Published online Oct 28, 2016. doi: 10.3748/wjg.v22.i40.9012
Peer-review started: June 3, 2016
First decision: July 12, 2016
Revised: July 20, 2016
Accepted: August 10, 2016
Article in press: August 10, 2016
Published online: October 28, 2016
Processing time: 146 Days and 0.2 Hours
To critically assess the available literature regarding the efficacy of thioguanine treatment in inflammatory bowel disease (IBD) patients, irrespective of the (hepato-) toxicity profile.
A systematic literature search of the MEDLINE database using PubMed was performed using the keywords “thioguanine”, “6-TG”, “thioguanine”, “inflammatory bowel disease”, “IBD”, “Crohn’s disease”, “Ulcerative colitis” and “effectiveness” in order to identify relevant articles published in English starting from 2000. Reference lists of the included articles were cross-checked for missing articles. Reviewed manuscripts concerning the effectiveness of thioguanine treatment in IBD were reviewed by the authors and the data were extracted. Data were subsequently analyzed with descriptive statistics. Due to the lack of standardized outcomes, a formal meta-analysis was not performed.
A total of 11 applicable studies were found that involved the effectiveness of thioguanine therapy in IBD. Eight studies were conducted in a prospective manner, in the remaining three studies, data was collected retrospectively. In total, 353 IBD-patients (225 patients with Crohn’s disease, 119 with ulcerative colitis and nine with unclassified IBD) with prior azathioprine/mercaptopurine resistance and/or intolerance (n = 321) or de novo thioguanine administration (n = 32) were included for analysis, of which 228 (65%) had clinical improvement on thioguanine therapy, based on standard IBD questionnaires, biochemical parameters or global physician assessments. Short-term results were based on 268 treatment years (median follow-up 9 mo, range 3-22 mo) with a median daily dose of 20 mg (range 10-80 mg). Discontinuation, mostly due to adverse events, was reported in 72 patients (20%).
The efficacy of thioguanine therapy in IBD patients intolerant to conventional thiopurine therapy is observed in 65%, with short term adverse events in 20% of patients.
Core tip: Whereas conventional thiopurines are globally accepted as second-line treatment of inflammatory bowel disease (IBD) patients, almost half of these patients discontinues this treatment due to ineffectiveness or intolerance. In this systematic review, the efficacy of thioguanine treatment, a thiopurine with a less extensive and complex metabolism, is systematically assessed to determine if this drug is an alternative in the treatment of IBD patients intolerant or ineffective to azathioprine and/or mercaptopurine. We showed that up to 65% of patients benefit of a switch to thioguanine, thus preserving these patients from potentially more harmful and expensive treatment with biologicals.
- Citation: Meijer B, Mulder CJ, Peters GJ, van Bodegraven AA, de Boer NK. Efficacy of thioguanine treatment in inflammatory bowel disease: A systematic review. World J Gastroenterol 2016; 22(40): 9012-9021
- URL: https://www.wjgnet.com/1007-9327/full/v22/i40/9012.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i40.9012
Inflammatory bowel disease (IBD) encompasses both Crohn’s disease (CD) and ulcerative colitis (UC) and forms a group of diseases characterized by idiopathic chronic inflammation of the gastrointestinal tract. It has worldwide a rising incidence[1]. IBD is characterized by recurrent periods of remission and relapse of disease and treatment of IBD is mainly aimed at induction and maintenance of remission[2,3]. Based on current step-up treatment guidelines (systemic) corticosteroids are the therapy of choice for inducing remission[2,3]. Thiopurines, such as azathioprine (AZA) or mercaptopurine (MP) may be added to corticosteroid therapy for maintaining remission and medication may be initiated during induction phase[4-6]. However, the use of thiopurines is limited, largely due to an extensive spectrum of adverse events witnessed in up to almost half of patients, especially within the first twelve months of treatment. Toxicity includes myelotoxicity, hepatotoxicity, pancreatitis and gastrointestinal (GI-) complaints[7,8].
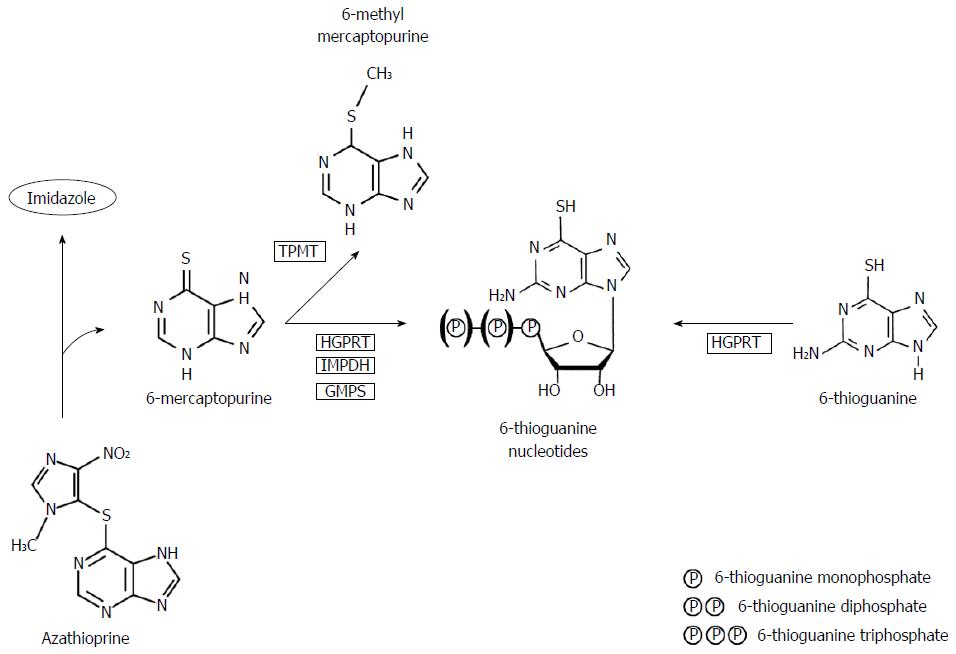
Thiopurines were first described in the 1950s by Gertrude Elion and George Hitchings and comprised three chemical structures: 6-thioguanine (6-TG), MP and AZA[9]. AZA and MP are frequently being used as treatment for IBD, while TG is currently only used as experimental or rescue therapy. Metabolism of conventional thiopurines is complicated, leading to formation of several, toxic and non-toxic metabolites, whereas the metabolism of TG is less complicated and more directly leading towards the intended pharmacologically active products (Figure 1)[10-12]. Effects of thiopurines may be characterized by two groups of metabolites; methylated thiopurines [e.g., 6-methylmercaptopurine (6-MMP)] and 6-thioguanine nucleotides (6-TGN). At relatively low dosages, as has been advocated in treatment of IBD, the anti-inflammatory effect of thiopurines is mainly mediated via inhibition of the small GTPase Rac1, leading to apoptosis of activated T-lymphocytes, whereas high dosages, as usual in oncological treatment, are associated with inhibition of DNA synthesis[13,14].
Based on these findings, it has been hypothesized that prescribing TG therapy instead of AZA/MP reduces generation of potentially toxic metabolites, such as the methylated metabolites, whilst it is primarily converted into the therapeutically aimed metabolite 6-TGN by bypassing several rate-limiting metabolic steps. The key reason for not introducing TG in the standard therapeutic armentarium of IBD appears to be the reported hepatotoxicity [i.e., nodular regenerative hyperplasia (NRH) and sinusoidal obstruction syndrome] which has been described to be highly prevalent, especially with higher dosages of TG (median 40 mg/d)[15]. Interestingly, these findings were not corroborated in subsequent studies in which lower dosages of TG were used (20 mg/d), justifying additional research regarding relatively efficacy of low-dose TG therapy in IBD patients[16-20]. These data are depicted in Table 1. Other adverse events probably associated with TG use, as described in previous literature, are summarized in Table 2.
| Dosage of thioguanine | 6-TGN level | Observed NRH | Ref. |
| About 20 mg per day (18-24 mg) | 278 (68-492) | 0% (0/12) | [20] |
| 20 mg per day | 564 ± 278 | 0% (0/28) | [18] |
| 20 mg per day | 802 (106-1092) | 0% (0/13) | [47] |
| About 21 mg per day (0.3 mg/kg) | 464 (65-1199) | 6% (7/111) | [48] |
| 40 mg per day | 807 (105-2545) | 0% (0/11) | [16] |
| About 40 mg per day (estimated) | 1230 (530-2310) | 62% (16/26) | [15] |
| 40-80 mg per day | Unknown | 36% (16/45) | [45] |
| Adverse event | Prevalence | Ref. |
| GI complaints | 1%-17% | [16,17,19,20,37,38] |
| Myelosuppression1 | 1%-15% | [17,20,36,38] |
| General malaise | 4%-22% | [17,38] |
| Allergic reaction | 1%-6% | [17,33] |
| Other AE (e.g., myalgia, alopecia) | 1%-38% | [16,17,19,20,33,36-38] |
Two years ago, there were approximately 1500 TG users in The Netherlands, with no serious toxicity being reported[21]. Since TG has recently been registered as certified treatment for IBD in The Netherlands, the question arises whether TG should be reconsidered as IBD treatment worldwide. The aim of this systematic review was to critically assess the available literature solely regarding the efficacy of TG treatment in IBD patients, irrespective of the alleged (hepato-)toxicity profile.
This study was executed using the PRISMA guidelines[22]. We conducted a systematic literature search in the MEDLINE database using PubMed. We applied the following search strategy: [“Thioguanine”(Mesh) OR 6-TG (tiab) OR thioguanine (tiab) OR tioguanine (tiab)] AND [“Inflammatory Bowel Diseases”(Mesh) OR IBD (tiab) OR Crohn (tiab) OR Colitis (tiab)] AND (efficacy OR effectivity OR effectiveness).
All studies were screened based on title and abstract. Full-text screening was performed in relevant studies by the same authors (BM and NdB). The following inclusion criteria were met: patients diagnosed with IBD, TG therapy, efficacy as outcome, studies available in full-text in English or Dutch. Exclusion criteria were: in vitro studies, efficacy not identified as outcome, patients receiving TG therapy for other reasons than IBD, article not available in English or Dutch. Furthermore, all references of the included original papers were cross-checked to complete the search. All studies published from 2000 till 2016 were included in the systematic review. All studies with original study populations were included for analysis. Finally, authors of the included manuscripts were contacted in case of missing or unclear data or to identify additional studies.
If articles were eligible, we collected the following data from the original papers: study design, number of patients, patient characteristics, disease characteristics [i.e., CD, UC or IBD unclassified (IBDu)], reason for initiation of TG, co-medication with corticosteroids, TG dose, duration of follow-up, efficacy of therapy, biochemical parameters [i.e., C-reactive protein (CRP) and/or fecal calprotectin] and thiopurine drug metabolites (6-TGN and/or 6-MMP) during AZA/MP and TG treatment. Effectiveness of therapy was determined using endoscopic/clinical scoring scales [i.e., Harvey-Bradshaw Index (HBI)[23], CD Activity Index (CDAI)[24], Colitis Activity Index (CAI)[25] or Simple Clinical Colitis Activity Index (SCCAI)[26]], as used in the different articles. Concentrations of 6-TGN were described using the method of Lennard et al[27] When the initial measurement was performed using the method described by Dervieux et al[28], this value was transposed into a calculated “Lennard value” as described by Shipkova et al[29].
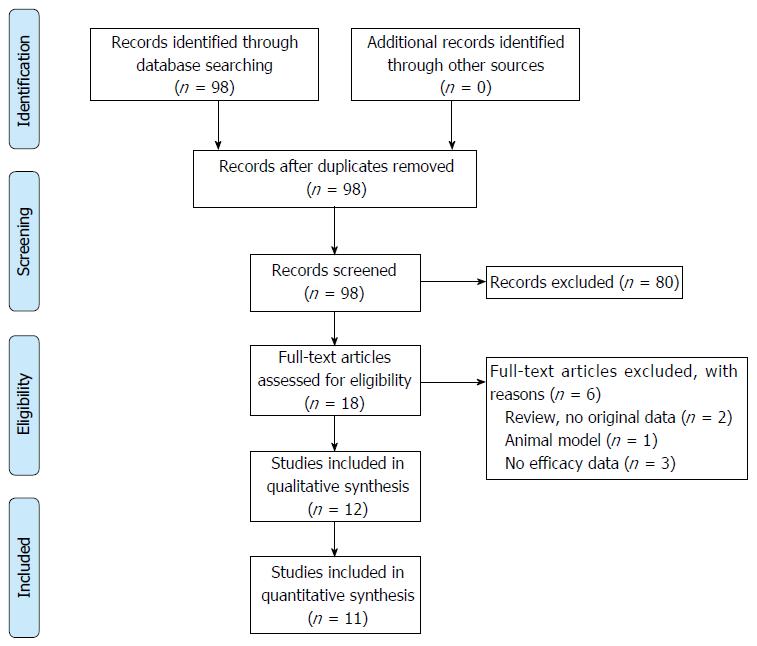
The search strategy resulted in 98 papers. Most articles were excluded since these articles described measuring 6-TGN in patients treated with AZA or MP, instead of TG therapy. Thirteen were selected for full-text screening. One additional article was excluded because efficacy was not described.
Finally, twelve relevant articles were included (see Figure 2). Of these twelve articles, eleven studies comprised different study populations. One of the included papers is the extended follow-up period of another included paper, and was therefore not visualized in our primary overview (Table 3).
| Author | Ref. | Year | Score1 | Number of patients | Number of IBD (CD/UC/IBDu) | Dose (med) | Follow-up (M) | Effective n (%) | Non-effective n (%) | Discontinuation | 6-TGN (med) |
| n (%) | |||||||||||
| Dubinsky | 17 | 2001 | Very low | 10 | 10/0/0 | 40 | 4 | 7 (70) | 1 (10) | 2 (20) | 15482 |
| Cheung | 18 | 2003 | Very low | 15 | 13/1/1 | 40 | 3 | 12 (79) | 1 (7) | 2 (14) | N/A |
| Herrlinger | 19 | 2003 | Low | 37 | 37/0/0 | 40 | 6 | 21 (57) | 7 (19) | 9 (24) | N/A |
| Bonaz | 21 | 2003 | Very low | 49 | 49/0/0 | 20 | 12 | 38 (78)3 | 6 (12) | 5 (10) | 6484 |
| Dubinsky | 22 | 2003 | Very low | 21 | 14/7/0 | 20 | 9 | 14 (67) | 3 (14) | 4 (19) | 13651 |
| Teml | 23 | 2005 | Low | 20 | 0/14/6 | 20 | 6 | 11 (55) | 3 (15) | 6 (30) | 8164 |
| Qasim | 24 | 2007 | Very low | 40 | 28/10/2 | 40 | 6 | 19 (48) | 8 (20) | 13 (32) | N/A |
| Ansari | 12 | 2008 | Low | 30 | 30/0/0 | 40 | 6 | 18 (60) | 5 (17) | 7 (23) | 8074 |
| Almer | 25 | 2009 | Low | 23 | 23/0/0 | 40 | 9 | 5 (22) | 5 (22) | 13 (56) | 11552 |
| Asseldonk | 16 | 2011 | Low | 46 | 0/46/0 | 20 | 22 | 37 (80)3 | 3 (7) | 6 (13) | 2784 |
| Pavlidis | 15 | 2014 | Moderate | 62 | 21/41/0 | 20 | 6 | 46 (78) | 11 (14) | 5 (8) | 8114 |
In the first study regarding TG-use[30] ten CD patients with therapeutic failure (i.e., CDAI-scores above 150 and/or steroid-dependent disease) to AZA/MP therapy, combined with a preferential metabolite profile [defined as 6-TGN levels below 235 and 6-MMP levels above 6000 pmol/8 x 108 red blood cells (RBC)] were included. Nine patients were adults in whom TG was initiated at a dose of 40 mg/d, the pediatric patient (9 years old) was started on 20 mg/d. After 16 wk follow-up, eight patients were still using TG, in whom seven patients had a good clinical response, defined as a reduction in CDAI of at least 70 points or steroid reduction of at least 50%. 6-TGN levels in these patients were median 1548 pmol/8 x 108 RBC (range 603-2073), with only one patient with a 6-TGN level below 1350. 6-MMP metabolites were undetectable in all patients. Biochemical parameters were not extensively reported. The two patients who discontinued TG treatment before week sixteen were excluded due to protocol violation, but were not reported to develop adverse events or to have an increase in IBD activity.
The next study[31] included fifteen IBD patients (13 CD/1 UC/1 IBDu) with either intolerance (n = 12) or inefficacy (n = 1) on AZA/MP therapy, as well as two thiopurine-naïve patients in which TG was started to “induce a quick therapeutic response”. Fourteen patients were adults (range 23-65 years old) and one adolescent (17 years old). All patients were started on 40 mg TG. Based on global physician assessment (GPA), eleven patients (73%) had a good clinical response to TG therapy after a mean duration of only 3 wk. One additional patient had no decrease in CDAI but was able to successfully reduce prednisolone with > 50% and was classified as a partial response. There was a median follow-up of 16 wk (range 3-21 wk). Adverse events were described in four patients (27%) and were classified as mild. One patient had to discontinue TG treatment due to suspected pancreatitis (i.e., slowly rising lipase concentration) and in three patients dosage was successfully reduced as diarrhea (n = 2) or leukopenia (n = 1) developed.
In a German study from 2003[32], 37 patients (22 with prior AZA intolerance, 15 thiopurine naive) with CD received 40 mg of TG daily. Dose was increased to 80 mg/d after 12 wk in non-responders and the effect was evaluated after a follow-up of 24 wk. Nine patients (24%) discontinued therapy before week 24 due to intolerance (n = 6), inefficacy (n = 2) or violation of protocol (n = 1). Of the remaining 28 patients, there were 21 patients (57%) with a clinical response, defined as a decrease in CDAI of > 70 points. Thirteen of these patients were in complete remission, of which twelve patients achieved this quiescent phase within four weeks of therapy. Twenty out of 27 patients (74%) on corticosteroids at initiation of TG were able to decrease steroids dosage with a median of 67% of initial steroid dose. CRP concentration was measured at baseline and at last follow-up, but there was no difference between these time points. In a second follow-up paper, the effect of maintenance treatment (total follow-up of one year) was evaluated[33]. Sixteen patients with continued use after six months of therapy were evaluated of which twelve were in remission with TG (i.e., CDAI < 150) and four showed clinical response (defined as ΔCDAI > 70). Shortly after six months, two additional patients came into complete remission and patients in remission after six months maintained in remission after 12 mo of treatment. One patient with initial clinical response to TG relapsed and was switched to methotrexate therapy.
In another study from 2003[34] 49 patients with CD either intolerant for or refractory to AZA/MP therapy were included. All patients were adults and were started on 20 mg TG daily. Five patients (10%) out of 39 patients with prior intolerance to AZA/MP had to discontinue TG treatment due to (mild) adverse events within three weeks of therapy: nausea (n = 1), increase of hepatic enzymes (n = 2), vertigo (n = 1) and paresthesia (n = 1). After a median period of seven months, complete remission (defined as HBI below 3 and cessation of corticosteroids or infliximab) was achieved in 21 patients (43%). It was described that six patients (12%) relapsed on TG therapy. The remaining seventeen patients were not more extensively described in this study.
In a second study by Dubinsky et al[35], 21 patients were included with either CD or UC (14:7) who experienced a hypersensitivity reaction on conventional thiopurine therapy. All patients were adults. The dose of TG was not standardized and varied between 10 and 40 mg daily (median 20 mg/d). Four patients (19%) experienced a (mild) hypersensitivity reaction on TG (two patients with gastrointestinal symptoms and two patients with flu-like illness). Of the remaining seventeen patients, fourteen (67%) improved on TG therapy after a median period of 9 mo, based on GPA. Two patients remained in remission and one patient had worsening of disease. 6-TGN concentrations were obtained in 14 of 17 patients and were all above 1100 pmol/8 × 108 RBC, irrespective of clinical response and not correlating with disease activity.
An Austrian research group[36] described fourteen UC patients and six IBDu patients with prior intolerance (n = 8) or inefficacy to previous AZA/MP treatment were reported. After a follow-up of 26 wk, eleven patients (55%) showed a therapeutic response (five with complete remission), defined as a CAI of 4 or lower. Three patients were classified as non-responders (15%), six patients discontinued treatment due to AE (n = 2) or non-compliance (n = 4, based on 6-TGN levels of below 250 pmol/8 × 108 RBC). Median 6-TGN level during therapy was 816 (range 279-2300), not correlating with response to therapy. Concentrations of CRP at follow-up did not differ from baseline concentrations.
An Irish population was included in another study[37] of 40 patients (28 CD, 10 UC and 2 IBDu) with prior inefficacy on AZA/MP in 21 patients or intolerance in 8 patients while de novo TG therapy was given in eleven patients. All patients were adults and started on 40 mg daily. After six months of therapy, TG had to be discontinued in thirteen patients (32%) due to AE, of which eight patients had hepatotoxicity (including thrombocytopenia, liver test abnormalities and splenomegaly). Nineteen patients (48%) had clinical benefit (i.e., modified HBI or modified UC disease activity index below 4) of TG therapy and eight patients (20%) displayed no therapeutic response. Eleven patients were able to continue therapy over 1 year time period with therapeutic effect (complete remission in 10 patients). Furthermore, concentrations of CRP decreased during TG treatment when compared to baseline levels (P = 0.001).
Ansari et al[16] studied 30 CD patients with a median age of 34 years (range 12-57) treated with a median dose of 40 mg daily (range 20-60). All patients were either nonresponsive (n = 16) or intolerant (n = 14) to prior AZA treatment. After 6 mo there was a clinical response (i.e., HBI < 5, in combination with successful withdrawal of steroids or infliximab) in eighteen patients (60%) and seven patients (23%) withdrew TG treatment due to AE. After six months another six patients developed AE leading to withdrawal of therapy. Eleven patients (37%) were able to continue therapy for a median period of 44 mo, leading to long-lasting remission. Five patients (17%) had no benefit from TG therapy. Median 6-TGN level was 807 pmol/8 × 108 RBC, there was no correlation between 6-TGN concentrations and clinical response.
In a Swedish study from 2009[38] 23 adult CD patients with prior thiopurine intolerance (n = 18) or resistance (n = 5) were treated with 40 mg (range 20-60 mg) TG once daily. After a median follow-up of 8 mo, thirteen patients (56%) had to discontinue treatment due to AE (n = 10) or unspecified safety concerns. Five patients (22%) had clinical response (defined as HBI < 5) on TG therapy, whilst five patients were non-responders. Median 6-TGN level in responding patients was 1155 (range 466-2488) pmol/8 × 108 RBC, however this result was not statistically different from non-responders [median 645 (range 551-1852), P = 0.73].
In a Dutch population[20] the sole focus was on UC patients and TG was introduced in a dose of approximately 0.3 mg/kg (median 20 mg/d, range 18-24) in 46 adult patients with either intolerance (n = 42) or refractoriness to AZA/MP. Within 6 mo, five patients had to discontinue treatment due to AE (n = 3) or were lost to follow-up. During follow-up, another three patients developed intolerance adding up to six patients (13%). Three patients experienced non-effectiveness on TG therapy and underwent colectomy. In the remaining 37 patients (80%), there was ongoing benefit and TG therapy was continued.
Finally, in a study of Pavlidis et al[19] performed in Australia and the United Kingdom, 62 adult patients (21 CD/41 UC) started on split-dose TG therapy of 20 mg once, twice or thrice daily after intolerance to conventional thiopurine therapy. After six months, 46 patients (78%) had a clinical response to TG therapy, defined as decrease in clinical activity scores (HBI ≤ 3 or SCCAI ≤ 2) and/or steroid use. Eleven patients (14%) did not benefit from treatment and had to undergo surgery. The remaining five patients discontinued treatment due to AE (n = 2) or were lost to follow-up. The median 6-TGN level was 811 pmol/8 × 108 RBC (range 340-2678) which did not correlate with disease activity.
In summary, a total number of 353 (CD: 225/UC: 119/IBDu: 9) patients were treated with TG with a starting dose of 20 to 40 mg daily. The dosing was per individual adjusted to 10-80 mg/d, based on the development of adverse events or efficacy. Based on the median follow-up in the different studies, TG was administered for an estimated 268 treatment years. In 228 patients (65%), there was a benefit of TG therapy, defined as decrease in clinical disease symptom scales or the opportunity to cease or clinically significantly decrease corticosteroids without relapse of disease. No benefit of therapy was reported in 15% of patients, whereas 20% of the patients had to discontinue TG, mostly due to AE, comprising mainly gastrointestinal complaints, hypersensitivity reactions and elevated liver enzymes. In a subgroup analysis, 52% of CD patients and 62% of UC patients benefitted from TG therapy, whereas 11% of CD patients and 13% of UC patients had no benefit of therapy (Table 4).
| Total number of patients | CD | UC | IBDu | Daily dose (mg)1 | Treatment years2 | Benefit3 | No benefit | Discontinuation | |
| 353 | 225 (64) | 119 (34) | 9 (2) | 20 | 268 | 228 (65) | 53 (15) | 72 (20) | |
| [10-80] | |||||||||
| Discontinuation | Response unknown4 | ||||||||
| Crohn’s disease (n = 225) | 141 | 118 (52) | 25 (11) | 40 (18) | 42 (19) | ||||
| Ulcerative colitis (n = 119) | 122 | 73 (62) | 16 (13) | 11 (9) | 19 (16) | ||||
In this study we systematically reviewed literature regarding the efficacy of TG treatment in IBD patients.
In 65% (range 22%-80%) of patients with active IBD treated with TG, mainly in patients failing prior conventional thiopurine therapy, clinical improvement was achieved. This was in line with recent reviews regarding efficacy of AZA or MP treatment in IBD: for maintenance therapy, efficacy of conventional thiopurine therapy was 73% and 50%, respectively. Induction therapy was effective in 30% and 51%, respectively[4,6,39,40].
Interestingly, most of the included patients experienced intolerance or inadequate response to previous conventional thiopurine therapy (i.e., AZA/MP). Therefore the result of 65% is primarily based on patients with prior thiopurine exposure.
Eight studies (73%) were conducted in a prospective way, however no randomized trials have been performed to date. The study of Almer et al[38] is a relative negative outlier with only 22% response rate. This might be due to a small sample size (n = 23) in combination with a high number of discontinuation (n = 13). Three patients had to discontinue due to unspecified “safety reasons” and ten patients had adverse events leading to discontinuation. Five of them discontinued due to pain or gastrointestinal intolerance and two had mild hepatotoxicity with increasing bilirubin concentration or aminotransferase activity. On the contrary, two positive outliers were the studies of Bonaz et al[34] and van Asseldonk et al[20] with response rates of 78% and 80%, respectively. Interestingly, in these studies patients were started on 20 mg/d instead of 40 mg/d. This lower dosage might be the reason for better tolerability and could contribute to longer usage and subsequent higher efficacy.
The aim of this paper was to assess the effectiveness of TG treatment by a systematic review of available literature. Safety issues have extensively been reviewed (and nuanced) elsewhere (Table 1)[18,41-45]. However, since the majority of the included patients experienced adverse events on more conventional thiopurine derivatives, we compared the number of patients discontinuing treatment due to adverse events. Overall, 72 of 353 patients (20%) had to discontinue TG treatment, mainly due to adverse events. Interestingly, there seemed to be no increased risk of developing clinically overt non-cirrhotic portal hypertension due to NRH as compared to the study by Dubinsky et al[15] Other reasons for discontinuation were (unspecified) “safety reasons” or violation of applicable study protocol.
Concentrations of 6-TGN during TG treatment in none of the included studies (if available) showed a correlation with efficacy; its value in the management of TG therapy (therapeutic drug monitoring) can therefore not be extracted from the current series and, thus, warrant further analysis and study. However, patients with benefit of TG therapy showed median 6-TGN levels 1155, 1365 and 1548 pmol/8 × 108 RBC, respectively, in those studies in which this benefitting subgroup specifically was analyzed[15,35,38]. Based on these results, one may hypothesize that the therapeutic range of 6-TGN levels as proposed for conventional AZA/MP treatment (i.e., > 230 pmol/8 × 108 RBC) is not applicable in patients treated with TG[46].
Several remarks have to be made about study design and patient population of the various included studies. All included studies are observational, open-label studies without control groups. A major part of discussion is the risk of bias in these kind of studies, especially publication bias. This type of bias is unavoidable in studies which are not previously registered in a trial registry, so the results in this review have to be interpret with this possible risk of bias taken into account. Furthermore, even though a larger part of the studies had a prospective design, no randomized trials are performed, yet, probably leading to confounding bias. Additionally, analyses in this paper were based on small patient groups (range 10-62) and effectiveness endpoints differed between the included studies, thwarting comparisons and robust conclusions.
Taken together, we critically reviewed the literature regarding effectiveness of TG treatment in IBD patients. Several small prospective trials showed encouraging results regarding therapeutic effect and tolerability of TG in a population of IBD patients with reported intolerance or refractoriness to conventional thiopurine therapy with AZA or MP. These findings warrant randomized trials in IBD patients.
Conventional thiopurines play an important role in maintenance therapy of patients with inflammatory bowel disease (IBD). In a large proportion of IBD patients conventional thiopurine therapy fails, mainly due to adverse events. Thioguanine, another thiopurine derivative, has a less complicated metabolism and generates less potentially toxic metabolites and might serve as a rescue thiopurine.
Thiopurines were first described by Gertrude Elion and George Hitchings in the 1950s. The conventional thiopurines (azathioprine and mercaptopurine) are frequently used in IBD treatment. The rediscovery of thioguanine led to the application of this drug as rescue treatment in IBD. In 2003, Dubinsky et al showed that over half of the patients treated with this drug (in high dose) developed nodular regenerative hyperplasia of the liver, leading to the immediate stop of thioguanine in IBD treatment. Over the years, these findings were not reproduced by other studies researching this topic.
Thioguanine has been successfully used in the treatment of IBD in various studies. Retrieved manuscripts (case series and observational cohort studies) concerning the effectiveness of thioguanine treatment in IBD were reviewed by the authors and the data were extracted.
This review suggests that thioguanine is an effective treatment option in IBD patients who experienced intolerance or ineffectiveness to conventional thiopurine treatment. This review may serve as fundament for prospective, randomized trials.
Thioguanine is one of the three thiopurine derivatives used in the treatment of inflammatory bowel disease. Over the past decade, this drug has been ‘rediscovered’, but its use was limited due to alleged hepatotoxicity. Since March 2016, thioguanine has been registered as certified IBD treatment in The Netherlands.
This is a well-conceived and executed study in which the authors systematically reviewed literature regarding the efficacy of TG treatment in IBD patients. The study is an important one, was performed in an exemplary manner and is very well presented.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Netherlands
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lakatos PL, Perse M, Tarnawski AS S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
| 1. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3527] [Article Influence: 271.3] [Reference Citation Analysis (5)] |
| 2. | Dignass A, Van Assche G, Lindsay JO, Lémann M, Söderholm J, Colombel JF, Danese S, D’Hoore A, Gassull M, Gomollón F. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Current management. J Crohns Colitis. 2010;4:28-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1118] [Cited by in RCA: 1032] [Article Influence: 68.8] [Reference Citation Analysis (1)] |
| 3. | Dignass A, Lindsay JO, Sturm A, Windsor A, Colombel JF, Allez M, D’Haens G, D’Hoore A, Mantzaris G, Novacek G. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012;6:991-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 702] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 4. | Chande N, Patton PH, Tsoulis DJ, Thomas BS, MacDonald JK. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2015;CD000067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 5. | Timmer A, McDonald JW, Tsoulis DJ, Macdonald JK. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012;CD000478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Chande N, Tsoulis DJ, MacDonald JK. Azathioprine or 6-mercaptopurine for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2013;CD000545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Gearry RB, Barclay ML, Burt MJ, Collett JA, Chapman BA. Thiopurine drug adverse effects in a population of New Zealand patients with inflammatory bowel disease. Pharmacoepidemiol Drug Saf. 2004;13:563-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Jharap B, Seinen ML, de Boer NK, van Ginkel JR, Linskens RK, Kneppelhout JC, Mulder CJ, van Bodegraven AA. Thiopurine therapy in inflammatory bowel disease patients: analyses of two 8-year intercept cohorts. Inflamm Bowel Dis. 2010;16:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 160] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 10. | van Asseldonk DP, Sanderson J, de Boer NK, Sparrow MP, Lémann M, Ansari A, Almer SH, Florin TH, Gearry RB, Mulder CJ. Difficulties and possibilities with thiopurine therapy in inflammatory bowel disease--proceedings of the first Thiopurine Task Force meeting. Dig Liver Dis. 2011;43:270-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Tiede I, Fritz G, Strand S, Poppe D, Dvorsky R, Strand D, Lehr HA, Wirtz S, Becker C, Atreya R. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest. 2003;111:1133-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 573] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 12. | Ben-Horin S, Goldstein I, Fudim E, Picard O, Yerushalmi Z, Barshack I, Bank I, Goldschmid Y, Meir SB, Mayer L. Early preservation of effector functions followed by eventual T cell memory depletion: a model for the delayed onset of the effect of thiopurines. Gut. 2009;58:396-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Quéméneur L, Gerland LM, Flacher M, Ffrench M, Revillard JP, Genestier L. Differential control of cell cycle, proliferation, and survival of primary T lymphocytes by purine and pyrimidine nucleotides. J Immunol. 2003;170:4986-4995. [PubMed] |
| 14. | de Boer NK, van Bodegraven AA, Jharap B, de Graaf P, Mulder CJ. Drug Insight: pharmacology and toxicity of thiopurine therapy in patients with IBD. Nat Clin Pract Gastroenterol Hepatol. 2007;4:686-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Dubinsky MC, Vasiliauskas EA, Singh H, Abreu MT, Papadakis KA, Tran T, Martin P, Vierling JM, Geller SA, Targan SR. 6-thioguanine can cause serious liver injury in inflammatory bowel disease patients. Gastroenterology. 2003;125:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 191] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 16. | Ansari A, Elliott T, Fong F, Arenas-Hernandez M, Rottenberg G, Portmann B, Lucas S, Marinaki A, Sanderson J. Further experience with the use of 6-thioguanine in patients with Crohn’s disease. Inflamm Bowel Dis. 2008;14:1399-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | de Boer NK, Derijks LJ, Gilissen LP, Hommes DW, Engels LG, de-Boer SY, den Hartog G, Hooymans PM, Mäkelburg AB, Westerveld BD. On tolerability and safety of a maintenance treatment with 6-thioguanine in azathioprine or 6-mercaptopurine intolerant IBD patients. World J Gastroenterol. 2005;11:5540-5544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | de Boer NK, Zondervan PE, Gilissen LP, den Hartog G, Westerveld BD, Derijks LJ, Bloemena E, Engels LG, van Bodegraven AA, Mulder CJ. Absence of nodular regenerative hyperplasia after low-dose 6-thioguanine maintenance therapy in inflammatory bowel disease patients. Dig Liver Dis. 2008;40:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Pavlidis P, Ansari A, Duley J, Oancea I, Florin T. Splitting a therapeutic dose of thioguanine may avoid liver toxicity and be an efficacious treatment for severe inflammatory bowel disease: a 2-center observational cohort study. Inflamm Bowel Dis. 2014;20:2239-2246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | van Asseldonk DP, Jharap B, Kuik DJ, de Boer NK, Westerveld BD, Russel MG, Kubben FJ, van Bodegraven AA, Mulder CJ. Prolonged thioguanine therapy is well tolerated and safe in the treatment of ulcerative colitis. Dig Liver Dis. 2011;43:110-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Mulder CJ, van Asseldonk DP, de Boer NK. Drug rediscovery to prevent off-label prescription reduces health care costs: the case of tioguanine in the Netherlands. J Gastrointestin Liver Dis. 2014;23:123-125. [PubMed] |
| 22. | Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47184] [Article Influence: 2949.0] [Reference Citation Analysis (0)] |
| 23. | Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514. [PubMed] |
| 24. | Best WR, Becktel JM, Singleton JW, Kern F. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439-444. [PubMed] |
| 25. | Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989;298:82-86. [PubMed] |
| 26. | Jowett SL, Seal CJ, Phillips E, Gregory W, Barton JR, Welfare MR. Defining relapse of ulcerative colitis using a symptom-based activity index. Scand J Gastroenterol. 2003;38:164-171. [PubMed] |
| 27. | Lennard L, Singleton HJ. High-performance liquid chromatographic assay of the methyl and nucleotide metabolites of 6-mercaptopurine: quantitation of red blood cell 6-thioguanine nucleotide, 6-thioinosinic acid and 6-methylmercaptopurine metabolites in a single sample. J Chromatogr. 1992;583:83-90. [PubMed] |
| 28. | Dervieux T, Chu Y, Su Y, Pui CH, Evans WE, Relling MV. HPLC determination of thiopurine nucleosides and nucleotides in vivo in lymphoblasts following mercaptopurine therapy. Clin Chem. 2002;48:61-68. [PubMed] |
| 29. | Shipkova M, Armstrong VW, Wieland E, Oellerich M. Differences in nucleotide hydrolysis contribute to the differences between erythrocyte 6-thioguanine nucleotide concentrations determined by two widely used methods. Clin Chem. 2003;49:260-268. [PubMed] |
| 30. | Dubinsky MC, Hassard PV, Seidman EG, Kam LY, Abreu MT, Targan SR, Vasiliauskas EA. An open-label pilot study using thioguanine as a therapeutic alternative in Crohn’s disease patients resistant to 6-mercaptopurine therapy. Inflamm Bowel Dis. 2001;7:181-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Cheung TK, Florin TH. 6-thioguanine: a new old drug to procure remission in inflammatory bowel disease. Intern Med J. 2003;33:44-46. [PubMed] |
| 32. | Herrlinger KR, Kreisel W, Schwab M, Schoelmerich J, Fleig WE, Ruhl A, Reinshagen M, Deibert P, Fellermann K, Greinwald R. 6-thioguanine--efficacy and safety in chronic active Crohn’s disease. Aliment Pharmacol Ther. 2003;17:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Herrlinger KR, Deibert P, Schwab M, Kreisel W, Fischer C, Fellermann K, Stange EF. Remission maintenance by tioguanine in chronic active Crohn’s disease. Aliment Pharmacol Ther. 2003;17:1459-1464. [PubMed] |
| 34. | Bonaz B, Boitard J, Marteau P, Lémann M, Coffin B, Flourié B, Belaiche J, Cadiot G, Metman EH, Cortot A. Tioguanine in patients with Crohn’s disease intolerant or resistant to azathioprine/mercaptopurine. Aliment Pharmacol Ther. 2003;18:401-408. [PubMed] |
| 35. | Dubinsky MC, Feldman EJ, Abreu MT, Targan SR, Vasiliauskas EA. Thioguanine: a potential alternate thiopurine for IBD patients allergic to 6-mercaptopurine or azathioprine. Am J Gastroenterol. 2003;98:1058-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Teml A, Schwab M, Harrer M, Miehsler W, Schaeffeler E, Dejaco C, Mantl M, Schneider B, Vogelsang H, Reinisch W. A prospective, open-label trial of 6-thioguanine in patients with ulcerative or indeterminate colitis. Scand J Gastroenterol. 2005;40:1205-1213. [PubMed] |
| 37. | Qasim A, McDonald S, Sebastian S, McLoughlin R, Buckley M, O’Connor H, O’Morain C. Efficacy and safety of 6-thioguanine in the management of inflammatory bowel disease. Scand J Gastroenterol. 2007;42:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Almer SH, Hjortswang H, Hindorf U. 6-Thioguanine therapy in Crohn’s disease--observational data in Swedish patients. Dig Liver Dis. 2009;41:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D’Haens G, Diamond RH, Broussard DL. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2539] [Cited by in RCA: 2376] [Article Influence: 158.4] [Reference Citation Analysis (1)] |
| 40. | Panés J, López-Sanromán A, Bermejo F, García-Sánchez V, Esteve M, Torres Y, Domènech E, Piqueras M, Gomez-García M, Gutiérrez A. Early azathioprine therapy is no more effective than placebo for newly diagnosed Crohn’s disease. Gastroenterology. 2013;145:766-774.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 181] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 41. | Teml A, Schwab M, Hommes DW, Almer S, Lukas M, Feichtenschlager T, Florin T, Seiderer J, Petritsch W, Bokemeyer B. A systematic survey evaluating 6-thioguanine-related hepatotoxicity in patients with inflammatory bowel disease. Wien Klin Wochenschr. 2007;119:519-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | de Boer NK, Reinisch W, Teml A, van Bodegraven AA, Schwab M, Lukas M, Ochsenkühn T, Petritsch W, Knoflach P, Almer S. 6-Thioguanine treatment in inflammatory bowel disease: a critical appraisal by a European 6-TG working party. Digestion. 2006;73:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Kverka M, Rossmann P, Tlaskalova-Hogenova H, Klimesova K, Jharap B, de Boer NK, Vos RM, van Bodegraven AA, Lukas M, Mulder CJ. Safety and efficacy of the immunosuppressive agent 6-tioguanine in murine model of acute and chronic colitis. BMC Gastroenterol. 2011;11:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Derijks LJ, de Jong DJ, Gilissen LP, Engels LG, Hooymans PM, Jansen JB, Mulder CJ. 6-Thioguanine seems promising in azathioprine- or 6-mercaptopurine-intolerant inflammatory bowel disease patients: a short-term safety assessment. Eur J Gastroenterol Hepatol. 2003;15:63-67. [PubMed] |
| 45. | Seiderer J, Zech CJ, Reinisch W, Lukas M, Diebold J, Wrba F, Teml A, Chalupna P, Stritesky J, Schoenberg SO. A multicenter assessment of liver toxicity by MRI and biopsy in IBD patients on 6-thioguanine. J Hepatol. 2005;43:303-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 46. | Van Asseldonk DP, de Boer NK, Peters GJ, Veldkamp AI, Mulder CJ, Van Bodegraven AA. On therapeutic drug monitoring of thiopurines in inflammatory bowel disease; pharmacology, pharmacogenomics, drug intolerance and clinical relevance. Curr Drug Metab. 2009;10:981-997. [PubMed] |
| 47. | Gilissen LP, Derijks LJ, Driessen A, Bos LP, Hooymans PM, Stockbrügger RW, Engels LG. Toxicity of 6-thioguanine: no hepatotoxicity in a series of IBD patients treated with long-term, low dose 6-thioguanine. Some evidence for dose or metabolite level dependent effects? Dig Liver Dis. 2007;39:156-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | van Asseldonk DP, Jharap B, Verheij J, Den Hartog G, Westerveld BD, Becx MC, Russel MG, Engels LG, De Jong DJ, Witte BI. The prevalence of nodular regenerative hyperplasia in inflammatory bowel disease patients treated with thioguanine is not associated with clinically significant liver disease. Inflamm Bowel Dis. 2016;17:265-572. [RCA] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 49. | Seinen ML, van Asseldonk DP, Mulder CJ, de Boer NK. Dosing 6-thioguanine in inflammatory bowel disease: expert-based guidelines for daily practice. J Gastrointestin Liver Dis. 2010;19:291-294. [PubMed] |
| 50. | Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4091] [Cited by in RCA: 5706] [Article Influence: 407.6] [Reference Citation Analysis (0)] |
| 51. | Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, Alonso-Coello P, Djulbegovic B, Atkins D, Falck-Ytter Y. GRADE guidelines: 5. Rating the quality of evidence--publication bias. J Clin Epidemiol. 2011;64:1277-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1043] [Cited by in RCA: 1369] [Article Influence: 97.8] [Reference Citation Analysis (0)] |