Published online Oct 28, 2016. doi: 10.3748/wjg.v22.i40.9035
Peer-review started: July 16, 2016
First decision: August 19, 2016
Revised: September 8, 2016
Accepted: September 28, 2016
Article in press: September 28, 2016
Published online: October 28, 2016
Processing time: 105 Days and 1.7 Hours
We recently reported several driver genes of biliary tract carcinoma (BTC) that are known to play important roles in oncogenesis and disease progression. Although the need for developing novel therapeutic strategies is increasing, there are very few BTC cell lines and xenograft models currently available for conducting preclinical studies. Using a total of 88 surgical BTC specimens and 536 immunodeficient mice, 28 xenograft models and 13 new BTC cell lines, including subtypes, were established. Some of our cell lines were found to be resistant to gemcitabine, which is currently the first choice of treatment, thereby allowing highly practical preclinical studies to be conducted. Using the aforementioned cell lines and xenograft models and a clinical pathological database of patients undergoing BTC resection, we can establish a preclinical study system and appropriate parameters for drug efficacy studies to explore new biomarkers for practical applications in the future studies.
Core tip: Although the need for developing novel therapeutic strategies for biliary tract carcinoma (BTC) is increasing, there are only few xenograft models and cell lines available for in vivo and in vitro studies, respectively. To conduct appropriate preclinical studies, we established 28 xenograft models and 13 new BTC cell lines using several surgical BTC specimens and immunodeficient mice. Using the aforementioned cell lines and xenograft models and a clinical pathological database of patients undergoing BTC resection, we can establish appropriate parameters for drug efficacy studies to explore new biomarkers for practical applications in the future studies.
- Citation: Ojima H, Yamagishi S, Shimada K, Shibata T. Establishment of various biliary tract carcinoma cell lines and xenograft models for appropriate preclinical studies. World J Gastroenterol 2016; 22(40): 9035-9038
- URL: https://www.wjgnet.com/1007-9327/full/v22/i40/9035.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i40.9035
Biliary tract carcinoma (BTC) is an extremely malignant tumor. The incidence and mortality rates of BTC are currently rising and are particularly high in Asian countries. Surgical resection is the only curative treatment; however, most cases are diagnosed to be at advanced and inoperable stages by the time patients visit a hospital. The most serious problem is that there are no efficient chemotherapeutic regimens for patients with inoperable or recurrent BTC. Worldwide, gemcitabine-cisplatin combination therapy is the first choice, but clinicians are not satisfied with its efficacy. New drugs are needed for BTC patients.
Recently, we conducted genomic analyses of clinical specimens from 260 patients, which is the largest study till date, wherein we identified genomic abnormalities, which could be potential therapeutic targets, in 32 driver genes that play important roles in oncogenesis and disease progression in approximately 40% of BTC patients[1]. Although the need for developing novel therapeutic strategies is increasing, there are very few BTC-related resources currently available for conducting preclinical studies. The main reasons are as follows: the number of surgical BTC patients is not high at a single institute, and there is no large clinicopathological database. It is difficult to obtain surgical specimens for basic research. Therefore, there are only few xenograft models and cell lines available for in vivo and in vitro studies.
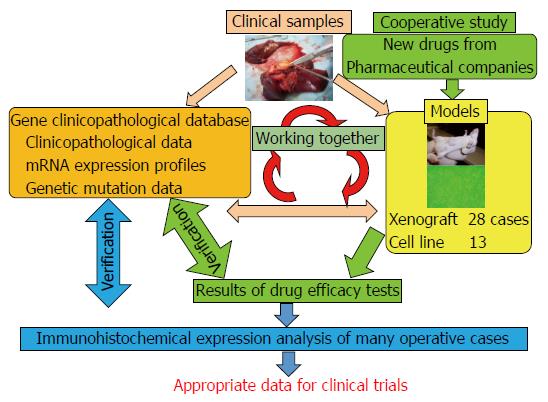
To conduct appropriate preclinical studies, surgical BTC specimens (collected from Japanese patients at the National Cancer Center Hospital, Tokyo, Japan since 2005 in an appropriate manner without any interference to pathological diagnosis) were directly transplanted into immunodeficient mice and subjected to cell culture medium to establish xenograft models and cell lines, respectively, as reported in 2010[2]. From a total of 88 BTC specimens and 536 immunodeficient mice during the period 2005-2013, we established 28 xenograft models (18 intrahepatic cholangiocarcinoma, four perihilar, and six distal BTC) and 13 new BTC cell lines, including subtypes (eight intrahepatic cholangiocarcinoma, two perihilar, and three distal BTC) (Table 1). Some of our established cell lines were found to be resistant to gemcitabine (Table 2), thereby allowing highly practical preclinical studies to be conducted. In addition, we conducted molecular pathology analyses of cell lines and constructed a clinical pathological database of patients undergoing BTC resection to establish appropriate parameters for drug efficacy studies to explore new biomarkers for practical applications (Figure 1)[2-5]. All experiments were approved by the Animal Care and Ethics Committee of the National Cancer Center (ID: T05-046). This study was approved by the Ethical Committee of the National Cancer Center (ID: 2007-022).
| Xenograft | Pathological diagnosis of original tumor | Age/sex | Histologic type | Prognosis (survival days) | Chemotherapy | Clinical evaluation of chemotherapy effect (effective days) | Established cell line |
| 1 | CCC | 70/F | Adeno, mod | Death (402) | Non | NCC-CC1 | |
| 2 | CCC | 71/F | Adeno, mod | Death (175) | Non | NCC-CC3-1 | |
| NCC-CC3-2 | |||||||
| 3 | CCC | 59/M | Adeno, mod | Alive (2172) | Non | NCC-CC4-1 | |
| NCC-CC4-2 | |||||||
| NCC-CC4-3(NCC-CC5) | |||||||
| 4 | CCC | 31/M | Adeno, mod + PSC | Death (386) | GEM + TS1 | SD (84 d) | NCC-CC6-1 |
| NCC-CC6-2 | |||||||
| 5 | Distal BDCa | 58/F | Adeno, mod | Death (299) | GEM | PD | NCC-BD1 |
| 6 | Distal BDCa | 77/F | Adeno, mod | Death (393) | GEM | PD | NCC-BD21 |
| 7 | Distal BDCa | 80/M | Adeno, mod | Death (212) | Non | NCC-BD3 | |
| 8 | Hilar BDCa | 74/M | Adeno, mod | Death (172) | Non | NCC-BD4-1 | |
| NCC-BD4-2 | |||||||
| 9 | Hilar BDCa | 48/M | Adeno, well | Alive (500) | GEM | PD | NA |
| 10 | Hilar BDCa | 43/M | Adeno, mod | Alive (1422) | Non | NA | |
| 11 | CCC | 69/M | Adeno, mod | Death (174) | Non | NA | |
| 12 | CCC | 54/F | Adeno, mod | Death (181) | Non | NA | |
| 13 | CCC | 56/M | Adeno, mod | Death (319) | GEM | PD | NA |
| 14 | CCC | 73/M | Adeno, mod | Death (53) | Non | NA | |
| 15 | CCC | 54/M | Adeno, mod | Alive (2608) | Non | NA | |
| 16 | CCC | 45/F | Adeno, mod | Alive (882) | GEM + CDDP | Unknown | NA |
| 17 | CCC | 72/M | Muc | Death (749) | GEM/GEM + TS1 | Unknown | NA |
| 18 | CCC | 78/M | Adeno, mod | Death (382) | GEM | Unknown | NA |
| 19 | CCC | 66/M | Adeno, mod | Death (168) | Non | NA | |
| 20 | CCC | 65/M | CoCC | Alive (1604) | Non | NA | |
| 21 | CCC | 70/M | Adeno, por | Death (851) | GEM | SD (49 d) | NA |
| 22 | CCC | 63/F | Adeno, mod | Alive (363) | Unknown | Unknown | NA |
| 23 | CCC | 72/M | Adeno, mod | Death (394) | GEM | PD | NA |
| 24 | CCC | 77/F | Adeno, mod | Death (445) | GEM | SD (105 d) | NA |
| 25 | Hilar BDCa | 66/M | Adeno, mod | Alive (102) | GEM + TS1 | Unknown | NA |
| 26 | Distal BDCa | 54/M | Adeno, mod | Alive (2096) | Non | NA | |
| 27 | Distal BDCa | 67/M | Adeno, mod | Death (672) | GEM + TS1 | PD | NA |
| 28 | Distal BDCa | 80/M | Adeno, mod | Alive (2024) | GEM | PR-CR (548 d) | NA |
| Cell line | Sensitivity to gemcitabine in cell line1 | |||
| IC50 (μmol/L) | IC60 (μmol/L) | IC70 (μmol/L) | IC80 (μmol/L) | |
| NCC-CC1 | 86.78 | N.A | N.A | N.A |
| NCC-CC3-1 | 0.04 | 1.82 | 9.31 | 85.21 |
| NCC-CC3-2 | 0.10 | 1.92 | 43.83 | N.A |
| NCC-CC4-1 | 0.05 | 4.08 | N.A | N.A |
| NCC-CC4-2 | 0.03 | 11.53 | N.A | N.A |
| NCC-CC4-3 (NCC-CC5) | 0.06 | 4.92 | 95.10 | N.A |
| NCC-CC6-1 | 0.01 | 0.02 | 0.06 | 3.76 |
| NCC-CC6-2 | 10.98 | 35.67 | N.A | N.A |
| NCC-BD1 | 7.66 | 58.00 | N.A | N.A |
| NCC-BD2 | N.A | N.A | N.A | N.A |
| NCC-BD3 | N.A | N.A | N.A | N.A |
| NCC-BD4-1 | 0.04 | 0.06 | 0.09 | 2.93 |
| NCC-BD4-2 | 0.06 | 0.07 | 0.19 | 5.37 |
Preclinical studies have found very little evidence regarding the combined effects of prospective anticancer combination therapies, including gemcitabine. Therefore, we continue to examine the combined effects of the utility of the Bliss method and combination index to assess the prognosis of BTC. Moreover, we are going to release some of our resources and data in the near future. We believe that our materials and data will not only aid in conducting appropriate preclinical studies but also accelerate basic research of BTC.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Cho YB, Peraldo-Neia C S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
| 1. | Nakamura H, Arai Y, Totoki Y, Shirota T, Elzawahry A, Kato M, Hama N, Hosoda F, Urushidate T, Ohashi S. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47:1003-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 651] [Cited by in RCA: 941] [Article Influence: 94.1] [Reference Citation Analysis (0)] |
| 2. | Ojima H, Yoshikawa D, Ino Y, Shimizu H, Miyamoto M, Kokubu A, Hiraoka N, Morofuji N, Kondo T, Onaya H. Establishment of six new human biliary tract carcinoma cell lines and identification of MAGEH1 as a candidate biomarker for predicting the efficacy of gemcitabine treatment. Cancer Sci. 2010;101:882-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Morofuji N, Ojima H, Onaya H, Okusaka T, Shimada K, Sakamoto Y, Esaki M, Nara S, Kosuge T, Asahina D. Macrophage-capping protein as a tissue biomarker for prediction of response to gemcitabine treatment and prognosis in cholangiocarcinoma. J Proteomics. 2012;75:1577-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Takahashi H, Ojima H, Shimizu H, Furuse J, Furukawa H, Shibata T. Axitinib (AG-013736), an oral specific VEGFR TKI, shows potential therapeutic utility against cholangiocarcinoma. Jpn J Clin Oncol. 2014;44:570-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Shirota T, Ojima H, Hiraoka N, Shimada K, Rokutan H, Arai Y, Kanai Y, Miyagawa S, Shibata T. Heat Shock Protein 90 Is a Potential Therapeutic Target in Cholangiocarcinoma. Mol Cancer Ther. 2015;14:1985-1993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |