Published online Nov 7, 2016. doi: 10.3748/wjg.v22.i41.9096
Peer-review started: June 19, 2016
First decision: August 8, 2016
Revised: August 22, 2016
Accepted: September 14, 2016
Article in press: September 14, 2016
Published online: November 7, 2016
Processing time: 141 Days and 21.9 Hours
The nonalcoholic fatty liver disease (NAFLD) is the hepatic manifestation of the metabolic syndrome. NAFLD encompasses a wide histological spectrum ranging from benign simple steatosis to non-alcoholic steatohepatitis (NASH). Sustained inflammation in the liver is critical in this process. Hepatic macrophages, including liver resident macropaghes (Kupffer cells), monocytes infiltrating the injured liver, as well as specific lymphocytes subsets play a pivotal role in the initiation and perpetuation of the inflammatory response, with a major deleterious impact on the progression of fatty liver to fibrosis. During the last years, Th17 cells have been involved in the development of inflammation not only in liver but also in other organs, such as adipose tissue or lung. Differentiation of a naïve T cell into a Th17 cell leads to pro-inflammatory cytokine and chemokine production with subsequent myeloid cell recruitment to the inflamed tissue. Th17 response can be mitigated by T regulatory cells that secrete anti-inflammatory cytokines. Both T cell subsets need TGF-β for their differentiation and a characteristic plasticity in their phenotype may render them new therapeutic targets. In this review, we discuss the role of the Th17 pathway in NAFLD progression to NASH and to liver fibrosis analyzing different animal models of liver injury and human studies.
Core tip: Interleukin-17 producing cells are important in maintaining inflammation since they are a source of pro-inflammatory cytokines and chemokines with a critical role in fighting extracellular bacteria. In the last years, this lymphocyte subset has been linked to the pathogenesis of multiple immune mediated diseases and in some cases to the progression to fibrosis. In this review, we discuss the role of the Th17 pathway in nonalcoholic fatty liver disease progression to non-alcoholic steatohepatitis and to liver fibrosis analyzing previously published data obtained from different animal models and human studies of liver injury.
- Citation: Chackelevicius CM, Gambaro SE, Tiribelli C, Rosso N. Th17 involvement in nonalcoholic fatty liver disease progression to non-alcoholic steatohepatitis. World J Gastroenterol 2016; 22(41): 9096-9103
- URL: https://www.wjgnet.com/1007-9327/full/v22/i41/9096.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i41.9096
Nonalcoholic fatty liver disease (NAFLD) is defined as an abnormal accumulation of fat in the liver, evidenced by either imaging or histology without any known cause of secondary hepatic fat accumulation such as alcohol consumption, steatogenic medication or hereditary disorders[1]. The histological spectrum of NAFLD comprises benign simple steatosis and a more severe form with inflammation, hepatocyte injury with or without fibrosis called Non-alcoholic steatohepatitis (NASH), this last entity can progress to cirrhosis, liver failure and hepatocellular carcinoma. The incidence of NAFLD and NASH is growing worldwide associated with obesity and diabetes, becoming a common cause of chronic liver disease and need of liver transplantation. The prevalence in the European general population is between 20%-30%, reaching 90% among obese patients[2]. Sustained inflammation in the liver is critical in the progression from benign simple steatosis to NASH. Hepatic macrophages, comprising liver resident macropaghes (Kupffer cells), monocytes infiltrating the injured liver, as well as specific lymphocytes subsets play a pivotal role in the initiation and perpetuation of the inflammatory response, with a major deleterious impact on key steps of fatty liver progression to fibrosis[3]. During the last years, a specific subset of CD4 T effector cells, Th17 subpopulation has been suggested to be involved in this process[4,5]. In this review, we discuss the role of the Th17 pathway in NAFLD progression to NASH and to liver fibrosis analyzing previously published data obtained from different animal models and human studies of liver injury
For this review, we used PubMed and Google Scholar databases to search for relevant articles using the following mesh terms: “Th17 cells”; “NASH”; “NAFLD”“liver inflammation”; “liver fibrosis”; “induced liver injury”“IL17”; “Tregs”; “CD4 T cells” and “regulatory T cells”. Only the articles published between 2006 and 2016 were included.
CD4 T helper cells that recognize antigens in the context of Mayor Histocompatibility Complex type II (MHC II) can be polarized into different types of effector T cells to coordinate different immunophatological responses[6]. Th17 cells play a role in pathogen clearance and tissue inflammation but are also implicated in the pathogenesis of autoimmune diseases[7,8]. The differentiation of naïve CD4 T cells into Th17 cells in humans is triggered by the combined action of transforming growth factor (TGF)-β, interleukin (IL)-6 and IL-1β, these cytokines induce the expression of the key lineage defining transcription factor orphan nuclear receptor (RORc). RORc is necessary and sufficient for the differentiation of Th17 cells whereas IL-23 is required only for the pathogenicity and expansion of this lineage[9,10]. Th17 pathway is suppressed by IFN-γ and IL-4 that promote Th1 or Th2 respectively[11]. The major target genes for IL-17 include pro-inflammatory chemokines, hematopoietic cytokines, acute phase response genes and anti-microbial substances[12].
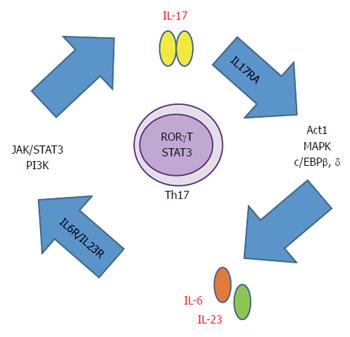
Though six IL-17 ligands have been described, IL-17A is the best characterized. IL-17F has 60% homology with IL-17A but it has 10 times less affinity for their receptors[13] (Table 1). They can form homo or heterodimers. Once they bind their cognate heterodimeric receptor IL-17RA, propagates a cascade of events that lead to neutrophil recruitment, inflammation and host defense[14]. Secretion of IL-17 is triggered and perpetuated by IL-6 and IL-23 through at least two transcription factors. The first one is Janus kinase - signal transducer and activator of transcription (JAK-STAT) and the second one is phosphoinositide-3-kinase (PI3K) through the nuclear factor-κB (NF-κB)[15,16]. STAT3 and/or NF-κB, respectively, translocate to the nucleus to promote IL-17 production (Figure 1).
| IL-17 family ligands | Binding receptor | Produced mainly by |
| IL-17 A | IL-17 RA, IL-17 RC | T cells |
| IL-17 A/F | IL-17 RA, IL-17 RC | T cells |
| IL-17 B | IL-17 RB | Numerous cells |
| IL-17 C | Unknown | Prostate, kidney cells |
| IL-17 D | Unknown | Numerous cells |
| IL-17 E (IL-25) | IL-17 RB (IL-25 R) | Numerous cells |
| IL-17 F | IL-17 RA, IL-17 RC | T cells |
Regarding IL-17 receptors, there are five different heterodimeric receptors for the IL-17 family ligands. IL-17 RA is ubiquitously expressed on a wide range of tissues (liver, intestine, lung, adipose tissue) and cell types (endothelial and immune cells). IL-17RA downstream signaling involves activation of NF-κB activator 1 (Act1), CCAAT/enhancer binding protein beta (C/EBPβ), CCAAT/enhancer binding protein delta (C/EBPδ) and mitogen-activated protein kinase (MAPK) activation, followed by NF-κB and JNK nuclear translocation. Thus, leading to the production of pro-inflammatory cytokines and chemokines and subsequent myeloid cell recruitment to the inflamed tissue[15,17].
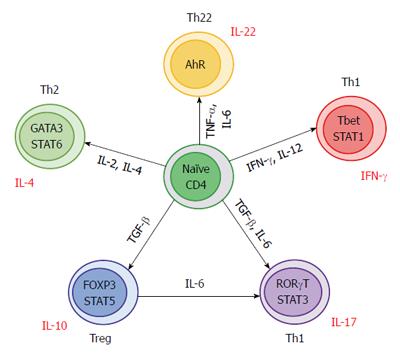
Even though Th17 and T regulatory cells (Tregs) have different functions, they do share some similarities. Depending on the stimulus, both T cells populations are capable to change their regulation and function[18]. TGF-β for example, is essential for differentiation of both cell types, but in the absence of pro-inflammatory signals promotes the expansion of inducible Tregs (iTregs)[19]. On the other hand, Th17 development requires the presence of both TGF-β and IL-6[16,17].
This effect could be explained by a TGF-β concentration-dependent function. TGF-β at low concentrations acts synergistically with IL-6 and IL-21 to promote IL-23 receptor (IL-23R) expression, favoring Th17 differentiation[20,21]. On the contrary, at high concentrations, TGF-β suppresses IL-23R and Tregs development is favored by Foxp3+ expression (which in turn inhibits RORγt function)[22,23].
Several studies have established that differentiation of Foxp3+ Tregs is not static and that they can transdifferentiate into Th17 cells[24,25]. In mice, IL-6 showed to convert Foxp3+ cells into Th17 cells in the absence of TGF-β[25] (Figure 2).
IL-17 has been linked to the pathogenesis of many immune mediated diseases like psoriasis, pulmonary fibrosis, systemic sclerosis, myocardial fibrosis, systemic lupus erythematosus, inflammatory bowel disease, rhino sinusitis, encephalomyelitis, multiple sclerosis, asthma, and uveitis[7,8,26-37]. Still, the role of the Th17 pathway in human liver disease is not fully understood.
The association between obesity and NAFLD/NASH implicates the crosstalk of many cells types and organs. Due to the limitation of using human samples, the best approach is to study deeply the different cell interactions in murine models.
There is evidence regarding IL-17 axis playing a broad role in multiple models of NAFLD via modulation of hepatic inflammation. Among resident hepatic cells, hepatic stellate cells (HSC), Kupffer cells, hepatocytes and endothelial cells express the IL-17RA and are known to activate inflammatory pathways which exacerbate the disease[38,39]. On the other hand, other studies showed that hepatocytes and endothelial cells do not transmit IL-17 signals despite IL-17RA expression and that they do not produce IL-17[39-41]. As regard the production of IL-17 in liver, is not only limited to CD4+ and CD8+ T cells. Natural Killer T cells, macrophages, neutrophils, γδ T cells and Innate Lymphoid Cells are also capable of producing IL-17[39,42,43]. At least for now, only Th17 CD4 T cells, macrophages and neutrophils are known to be involved in the development of steatohepatitis inflammation process.
As mentioned before, the progression from NAFLD to NASH involves a wide spectrum of events such as lipid deposition, inflammation, oxidative stress, fibrosis[44]. To study the mediators involved in this process, were characterized and described several animal models.
One of the oldest model for liver fibrosis is the CCL4 toxin-based damage. During the development of liver fibrosis by this approach, CD4+ and CD8+ T cells both exhibited increased IL-17A expression. However the major source of this interleukin was represented by neutrophils. Moreover, HSC were activated and responded by increasing IL-6, α-SMA, TNF-α and TGF-β mRNA expression[39,45,46]. Therefore, when studied the balance of Th17/Treg in the liver, it was favored toward Th17, thus promoting inflammation[45].
In vivo and in vitro analysis of this model demonstrated that in HSC, IL-17 increases the expression of Collagen-α1 through STAT3 signaling. Stimulation of HSCs with IL-17 results in Collagen-α1 up-regulation via IL-17RA. Moreover, in a STAT3-deficient mice, HSCs do not up-regulate Collagen-α1 in response to IL-17A, confirming that this mediator is a required target of IL-17 signaling[39,47].
Another model of liver injury is the bile duct ligation (BDL) where the bile flow is disrupted, resulting in severe inflammatory cholestatic liver injury that induces a strong fibrotic response after 21 to 28 d[48]. During the inflammatory process CD4+ T cells exhibited an increase in IL-17 expression in the liver. For the CD8+ T cells controversial results were observed, in some studies was reported that IL-17 was produced whereas others indicated the opposite[39,49]. However, neutrophils keep on representing the major source of IL-17 among the infiltrating cells in liver after BDL[49].
Inflammatory cytokines, TGF-β, IL-6, IL-1β , and TNF-α were increased after BDL, but when anti-IL-17mAb treatment or knock out (KO) IL-17RA mice was performed, a marked improvement in liver function was observed. Suppressed Kupffer cells and HSC activation (collagen-α1 production through STAT3), macrophages infiltration and decreased proinflammatory mediators level in serum and injured liver in mice were shown[39,49].
Diet induced models of liver damage have been characterized. One of the most used is the Methionine Choline deficient diet (MCDD) where steatohepatitis occurs at day 10 and fibrosis is observed by 8-10 wk in mice[50]. The main disadvantage of this model is that obesity and insulin resistance are not present. MCDD-driven NAFLD was related to increased hepatic IL-17RA expression and IL-17A/IL-17F production. Moreover, was observed an increase of Tregs (peak at 4 wk of diet) and Th17 (peak 8 wk of diet or further)[51]. When MCDD animals were treated in-vivo with neutralizing antibodies against CD25 or IL-17, the liver injury (measured by ALT and AST levels) was alleviated or worsen respectively. However, no evident histological changes were found[51]. On the other hand, when KO mice of IL-17RA, IL-17A or IL-17F were challenged with the diet, a reduction in proinflammatory cytokine and chemokine production, immune cell infiltration and hepatocellular damage was observed[52,53]. The anti-inflammatory and/or immune-regulatory mediators normally inhibited by the IL-17 axis were restored, for instance when IL-17A or IL-17F were missing Treg cell expansion and activation returned to normal. Rolla et al[52] described no changes in Treg cells but observed the presence of Th22 cells. Interestingly, was shown in IL-17 KO mice that Th22 cells seemed to be protective in NASH preventing from lipotoxicity[52].
Another widely used diet induced model of liver injury in mice is the high fat diet (HFD). Even if it is a good model for glucose intolerance and obesity, fibrosis is rarely observed and usually additional events such as LPS challenge are required to develop it. The increased oxidative stress produced in the fatty liver causes the apoptosis of Tregs, and increase the Th17 cells[54,55]. When IL-17 is neutralized in HFD mice the challenge with LPS promotes a decrease in serum transaminases levels and a reduced hepatic inflammatory cell infiltrate[55]. In in vitro high fat models (HepG2 and primary mice hepatocytes) the exposure to IL-17 induced a higher IL-6 release in the culture medium, higher triglyceride intracellular content and interfered insulin-signaling pathway[55] (Table 2).
NAFLD prevalence is higher in morbid obese (MO) patients than in the lean population, and these patients present a higher risk for developing NASH and its complications. In a prospective study that included 112 obese patients with NAFLD, the Th17/Tregs ratio correlated positively with NASH progression (by histology) and CK-18 expression (one of the proposed biomarkers of NAFLD progression) analyzed in peripheral blood and in intra hepatic lymphocytes. One year after bariatric surgery, there was a decrease in the Th17/Tregs ratio that became similar to healthy lean controls[4]. In Vonghia et al[56] prospective study, a decrease in the IL-10/IL-17A ratio marked an accentuated pro-inflammatory state in obese patients with NASH in comparison to those without NASH.
Studies with MO patients evaluated subcutaneous adipose tissue CD4 T cells content from lean, metabolically normal obese and metabolically abnormal obese subjects. They found that CD4+ gene expression was increased progressively and skewed towards Th17 phenotype. JNK activation was proposed as the mechanism responsible for IL-17 induced insulin resistance[57].
IL-17 mRNA expression from visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) of MO patients was increased in comparison to normal weight women being higher in VAT than in SAT[58]. Moreover, SAT, VAT and peripheral blood mononuclear cells (PBMC) from overweight/moderately obese and MO subjects presented a marked increase in the Th17 population (VAT higher than SAT and peripheral blood)[59]. Positive correlations between IL-17 vs IL-6 or Resistin at mRNA levels were found but not correlations for the percentages of Th17 cell with insulin resistance values have been established[58,59].
Contrarily to what is reported in mice[52], to our knowledge the study published by Zapata-Gonzalez et al[58] is the only one that reported higher plasmatic IL-17 concentration in the normal weight group than in MO patients.
Diabetes mellitus type II (T2D) is a common disorder among NAFLD patients. In the work of Zeng et al[60], CD4 T cells from PBMC were analyzed by flow cytometry. A reduction in the absolute number and in the percentage of Tregs was shown favoring the Th17/Tregs ratio toward Th17 cells[60]. Even though functionality of Tregs cells was conserved, their number was decreased because of impaired survival ability. Interestingly, Th17 cells were higher in patients that presented more T2D complications[57]. Conversely, no differences were found in IL-17 plasma of T2D compared to age-matched healthy controls[61].
In liver fibrosis secondary to primary biliary cirrhosis (PBC), patients presented higher peripheral Th17 cells when compared to healthy controls. In the liver, IL-17+ cells gathered around the portal areas[62]. Furthermore, in cirrhotic liver tissue IL-17+ cell infiltration was higher than controls[46].
In vitro studies of human hepatic stellate cells (HSC) exposed to IL-17 showed a dose dependent activation and proliferation response that was neutralized by an IL-17 antagonist[62]. Fabre et al[63] evaluated HSC activation (LX2 cell line and primary human hepatic stellate cells) by IL-17. They observed that IL-17 by itself was insufficient to activate the cells, but when combined with a suboptimal TGF-β dose generated a strong activation enhancing TGF-β response by increasing cell surface expression of its receptor and the profibrotic signaling[63].
Regarding the pediatric population, much less is known; we found only a study conducted by Łuczyński et al[64] in children with central obesity. They showed higher percentages of Th17 cells in the peripheral blood in comparison with healthy lean children[61]. In other pediatric diseases these T cells were involved, principally in inflammation, such as autoimmune thyroid disease or Mycoplasma pneumoniae infection[65,66] (Table 3).
| Th17 cells | Th17/Tregs | IL-17 expression | Disease | Ref. | |
| Liver | ↑ | ↑ | ↑ | NAFLD - MO | Rau et al[4] |
| PBC | Shi et al[62] | ||||
| CH - CIRR | Tan et al[46] | ||||
| VAT | ↑↑ | MO | McLaughlin et al[59] | ||
| MO | Zapata-Gonzalez et al[58] | ||||
| SAT | ↑ | MAO | Fabbrini et al[57] | ||
| MO | McLaughlin et al[59] | ||||
| PBMC | ↑↑↑ | ↑↑ | NAFLD - MO | Rau et al[4] | |
| T2D | Zeng et al[60] | ||||
| Obesity | Łuczyński et al[64] | ||||
| PBC | Shi et al[62] |
A pro-inflammatory state is crucial for the initiation and maintenance of inflammation in the onset and progression of NAFLD/NASH. T cells resident in non-lymphoid tissues are able to regulate local inflammation by modulating immunological and non-immunological responses. Many studies in different animal models have proved the important role of the Th17 pathway in inflammation and HSC activation. Much less is known about human physiopathology of NAFLD due to the limitations and difficulty to obtain samples. Studies with obese or diabetic patients obtained higher Th17 cells in blood with no changes or decrease in Tregs. If IL-17 is elevated or not in plasma is still controversial. Adipose tissue and intrahepatic Th17 lymphocyte subsets have been assessed in NAFLD/obese/PBC patients, being higher compared to control individuals.
It has been widely argued if inflammation occurs first in liver than in adipose tissue or the other way around. Until now, this is still unraveled but it is known that the adipose tissue inflammation and their adipokines, free fatty acids, and gut derived microbial products could promote Th17 differentiation in the liver, with the consequent imbalance towards inflammation. Obesity may maintain a positive feedback loop that promotes Th17 survival in the inflamed liver. This would explain how weight loss after bariatric surgery can reverse clinical and histopathological features of NASH. On the other hand, it seems that the T cell imbalance occurs in situ, but to date there is not enough evidence to explain the connection between adipose tissue inflammation and hepatic injury progression.
Studies that analyze the crosstalk between the different organs during the NAFLD/NASH progression should be promoted in order to evaluate and establish the main players in this disease.
Although there is evidence that implicates the Th17 pathway as a key player in the progression of NALFD, it seems that there is a lot more to be elucidated. Plasticity of this cell subtype may render it a therapeutic target.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Khedmat H, Laguna JC, Streba LA S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
| 1. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ; American Association for the Study of Liver Diseases, American College of Gastroenterology, American Gastroenterological Association. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Am J Gastroenterol. 2012;107:811-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 305] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 2. | LaBrecque DR, Abbas Z, Anania F, Ferenci P, Khan AG, Goh KL, Hamid SS, Isakov V, Lizarzabal M, Peñaranda MM. World Gastroenterology Organisation global guidelines: Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Clin Gastroenterol. 2014;48:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 275] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 3. | Marra F, Lotersztajn S. Pathophysiology of NASH: perspectives for a targeted treatment. Curr Pharm Des. 2013;19:5250-5269. [PubMed] |
| 4. | Rau M, Schilling AK, Meertens J, Hering I, Weiss J, Jurowich C, Kudlich T, Hermanns HM, Bantel H, Beyersdorf N. Progression from Nonalcoholic Fatty Liver to Nonalcoholic Steatohepatitis Is Marked by a Higher Frequency of Th17 Cells in the Liver and an Increased Th17/Resting Regulatory T Cell Ratio in Peripheral Blood and in the Liver. J Immunol. 2016;196:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 232] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 5. | Harley IT, Stankiewicz TE, Giles DA, Softic S, Flick LM, Cappelletti M, Sheridan R, Xanthakos SA, Steinbrecher KA, Sartor RB. IL-17 signaling accelerates the progression of nonalcoholic fatty liver disease in mice. Hepatology. 2014;59:1830-1839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 207] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 6. | Nati M, Haddad D, Birkenfeld AL, Koch CA, Chavakis T, Chatzigeorgiou A; World Gastroenterology Organisation. The role of immune cells in metabolism-related liver inflammation and development of non-alcoholic steatohepatitis (NASH). Rev Endocr Metab Disord. 2016;17:29-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 7. | Rother N, van der Vlag J. Disturbed T Cell Signaling and Altered Th17 and Regulatory T Cell Subsets in the Pathogenesis of Systemic Lupus Erythematosus. Front Immunol. 2015;6:610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Marinoni B, Ceribelli A, Massarotti MS, Selmi C. The Th17 axis in psoriatic disease: pathogenetic and therapeutic implications. Auto Immun Highlights. 2014;5:9-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 978] [Cited by in RCA: 982] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 10. | Stockinger B, Veldhoen M, Martin B. Th17 T cells: linking innate and adaptive immunity. Semin Immunol. 2007;19:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 202] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 11. | Hammerich L, Heymann F, Tacke F. Role of IL-17 and Th17 cells in liver diseases. Clin Dev Immunol. 2011;2011:345803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 199] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 12. | Gaffen SL. An overview of IL-17 function and signaling. Cytokine. 2008;43:402-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 268] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 13. | Shen F, Gaffen SL. Structure-function relationships in the IL-17 receptor: implications for signal transduction and therapy. Cytokine. 2008;41:92-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 211] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 14. | Gu C, Wu L, Li X. IL-17 family: cytokines, receptors and signaling. Cytokine. 2013;64:477-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 450] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 15. | Eljaafari A, Robert M, Chehimi M, Chanon S, Durand C, Vial G, Bendridi N, Madec AM, Disse E, Laville M. Adipose Tissue-Derived Stem Cells From Obese Subjects Contribute to Inflammation and Reduced Insulin Response in Adipocytes Through Differential Regulation of the Th1/Th17 Balance and Monocyte Activation. Diabetes. 2015;64:2477-2488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 16. | Cho ML, Kang JW, Moon YM, Nam HJ, Jhun JY, Heo SB, Jin HT, Min SY, Ju JH, Park KS. STAT3 and NF-kappaB signal pathway is required for IL-23-mediated IL-17 production in spontaneous arthritis animal model IL-1 receptor antagonist-deficient mice. J Immunol. 2006;176:5652-5661. [PubMed] |
| 17. | Chang SH, Dong C. Signaling of interleukin-17 family cytokines in immunity and inflammation. Cell Signal. 2011;23:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 175] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 18. | Brucklacher-Waldert V, Carr EJ, Linterman MA, Veldhoen M. Cellular Plasticity of CD4+ T Cells in the Intestine. Front Immunol. 2014;5:488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5130] [Cited by in RCA: 5475] [Article Influence: 288.2] [Reference Citation Analysis (0)] |
| 20. | Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1566] [Cited by in RCA: 1702] [Article Influence: 94.6] [Reference Citation Analysis (0)] |
| 21. | Korn T, Bettelli E, Gao W, Awasthi A, Jäger A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484-487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1499] [Cited by in RCA: 1469] [Article Influence: 81.6] [Reference Citation Analysis (0)] |
| 22. | Ueno A, Ghosh A, Hung D, Li J, Jijon H. Th17 plasticity and its changes associated with inflammatory bowel disease. World J Gastroenterol. 2015;21:12283-12295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 23. | Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1569] [Cited by in RCA: 1542] [Article Influence: 90.7] [Reference Citation Analysis (0)] |
| 24. | Omenetti S, Pizarro TT. The Treg/Th17 Axis: A Dynamic Balance Regulated by the Gut Microbiome. Front Immunol. 2015;6:639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 362] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 25. | Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725-6729. [PubMed] |
| 26. | Wilson MS, Madala SK, Ramalingam TR, Gochuico BR, Rosas IO, Cheever AW, Wynn TA. Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med. 2010;207:535-552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 476] [Cited by in RCA: 606] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 27. | Gasse P, Riteau N, Vacher R, Michel ML, Fautrel A, di Padova F, Fick L, Charron S, Lagente V, Eberl G. IL-1 and IL-23 mediate early IL-17A production in pulmonary inflammation leading to late fibrosis. PLoS One. 2011;6:e23185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 172] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 28. | Okamoto Y, Hasegawa M, Matsushita T, Hamaguchi Y, Huu DL, Iwakura Y, Fujimoto M, Takehara K. Potential roles of interleukin-17A in the development of skin fibrosis in mice. Arthritis Rheum. 2012;64:3726-3735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 29. | Feng W, Li W, Liu W, Wang F, Li Y, Yan W. IL-17 induces myocardial fibrosis and enhances RANKL/OPG and MMP/TIMP signaling in isoproterenol-induced heart failure. Exp Mol Pathol. 2009;87:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Cătană CS, Berindan Neagoe I, Cozma V, Magdaş C, Tăbăran F, Dumitraşcu DL. Contribution of the IL-17/IL-23 axis to the pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2015;21:5823-5830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 141] [Cited by in RCA: 144] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 31. | Gálvez J. Role of Th17 Cells in the Pathogenesis of Human IBD. ISRN Inflamm. 2014;2014:928461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 232] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 32. | Kolbinger F, Huppertz C, Mir A, Di Padova F. IL-17A and multiple sclerosis: signaling pathways, producing cells and target cells in the central nervous system. Curr Drug Targets. 2016; Epub ahead of print. [PubMed] |
| 33. | Melnikov M, Belousova O, Murugin V, Pashenkov М, Boyко A. The role of dopamine in modulation of Th-17 immune response in multiple sclerosis. J Neuroimmunol. 2016;292:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Dos Passos GR, Sato DK, Becker J, Fujihara K. Th17 Cells Pathways in Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorders: Pathophysiological and Therapeutic Implications. Mediators Inflamm. 2016;2016:5314541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 35. | Qin L, Feng J, Hu C, Li Y, Niu R. [Th17/Treg imbalance mediated by IL-8 in RSV-infected bronchial epithelial cells]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2016;41:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 36. | Gavino AC, Nahmod K, Bharadwaj U, Makedonas G, Tweardy DJ. STAT3 inhibition prevents lung inflammation, remodeling, and accumulation of Th2 and Th17 cells in a murine asthma model. Allergy. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 37. | Bi HS, Liu ZF, Cui Y. Pathogenesis of innate immunity and adaptive immunity in the mouse model of experimental autoimmune uveitis. J Chin Med Assoc. 2015;78:276-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Peverill W, Powell LW, Skoien R. Evolving concepts in the pathogenesis of NASH: beyond steatosis and inflammation. Int J Mol Sci. 2014;15:8591-8638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 290] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 39. | Meng F, Wang K, Aoyama T, Grivennikov SI, Paik Y, Scholten D, Cong M, Iwaisako K, Liu X, Zhang M. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology. 2012;143:765-766.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 564] [Article Influence: 43.4] [Reference Citation Analysis (1)] |
| 40. | Kang Z, Altuntas CZ, Gulen MF, Liu C, Giltiay N, Qin H, Liu L, Qian W, Ransohoff RM, Bergmann C. Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity. 2010;32:414-425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 238] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 41. | Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647-659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 527] [Cited by in RCA: 547] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 42. | Jie Z, Liang Y, Hou L, Dong C, Iwakura Y, Soong L, Cong Y, Sun J. Intrahepatic innate lymphoid cells secrete IL-17A and IL-17F that are crucial for T cell priming in viral infection. J Immunol. 2014;192:3289-3300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 43. | Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1091] [Cited by in RCA: 1244] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 44. | Rosso N, Chavez-Tapia NC, Tiribelli C, Bellentani S. Translational approaches: from fatty liver to non-alcoholic steatohepatitis. World J Gastroenterol. 2014;20:9038-9049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 45. | Sun XF, Gu L, Deng WS, Xu Q. Impaired balance of T helper 17/T regulatory cells in carbon tetrachloride-induced liver fibrosis in mice. World J Gastroenterol. 2014;20:2062-2070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 46. | Tan Z, Qian X, Jiang R, Liu Q, Wang Y, Chen C, Wang X, Ryffel B, Sun B. IL-17A plays a critical role in the pathogenesis of liver fibrosis through hepatic stellate cell activation. J Immunol. 2013;191:1835-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 256] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 47. | Ogata H, Chinen T, Yoshida T, Kinjyo I, Takaesu G, Shiraishi H, Iida M, Kobayashi T, Yoshimura A. Loss of SOCS3 in the liver promotes fibrosis by enhancing STAT3-mediated TGF-beta1 production. Oncogene. 2006;25:2520-2530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 212] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 48. | Tag CG, Sauer-Lehnen S, Weiskirchen S, Borkham-Kamphorst E, Tolba RH, Tacke F, Weiskirchen R. Bile duct ligation in mice: induction of inflammatory liver injury and fibrosis by obstructive cholestasis. J Vis Exp. 2015;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 169] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 49. | Zhang S, Huang D, Weng J, Huang Y, Liu S, Zhang Q, Li N, Wen M, Zhu G, Lin F. Neutralization of Interleukin-17 Attenuates Cholestatic Liver Fibrosis in Mice. Scand J Immunol. 2016;83:102-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 50. | Caballero F, Fernández A, Matías N, Martínez L, Fucho R, Elena M, Caballeria J, Morales A, Fernández-Checa JC, García-Ruiz C. Specific contribution of methionine and choline in nutritional nonalcoholic steatohepatitis: impact on mitochondrial S-adenosyl-L-methionine and glutathione. J Biol Chem. 2010;285:18528-18536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 186] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 51. | Liu Y, She W, Wang F, Li J, Wang J, Jiang W. 3, 3’-Diindolylmethane alleviates steatosis and the progression of NASH partly through shifting the imbalance of Treg/Th17 cells to Treg dominance. Int Immunopharmacol. 2014;23:489-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 52. | Rolla S, Alchera E, Imarisio C, Bardina V, Valente G, Cappello P, Mombello C, Follenzi A, Novelli F, Carini R. The balance between IL-17 and IL-22 produced by liver-infiltrating T-helper cells critically controls NASH development in mice. Clin Sci (Lond). 2016;130:193-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 53. | Giles DA, Moreno-Fernandez ME, Stankiewicz TE, Cappelletti M, Huppert SS, Iwakura Y, Dong C, Shanmukhappa SK, Divanovic S. Regulation of Inflammation by IL-17A and IL-17F Modulates Non-Alcoholic Fatty Liver Disease Pathogenesis. PLoS One. 2016;11:e0149783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 54. | Ma X, Hua J, Mohamood AR, Hamad AR, Ravi R, Li Z. A high-fat diet and regulatory T cells influence susceptibility to endotoxin-induced liver injury. Hepatology. 2007;46:1519-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 55. | Tang Y, Bian Z, Zhao L, Liu Y, Liang S, Wang Q, Han X, Peng Y, Chen X, Shen L. Interleukin-17 exacerbates hepatic steatosis and inflammation in non-alcoholic fatty liver disease. Clin Exp Immunol. 2011;166:281-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 242] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 56. | Vonghia L, Magrone T, Verrijken A, Michielsen P, Van Gaal L, Jirillo E, Francque S. Peripheral and Hepatic Vein Cytokine Levels in Correlation with Non-Alcoholic Fatty Liver Disease (NAFLD)-Related Metabolic, Histological, and Haemodynamic Features. PLoS One. 2015;10:e0143380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 57. | Fabbrini E, Cella M, McCartney SA, Fuchs A, Abumrad NA, Pietka TA, Chen Z, Finck BN, Han DH, Magkos F. Association between specific adipose tissue CD4+ T-cell populations and insulin resistance in obese individuals. Gastroenterology. 2013;145:366-374.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 215] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 58. | Zapata-Gonzalez F, Auguet T, Aragonès G, Guiu-Jurado E, Berlanga A, Martinez S, Martí A, Sabench F, Hernandez M, Aguilar C. Interleukin-17A Gene Expression in Morbidly Obese Women. Int J Mol Sci. 2015;16:17469-17481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 59. | McLaughlin T, Liu LF, Lamendola C, Shen L, Morton J, Rivas H, Winer D, Tolentino L, Choi O, Zhang H. T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler Thromb Vasc Biol. 2014;34:2637-2643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 268] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 60. | Zeng C, Shi X, Zhang B, Liu H, Zhang L, Ding W, Zhao Y. The imbalance of Th17/Th1/Tregs in patients with type 2 diabetes: relationship with metabolic factors and complications. J Mol Med (Berl). 2012;90:175-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 195] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 61. | Roohi A, Tabrizi M, Abbasi F, Ataie-Jafari A, Nikbin B, Larijani B, Qorbani M, Meysamie A, Asgarian-Omran H, Nikmanesh B. Serum IL-17, IL-23, and TGF-β levels in type 1 and type 2 diabetic patients and age-matched healthy controls. Biomed Res Int. 2014;2014:718946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 62. | Shi T, Zhang T, Zhang L, Yang Y, Zhang H, Zhang F. The Distribution and the Fibrotic Role of Elevated Inflammatory Th17 Cells in Patients With Primary Biliary Cirrhosis. Medicine (Baltimore). 2015;94:e1888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 63. | Fabre T, Kared H, Friedman SL, Shoukry NH. IL-17A enhances the expression of profibrotic genes through upregulation of the TGF-β receptor on hepatic stellate cells in a JNK-dependent manner. J Immunol. 2014;193:3925-3933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 64. | Łuczyński W, Grubczak K, Moniuszko M, Głowińska-Olszewska B, Bossowski A. Elevated levels of Th17 cells in children with central obesity. Scand J Clin Lab Invest. 2015;75:595-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 65. | Bossowski A, Moniuszko M, Idźkowska E, Grubczak K, Singh P, Bossowska A, Diana T, Kahaly GJ. Decreased proportions of CD4 + IL17+/CD4 + CD25 + CD127- and CD4 + IL17+/CD4 + CD25 + CD127 - FoxP3+ T cells in children with autoimmune thyroid diseases (.). Autoimmunity. 2016;49:320-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 66. | Wang X, Chen X, Tang H, Zhu J, Zhou S, Xu Z, Liu F, Su C. Increased Frequency of Th17 Cells in Children With Mycoplasma pneumoniae Pneumonia. J Clin Lab Anal. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |