore suggesting that ZnPPIX may exert its effects by inhibiting the EFS-mediated release of endogenous ACh (Figure 5B). However, ZnPPIX did not affect the contractile response to exogenous ACh (0.1-100 μmol/L) compared to vehicle (Ftreatments = 0.006, df = 1/9, P = 0.94; Fconcentrations = 36.89, df = 3/27, P < 0.0001; Ftreatments x concentrations = 0.84, df = 3/27, P = 0.45) (Figure 5C).
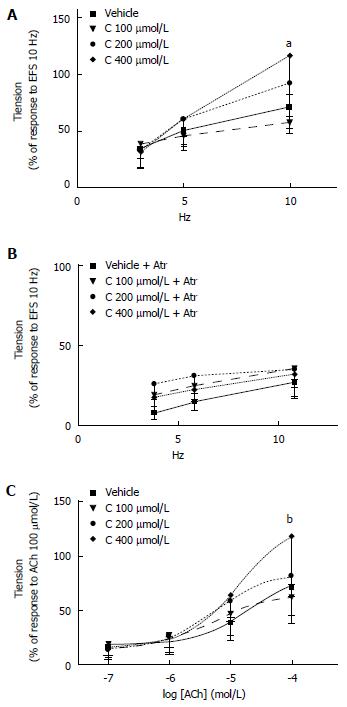
Effects of CORM-3 on EFS-induced and ACh-induced contractile response of colon preparations: Assessment of the EFS-induced contractile response after CORM-3 (100-400 μmol/L) administration shows that CORM-3 (400 μmol/L) significantly increased the EFS-induced contractile response compared to vehicle at 10 Hz [Ftreatments = 2.75, df = 3/20, P = 0.07; Ffrequencies = 55.38, df = 2/40, P < 0.0001; Ftreatments x frequencies = 4.36, df = 6/40, P = 0.002; at 10 Hz: CORM-3 (400 μmol/L) vs vehicle aP = 0.01] (Figure 6A).
When repeated after 20-min incubation with atropine (3 μmol/L, 20 min), the increased EFS-induced contractile response by CORM-3 (400 μmol/L) administration was abolished: Ftreatments = 3.06, df = 3/20, P = 0.052; Ffrequencies = 50.05, df = 2/40, P < 0.0001; Ftreatments x frequencies = 1.14, df = 6/40, P = 0.36. Consistent with the results obtained with ZnPPIX, these observations suggest that CORM-3 may enhance the EFS-induced release of endogenous ACh (Figure 6B).
Analysis performed to determine the effect of CORM-3 administration (100-400 μmol/L) on the contractile response to exogenous ACh (0.1-100 μmol/L) showed that incubation with CORM-3 (400 μmol/L) increases the contractile response to the highest ACh concentration (100 μmol/L) compared to vehicle-treated samples [Ftreatments = 2.28, df = 3/22, P = 0.11; Fconcentrations = 86.22, df = 3/66, P < 0.0001; Ftreatments x concentrations = 3.49, df = 9/66, P = 0.02; for ACh 100 μmol/L: CORM-3 (400 μmol/L) vs vehicle: P = 0.012] (Figure 6C).
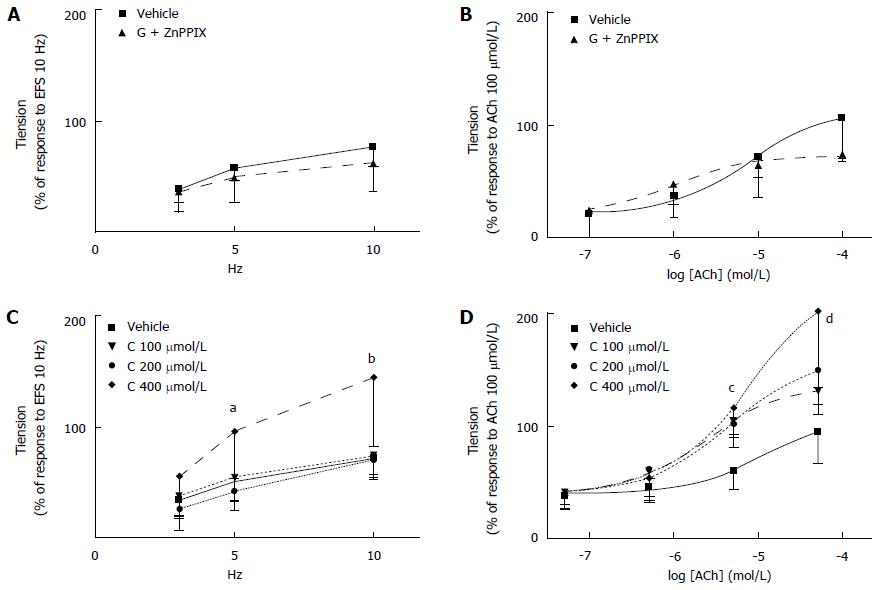
Effects of co-administration of granisetron with ZnPPIX or CORM-3 on EFS-induced and ACh-induced contractile response of colon preparations: When co-administered with granisetron (3 μmol/L, 15 min), incubation with ZnPPIX (10 μmol/L, 60 min) did not significantly modify the EFS-induced contraction compared to vehicle-treated samples (Ftreatments = 0.43, df = 1/8, P = 0.53; Ffrequencies = 55.35, df = 2/16, P < 0.0001; Ftreatments x frequencies = 1.66, df = 2/16, P = 22) (Figure 7A). Because incubation with ZnPPIX alone decreased the contractile response to EFS (Figure 5A), it is plausible to infer that co-administration of granisetron was responsible for the abolished effects of ZnPPIX on EFS-induced colon contraction.
Co-administration of ZnPPIX (10 μmol/L, 60 min) and granisetron (3 μmol/L, 15 min) did not modify the myogenic contractile response to ACh (Ftreatments = 0.22, df = 1/8, P = 0.65; Fconcentrations = 39.19, df = 3/24, P < 0.0001; Ftreatments x concentrations = 4.06, df = 3/24, P = 0.02). (Figure 7B).
When the effects of CORM-3 (100-400 μmol/L) on the EFS-induced contractile response were analyzed in combination with granisetron (3 μmol/L, 15 min), the results showed that coincubation of CORM-3 (400 μmol/L) and granisetron significantly increased the EFS-induced contractile response when compared to vehicle-treated samples at 5 and 10 Hz [Ftreatments = 5.47, df = 3/19, P < 0.01; Ffrequencies = 55.40, df = 2/38, P < 0.0001; Ftreatments x frequencies = 3.05, df = 6/38, P = 0.04; granisetron (3 μmol/L, 15 min) and CORM-3 (400 μmol/L) vs vehicle at 5 Hz: P = 0.007 and at 10 Hz: P = 0.001 (Figure 7C).
Interestingly, when compared to vehicle-treated samples, the concomitant incubation of CORM-3 (400 μmol/L) with granisetron significantly increased the myogenic response to ACh at 10 and 100 μmol/L (Ftreatments = 7.40, df = 3/19, P = 0.002; Fconcentrations = 61.69, df = 3/57, P < 0.0001; Ftreatments x concentrations = 3.55, df = 9/57, P = 0.027; at ACh 10 μmol/L: P = 0.001 and at ACh 100 μmol/L: P = 0.001) (Figure 7D).
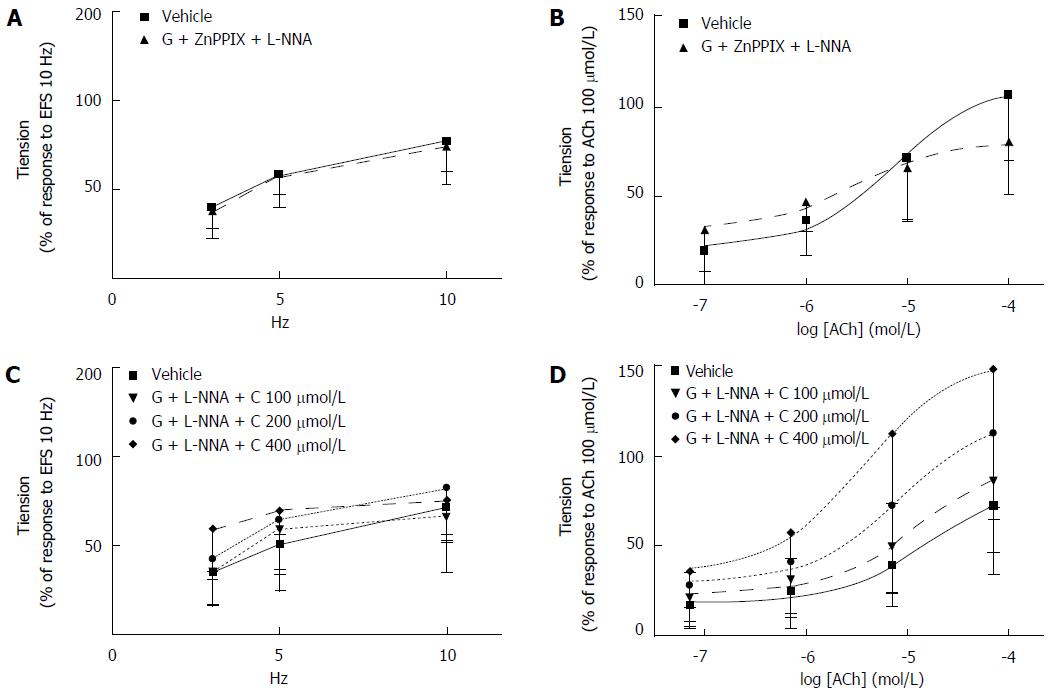
Effects of co-administration of granisetron, ZnPPIX, L-NNA and granisetron, CORM-3, L-NNA on EFS-induced and ACh-induced contractile response of colon preparations: When co-administration of granisetron (3 μmol/L, 15 min) and ZnPPIX (10 μmol/L, 60 min) was combined with L-NNA (100 μmol/L, 20 min), no difference in EFS-induced contractile effects was observed compared to vehicle-treated samples (Ftreatments = 0.08, df = 1/9, P = 0.79, Ffrequencies = 24.89, df = 2/18, P < 0.0001; Ftreatments x frequencies = 0.03, df = 2/18, P = 0.91) (Figure 8A). Similarly, contractile responses to exogenous ACh administration were not modified by concomitant administration of granisetron, ZnPPIX and L-NNA (vs vehicle-treated samples) (Ftreatments = 0.03, df = 1/9, P = 0.87; Fconcentrations = 45.18, df = 3/27, P < 0.0001; Ftreatments x concentrations = 3.90, df = 3/27, P = 0.04) (Figure 8B).
Co-administration of granisetron (3 μmol/L, 15 min) and CORM-3 (100-400 μmol/L) with L-NNA (100 μmol/L, 20 min) did not affect the EFS-induced contractile response at any frequency investigated (vs vehicle-treated samples) (Ftreatments = 0.83, df = 3/18, P = 0.49, Ffrequencies = 25.51, df = 2/36, P < 0.0001; Ftreatments x frequencies = 0.89, df = 6/36, P = 0.50) (Figure 8C) and did not modify the contractile responses to exogenous ACh administration (vs vehicle-treated samples) (Ftreatments = 3.38, df = 3/17, P = 0.04; Fconcentrations = 33.08, df = 3/57, P < 0.0001; Ftreatments x concentrations = 1.47, df = 9/51, P = 0.25, pre-planned contrast not significant) (Figure 8D).
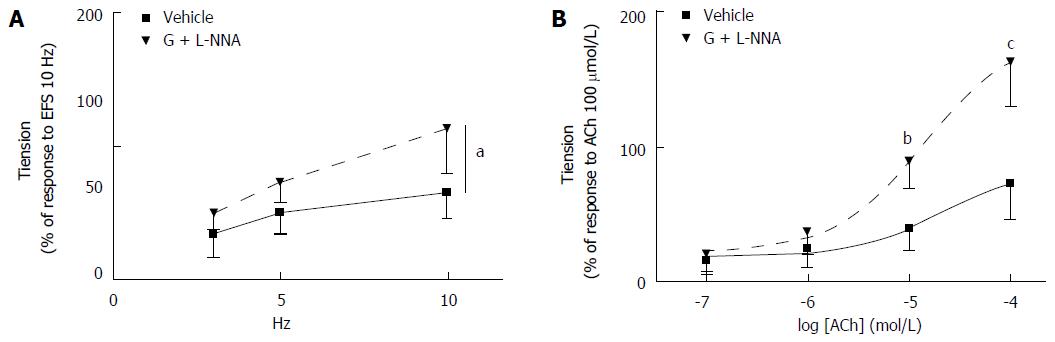
Effects of co-administration of granisetron and L-NNA on EFS-induced and ACh-induced contractile response of colon preparations: Co-administration of granisetron (3 μmol/L, 15 min) and L-NNA (100 μmol/L, 20 min) increased the contractile response to EFS compared to vehicle-treated samples (Ftreatments = 6.73, df = 1/11, P = 0.025; Ffrequencies = 16.80, df = 2/22, P = 0.001; Ftreatments x frequencies = 1.26, df = 2/22, P = 0.30) (Figure 9A).
Likewise, administration of granisetron (3 μmol/L, 15 min) and L-NNA (100 μmol/L, 20 min) increased the myogenic response to ACh compared to vehicle-treated samples (Ftreatments = 25.33, df = 1/11, P < 0.001; Fconcentrations = 80.22, df = 3/33, P < 0.0001; Ftreatments x concentrations = 15.8, df = 3/33, P = 0.001; t-test for ACh 10 μmol/L: t = 5.06, P = 0.000 and for ACh 100 μmol/L: t = 4.99, P = 0.000) (Figure 9B).
This study was planned to clarify the mechanisms underlying the potential contribution of the HO/CO pathway in the constipating effects of granisetron in rats. In a previous report, we found that inhibition of HO or increased expression of HO-1 in rat duodenum was able to influence the granisetron effects on the EFS-dependent response[9]. These findings provided a first evidence that the HO/CO pathway may play a role in the constipating activity of granisetron. However, because constipation is more closely related to abnormalities of colon motility, rather than in the duodenum[16-19], we planned to focus directly on the colon contractile responses. Moreover, in our previous study, the role of the HO/CO pathway on rat duodenum was evaluated under NANC conditions[9] to avoid the overwhelming effects of the main neurotransmitters at the gastrointestinal level, namely ACh and noradrenaline (NA). However, neurogenic gastrointestinal motility is strictly dependent on ACh and NA-mediated effects, and the functional relevance of NANC neurotransmission in vivo is still largely unknown[30]. Thus, in this work, the assessment of colon neurogenic response to granisetron was investigated under conditions directly resembling the existing intestinal environment.
Consistent with literature data reporting constipation in patients treated with granisetron as an antiemetic therapy[11,15], we observed an increased time to first defecation, a recognized indicator of whole-gut transit[24,25], after acute administration of granisetron in rats. Granisetron-induced constipation was abolished by in vivo co-administration with ZnPPIX (HO inhibitor), whereas co-administration of hemin (HO-1 inducer) did not decrease the delayed time to first defecation observed in granisetron-treated rats. These data support an active role of the HO/CO system in the constipating effect of granisetron[9]. Interestingly, neither ZnPPIX nor hemin was able to affect rat gastrointestinal motility when administered alone in vivo. This is not surprising because the HO/CO pathway is likely to be a fine-tuning mechanism whose activity may enhance or limit the extension of major signals involved in the integrated control of colon motility.
Consistent with this view, and with studies reporting a substantial effect of 5-HT3 antagonists only in the presence of high levels of 5-HT, either exogenously administered or endogenously released from enterochromaffin cells (for example, by mucosal pressure, distortion and/or chemical stimuli[31-33]), granisetron administration in vitro did not significantly inhibit the contractile response to EFS and showed a borderline trend to increase the contraction mediated by ACh (P = 0.09). Interestingly, colon contractile responses to EFS were decreased in vitro by incubation with ZnPPIX alone. Because ZnPPIX inhibits the HO-mediated production of CO, it is plausible to infer that the EFS-dependent contraction is mediated, at least in part, by CO. This hypothesis is consistent with studies reporting an almost completely abolished inhibitory response to EFS in jejunal smooth muscle strips of mice with targeted genomic deletion of HO-2. Concomitantly, in these animals, an exogenous administration of CO restores the EFS response[34].
CO appears to have a facilitatory effect on EFS-mediated ACh release, as suggested by the impaired ACh release observed in frog neuromuscular junctions under ZnPPIX incubation[35]. Analogous behavior was observed in our study in which the impaired contractile response to EFS obtained under ZnPPIX was restored by concomitant incubation with the muscarinic antagonist atropine. This finding, together with the lack of any effect of ZnPPIX on the myogenic contractile response to exogenous ACh, implies that a phasic CO production is required for physiological ACh release in rat colon.
The potential role of CO on granisetron effects, investigated in vivo by co-administration of hemin, was mimicked in vitro by co-administration of CORM-3, a CO-releasing molecule able to replicate the effects of HO-1 stimulation with hemin[3,36]. At the highest dose used (400 μmol/L) CORM-3 significantly increases the contractile response to both EFS (10 Hz) and exogenous ACh (100 μmol/L). These findings suggest that one mechanism by which CO may enhance the contractile response in rat colon is by facilitating the release of endogenous ACh. In addition, CO may indirectly potentiate the ACh contractile effects, as proposed by Lim et al[37], by concurrently activating L-type calcium channels in human intestinal smooth muscle via a nitric oxide (NO)-dependent mechanism. The binding of NO to guanylyl cyclase with subsequent changes in cAMP and intracellular Ca2+ levels will eventually lead to activation of the “contractile apparatus” [37].
When granisetron and CORM-3 were co-administered, the colon’s contractile responses to both EFS and ACh were further increased, suggesting a synergistic effect between these two substances. Similarly, when granisetron and ZnPPIX were co-administered, the effects of ZnPPIX alone were lost. Although the exact mechanism of granisetron and HO/CO system interplay remains to be clearly established, some explanations may be proposed: one is that, as suggested by the bell-shaped curve for in vivo response[38], granisetron may behave as a partial agonist at the concentrations used for the present in vitro and in vivo studies[39,40]. In this case, the activation of 5-HT3 receptors followed by subsequent increased release of ACh may have overcome the inhibition of ACh release secondary to ZnPPIX. Concomitantly, acting as a partial 5-HT3 agonist, granisetron may synergistically potentiate CORM-3 effects by increasing calcium influx.
Because the activation of L-type Ca2+ channels operated by CO is a NO-dependent mechanism, inhibition of NO production is expected to decrease the CORM-3-mediated effects. Indeed, in the presence of NO synthase inhibitor L-NNA, the potentiating effect of CORM-3 on granisetron activity was lost, confirming the necessary role of NO for the observed activities.
Because of the nature of the study, the following limitations must be considered. First, we cannot conclusively exclude that the colon response to granisetron/ZnPPIX treatment might be related to changes in the serotonergic system; nevertheless, the results obtained strongly suggest that the constipating effect of granisetron is only indirectly affected by ZnPPIX, which acts through reduction of EFS-induced acetylcholine release. Second, it is not clear whether the alleviation of granisetron-induced constipation might affect the antiemetic potential of this drug; studies directly evaluating this parameter would require a specific animal model and a completely different experimental approach, both of which are unavailable at this time. However, our perception is that alleviation of granisetron-induced constipation does not interfere with its antiemetic activity because this last effect relates to granisetron’s ability to reach the CNS. In this regard, it has been reported that ZnPPIX does not cross the blood-brain barrier[1,41]. Thus, it is plausible that the effects of ZnPPIX to reduce granisetron-induced constipation are related to peripheral mechanisms not involving the CTZ. Third, gastrointestinal transit (GIT) was measured by observing the time to first defecation after food ingestion; although intragastric administration of a non-absorbable, colored marker is considered the reference method to measure GIT, additional gavage administration would increase stress in animals and potentially affect the parameter evaluated. In our study, we considered the delayed GIT in rats treated with granisetron (compared to rats treated with vehicle) as a positive control to evaluate the effects of ZnPPIX and CORM-3 on the “time to first defecation” after food ingestion.
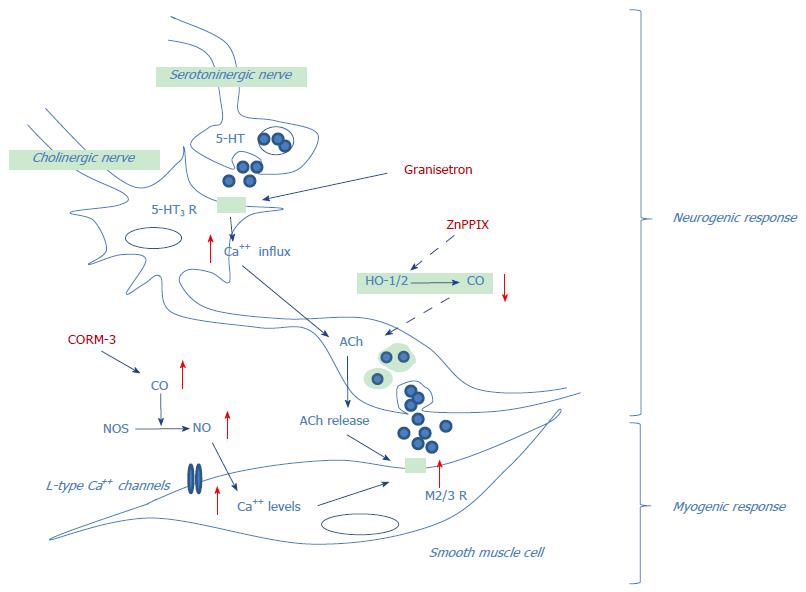
In conclusion, findings from the present study may shed light on the involvement of the HO/CO pathway in the neurogenic and myogenic contractile responses in rat colon and propose potential mechanisms underlying the interaction of granisetron and CO on colon motility (Figure 10).
Considering that granisetron is mainly used to prevent chemotherapy-induced nausea and vomiting in cancer patients and that increased expression of HO-1 has been observed in several cancer types[42], our findings suggest that HO inhibitors may be a reasonable therapeutic approach to reduce the unwanted constipating effects of granisetron.
The authors would like to thank Mr. Domenico Gallo and Dr. Anna De Salvia for their technical support.
In recent decades, the role played by carbon monoxide (CO) in several biochemical processes has been increasingly recognized. Once considered only for its lethal effects, the therapeutic use of CO has been proposed after the discovery of its potential "positive" functions. Ion channels have been shown to be, among others, the target of CO; thus, it is possible that CO may modulate the effects of other signals by acting directly on the same target or indirectly on the shared pool of secondary messengers. A similar modulating activity of CO might also be plausible toward specific drugs.
In a previous report, authors observed the involvement of the heme oxygenase (HO)/CO pathway in granisetron-mediated effects on duodenal motility.
Findings from the present study may shed light on the involvement of the HO/CO pathway in the neurogenic and myogenic contractile responses in rat colon and propose potential mechanisms underlying the interaction of granisetron and CO on colon motility.
Considering that granisetron is mainly used to prevent chemotherapy-induced nausea and vomiting in cancer patients and that increased expression of HO-1 has been observed in several cancer types[42], the authors findings suggest that HO inhibitors may be a reasonable therapeutic approach to reduce the unwanted constipating effects of granisetron.
Electrical field stimulation allows measurement of the neurogenic contractile response. In rat colon preparations, the electrical field stimulation (EFS) induces an immediate relaxation of specimens followed, at the end of EFS, by a contraction called off-contraction. This contractile response is indicative of a nervous reflex. Moreover, activation of enteric nerves by electrical field stimulation mimics the in vivo conditions because neurotransmitters are released by motor neurons to the neuroeffector apparatus in which interstitial cells of Cajal, neurons, glial cells and smooth muscle cells interact and induce contraction.
The authors present interesting data about HO/CO pathway and granisetoron. The authors report detailed data and proposed potential mechanisms underlying the interaction of granisetron and CO. Overall, it is an important study, and should be considered for publication.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Garcia-Olmo D, Sacharczuk M, Takano S S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Wu L, Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev. 2005;57:585-630. [PubMed] |
| 2. | Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1689] [Cited by in RCA: 1802] [Article Influence: 94.8] [Reference Citation Analysis (0)] |
| 3. | Gibbons SJ, Verhulst PJ, Bharucha A, Farrugia G. Review article: carbon monoxide in gastrointestinal physiology and its potential in therapeutics. Aliment Pharmacol Ther. 2013;38:689-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Zakhary R, Poss KD, Jaffrey SR, Ferris CD, Tonegawa S, Snyder SH. Targeted gene deletion of heme oxygenase 2 reveals neural role for carbon monoxide. Proc Natl Acad Sci USA. 1997;94:14848-14853. [PubMed] |
| 5. | Farrugia G, Szurszewski JH. Carbon monoxide, hydrogen sulfide, and nitric oxide as signaling molecules in the gastrointestinal tract. Gastroenterology. 2014;147:303-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 6. | Peers C. Modulation of ion channels and transporters by carbon monoxide: causes for concern? Front Physiol. 2012;3:477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Wilkinson WJ, Kemp PJ. Carbon monoxide: an emerging regulator of ion channels. J Physiol. 2011;589:3055-3062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Peers C, Boyle JP, Scragg JL, Dallas ML, Al-Owais MM, Hettiarachichi NT, Elies J, Johnson E, Gamper N, Steele DS. Diverse mechanisms underlying the regulation of ion channels by carbon monoxide. Br J Pharmacol. 2015;172:1546-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Zigrino A, Leo V, Renna G, Montagnani M, De Salvia MA. Hemin and Zinc Protoporphyrin IX Affect Granisetron Constipating Effects In Vitro and In Vivo. ISRN Gastroenterol. 2013;2013:612037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Derkach V, Surprenant A, North RA. 5-HT3 receptors are membrane ion channels. Nature. 1989;339:706-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 467] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 11. | Aapro M. 5-HT(3)-receptor antagonists in the management of nausea and vomiting in cancer and cancer treatment. Oncology. 2005;69:97-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Navari RM, Aapro M. Antiemetic Prophylaxis for Chemotherapy-Induced Nausea and Vomiting. N Engl J Med. 2016;374:1356-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 298] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 13. | Chetty N, Irving HR, Coupar IM. Activation of 5-HT3 receptors in the rat and mouse intestinal tract: a comparative study. Br J Pharmacol. 2006;148:1012-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Camilleri M, Northcutt AR, Kong S, Dukes GE, McSorley D, Mangel AW. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet. 2000;355:1035-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 405] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 15. | Beattie DT, Smith JA. Serotonin pharmacology in the gastrointestinal tract: a review. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:181-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Koch KL, Bitar KN, Fortunato JE. Tissue engineering for neuromuscular disorders of the gastrointestinal tract. World J Gastroenterol. 2012;18:6918-6925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Bassotti G, Imbimbo BP, Betti C, Dozzini G, Morelli A. Impaired colonic motor response to eating in patients with slow-transit constipation. Am J Gastroenterol. 1992;87:504-508. [PubMed] |
| 18. | Bazzocchi G, Ellis J, Villanueva-Meyer J, Jing J, Reddy SN, Mena I, Snape WJ. Postprandial colonic transit and motor activity in chronic constipation. Gastroenterology. 1990;98:686-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 121] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Wiskur B, Greenwood-Van Meerveld B. The aging colon: the role of enteric neurodegeneration in constipation. Curr Gastroenterol Rep. 2010;12:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Poli E, Lazzaretti M, Grandi D, Pozzoli C, Coruzzi G. Morphological and functional alterations of the myenteric plexus in rats with TNBS-induced colitis. Neurochem Res. 2001;26:1085-1093. [PubMed] |
| 21. | Fornai M, Pellegrini C, Antonioli L, Segnani C, Ippolito C, Barocelli E, Ballabeni V, Vegezzi G, Al Harraq Z, Blandini F. Enteric Dysfunctions in Experimental Parkinson’s Disease: Alterations of Excitatory Cholinergic Neurotransmission Regulating Colonic Motility in Rats. J Pharmacol Exp Ther. 2016;356:434-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Motterlini R, Gonzales A, Foresti R, Clark JE, Green CJ, Winslow RM. Heme oxygenase-1-derived carbon monoxide contributes to the suppression of acute hypertensive responses in vivo. Circ Res. 1998;83:568-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 202] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 23. | Gan HT, Chen JD. Induction of heme oxygenase-1 improves impaired intestinal transit after burn injury. Surgery. 2007;141:385-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Marona HR, Lucchesi MB. Protocol to refine intestinal motility test in mice. Lab Anim. 2004;38:257-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 108] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Bove GM. A non-invasive method to evaluate gastrointestinal transit behavior in rat. J Pharmacol Toxicol Methods. 2015;74:1-6. [PubMed] |
| 26. | Mitolo-Chieppa D, Mansi G, Nacci C, De Salvia MA, Montagnani M, Potenza MA, Rinaldi R, Lerro G, Siro-Brigiani G, Mitolo CI. Idiopathic chronic constipation: tachykinins as cotransmitters in colonic contraction. Eur J Clin Invest. 2001;31:349-355. [PubMed] |
| 27. | Martín-Cano FE, Camello PJ, Pozo MJ. Characterization of the motor inhibitory role of colonic mucosa under chemical stimulation in mice. Am J Physiol Gastrointest Liver Physiol. 2014;306:G614-G621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Sanders KM, Hwang SJ, Ward SM. Neuroeffector apparatus in gastrointestinal smooth muscle organs. J Physiol. 2010;588:4621-4639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 29. | McLean S, Begg R, Jesse HE, Mann BE, Sanguinetti G, Poole RK. Analysis of the bacterial response to Ru(CO)3Cl(Glycinate) (CORM-3) and the inactivated compound identifies the role played by the ruthenium compound and reveals sulfur-containing species as a major target of CORM-3 action. Antioxid Redox Signal. 2013;19:1999-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Lecci A, Capriati A, Altamura M, Maggi CA. Tachykinins and tachykinin receptors in the gut, with special reference to NK2 receptors in human. Auton Neurosci. 2006;126-127:232-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Bertrand PP, Hu X, Mach J, Bertrand RL. Serotonin (5-HT) release and uptake measured by real-time electrochemical techniques in the rat ileum. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1228-G1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Smith TK, Gershon MD. CrossTalk proposal: 5-HT is necessary for peristalsis. J Physiol. 2015;593:3225-3227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Spencer NJ, Sia TC, Brookes SJ, Costa M, Keating DJ. CrossTalk opposing view: 5-HT is not necessary for peristalsis. J Physiol. 2015;593:3229-3231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | Xue L, Farrugia G, Miller SM, Ferris CD, Snyder SH, Szurszewski JH. Carbon monoxide and nitric oxide as coneurotransmitters in the enteric nervous system: evidence from genomic deletion of biosynthetic enzymes. Proc Natl Acad Sci USA. 2000;97:1851-1855. [PubMed] |
| 35. | Sitdikova GF, Islamov RR, Mukhamedyarov MA, Permyakova VV, Zefirov AL, Palotás A. Modulation of neurotransmitter release by carbon monoxide at the frog neuro-muscular junction. Curr Drug Metab. 2007;8:177-184. [PubMed] |
| 36. | Wang G, Hamid T, Keith RJ, Zhou G, Partridge CR, Xiang X, Kingery JR, Lewis RK, Li Q, Rokosh DG. Cardioprotective and antiapoptotic effects of heme oxygenase-1 in the failing heart. Circulation. 2010;121:1912-1925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 37. | Lim I, Gibbons SJ, Lyford GL, Miller SM, Strege PR, Sarr MG, Chatterjee S, Szurszewski JH, Shah VH, Farrugia G. Carbon monoxide activates human intestinal smooth muscle L-type Ca2+ channels through a nitric oxide-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2005;288:G7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Darmani NA, Chebolu S, Amos B, Alkam T. Synergistic antiemetic interactions between serotonergic 5-HT3 and tachykininergic NK1-receptor antagonists in the least shrew (Cryptotis parva). Pharmacol Biochem Behav. 2011;99:573-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 39. | Mourad FH, O’Donnell LJ, Dias JA, Ogutu E, Andre EA, Turvill JL, Farthing MJ. Role of 5-hydroxytryptamine type 3 receptors in rat intestinal fluid and electrolyte secretion induced by cholera and Escherichia coli enterotoxins. Gut. 1995;37:340-345. [PubMed] |
| 40. | Schulzke JD, Pfaffenbach S, Fromm A, Epple HJ, Troeger H, Fromm M. Prostaglandin I(2) sensory input into the enteric nervous system during distension-induced colonic chloride secretion in rat colon. Acta Physiol (Oxf). 2010;199:305-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Li X, Clark JD. The role of heme oxygenase in neuropathic and incisional pain. Anesth Analg. 2000;90:677-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Fest S, Soldati R, Christiansen NM, Zenclussen ML, Kilz J, Berger E, Starke S, Lode HN, Engel C, Zenclussen AC. Targeting of heme oxygenase-1 as a novel immune regulator of neuroblastoma. Int J Cancer. 2016;138:2030-2042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |