Published online Oct 28, 2016. doi: 10.3748/wjg.v22.i40.8978
Peer-review started: June 29, 2016
First decision: July 29, 2016
Revised: August 14, 2016
Accepted: September 6, 2016
Article in press: September 6, 2016
Published online: October 28, 2016
Processing time: 119 Days and 21.9 Hours
To identify certain clinical factors other than the type of gastrectomy which affect the postoperative quality of life (QOL) of patients after gastrectomy.
The postgastrectomy syndrome assessment scale (PGSAS)-45 was designed to assess the severity of symptoms, the living status and the QOL of gastrectomized patients. It consists of 45 items, of which 22 are original items while 23 were retrieved from the SF-8 and Gastrointestinal Symptoms Rating Scale questionnaires with permission. A nationwide surveillance study to validate PGSAS was conducted and 2368 gastric cancer patients who underwent various types of gastrectomy at 52 medical institutions were enrolled. Of these, 1777 patients who underwent total gastrectomy (TG) reconstructed with Roux-Y (n = 393), distal gastrectomy (DG) reconstructed with Billroth-I (n = 909), or DG reconstructed with Roux-Y (n = 475) were evaluated in the current study. The influence of the type of gastrectomy and other clinical factors such as age, sex, duration after surgery, the symptom severity, the degree of weight loss, dietary intake, and the ability for working on the postoperative QOL (i.e., dissatisfaction for daily life subscale, physical component summary and mental component summary of the SF-8) were examined by multiple regression analysis (MRA). In addition, importance of various symptoms such as esophageal reflux, abdominal pain, meal-related distress, indigestion, diarrhea, constipation and dumping on the postoperative living status and QOL were also appraised by MRA.
The postoperative QOL were significantly deteriorated in patients who underwent TG compared to those after DG. However, the extent of gastrectomy was not an influential factor on patients’ QOL when adjusted by the MRA. Among various clinical factors, the symptom severity, ability for working, and necessity for additional meals were the most influential factors to the postoperative QOL. As for the individual symptoms, meal-related distress, dumping, abdominal pain, and esophageal reflux significantly affected the postoperative QOL in that order, while the influence of indigestion, diarrhea and constipation was insignificant.
Several clinical factors such as the symptom severity (especially in meal-related distress and dumping), ability for working and necessity for additional meals were the main factors which affected the patients’ well-being after gastrectomy.
Core tip: The extent of gastrectomy has been reported to substantially affect the postoperative quality of life (QOL). However, considerable differences in the QOL have been observed among patients who underwent the same type of gastrectomy, implicating that other clinical factors may have major influence over the postoperative QOL. In the present study, we first found that several clinical factors such as the symptom severity, ability for working and necessity for additional meals had significant impact on the postoperative QOL, while the influence of the extent of gastrectomy was unexpectedly small. These findings give us deeper understanding to manage the postgastrectomy syndrome appropriately.
- Citation: Nakada K, Takahashi M, Ikeda M, Kinami S, Yoshida M, Uenosono Y, Kawashima Y, Nakao S, Oshio A, Suzukamo Y, Terashima M, Kodera Y. Factors affecting the quality of life of patients after gastrectomy as assessed using the newly developed PGSAS-45 scale: A nationwide multi-institutional study. World J Gastroenterol 2016; 22(40): 8978-8990
- URL: https://www.wjgnet.com/1007-9327/full/v22/i40/8978.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i40.8978
Gastrectomy is widely used as an effective curative treatment modality in patients with gastric cancer. In Japan, the rate of diagnosis of gastric cancer at an early stage has been increasing[1], and with the consequent improvement of the treatment results, greater attention is being paid to the postoperative quality of life (QOL) of patients who underwent gastrectomy. Various clinical problems may occur after gastrectomy, including various abdominal and systemic symptoms, restriction of food intake, weight loss, decrease in physical activity, etc., which can interfere with the QOL of gastrectomized patients. Thus, there is need to prevent and manage these sequelae after gastrectomy, collectively labeled as postgastrectomy syndrome (PGS)[2-7].
Until date, the influence of the type of gastrectomy on the risk of development of PGS has been mainly investigated[8-23]. Multiple studies have reported a greater deterioration of the QOL after total gastrectomy (TG) than distal gastrectomy (DG)[8,10,13,18,19], and it is thought that the more extensive the resection of the stomach, the greater the severity of PGS[11]. On the other hand, function-preserving gastrectomy, in which the extent of gastrectomy is reduced, such as pylorus-preserving gastrectomy (PPG)[9,11,16,17,23] and proximal gastrectomy[20], is often used for treating early gastric cancer and has been reported to be useful to improve the QOL of patients after surgery. Thus, improvement in the gastrectomy procedures is recognized as one of the reliable means to reduce the risk of development of PGS. However, at least at present, it is difficult to eliminate PGS completely only by improving the gastrectomy procedures.
It has been observed that there are considerable individual differences in the postoperative QOL among patients who underwent the same type of gastrectomy; therefore, it appears likely that clinical factors other than the type of gastrectomy may also significantly influence the postoperative QOL, although not much information on this is available yet. Therefore, it seems important to identify undiscovered clinical factors which might affect the postoperative QOL of patients who underwent gastrectomy, besides the type of gastrectomy performed, in order to obtain a deeper understanding of PGS and to develop effective methods of prevention and management. Various symptoms are known to develop after gastrectomy, which cause much discomfort to the patients and place a burden on their lives. Although the degree of influence of these symptoms on the patients’ daily lives and QOL appears to differ depending on the nature of symptoms, the differences in the influences of each symptom on the patients’ daily lives and QOL have not yet been clarified. Therefore, this study was conducted to clarify these issues in the patients who underwent gastrectomy.
Fifty-two institutions participated in this study. Patient eligibility criteria were: (1) diagnosis of pathologically-confirmed stage IA or IB gastric cancer; (2) first-time gastrectomy status; (3) age ≥ 20 and ≤ 75 years; (4) no history of chemotherapy; (5) no recurrence or distant metastasis indicated; (6) gastrectomy conducted one or more years prior to enrollment date; (7) performance status ≤ 1 on the Eastern Cooperative Oncology Group scale; (8) full capacity to understand and respond to the questionnaire; (9) no history of other diseases or surgeries which might influence responses to the questionnaire; (10) no presence of organ failure or mental illness; and (11) written informed consent. Patients with dual malignancy or concomitant resection of other organs (with co-resection equivalent to cholecystectomy being the exception) were excluded.
The Postgastrectomy Syndrome Assessment Scale (PGSAS)-45[24] is a newly developed, multidimensional quality of life questionnaire (QLQ) based on the 8-item short-form health survey (SF-8)[25] and the Gastrointestinal Symptom Rating Scale (GSRS)[26]. The PGSAS-45 questionnaire consists of a total of 45 questions (Table 1), with eight items from the SF-8, 15 items from the GSRS, and 22 clinically-important items selected by the Japan Postgastrectomy Syndrome Working Party (JPGSWP) (Table 2). The PGSAS-45 questionnaire (Table 1) includes 23 items pertaining to postoperative symptoms (items 9-33), including 15 items from the GSRS and 8 newly selected items. In addition, 12 questionnaire items pertaining to dietary intake (8 items), work (1 item), and level of satisfaction with daily life (3 items) were selected. Twenty-three symptom items were clustered into seven symptom subscales (SS), i.e., the esophageal reflux SS, abdominal pain SS, meal-related distress SS, indigestion SS, diarrhea SS, constipation SS, and dumping SS by factor analysis. Details of the PGSAS-45 have been reported previously[24].
| Domains | Subdomains | Items | Subscales | ||
| QOL | SF-8 (QOL) | 1 | Physical functioning1 | Five or six-point Likert scale | Physical component summary1 |
| 2 | Role physical1 | Mental component summary1 | |||
| 3 | Bodily pain1 | ||||
| 4 | General health1 | ||||
| 5 | Vitality1 | ||||
| 6 | Social functioning1 | ||||
| 7 | Role emotional1 | ||||
| 8 | Mental health1 | ||||
| Symptoms | GSRS | 9 | Abdominal pains | Seven-point Likert scale | Esophageal reflux subscale (item 10, 11, 13, 24) |
| symptoms | 10 | Heartburn | Except item 29 and 32 | Abdominal pain subscale (item 9, 12, 28) | |
| 11 | Acid regurgitation | Meal-related distress subscale (item 25-27) | |||
| 12 | Sucking sensations in the epigastrium | Indigestion subscale (item 14-17) | |||
| 13 | Nausea and vomiting | Diarrhea subscale (item 19, 20, 22) | |||
| 14 | Borborygmus | Constipation subscale (item 18, 21, 23) | |||
| 15 | Abdominal distension | Dumping subscale (item 30, 31, 33) | |||
| 16 | Nausea and vomiting | ||||
| 17 | Increased flatus | Total symptom scale (above seven subscales) | |||
| 18 | Decreased passage of stools | ||||
| 19 | Increased passage of stools | ||||
| 20 | Loose stools | ||||
| 21 | Hard stools | ||||
| 22 | Urgent need for defecation | ||||
| 23 | Feeling of incomplete evacuation | ||||
| PGSAS original | 24 | Bile regurgitation | |||
| symptoms | 25 | Sense of foods sticking | |||
| 26 | Postprandial fullness | ||||
| 27 | Early satiation | ||||
| 28 | Lower abdominal pains | ||||
| 29 | Number and type of early dumping symptoms | ||||
| 30 | Early dumping general symptoms | ||||
| 31 | Early dumping abdominal symptoms | ||||
| 32 | Number and type of late dumping symptoms | ||||
| 33 | Late dumping symptoms | ||||
| Living status | Meals (amount) 1 | 34 | Ingested amount of food per meal1 | - | |
| 35 | Ingested amount of food per day1 | ||||
| 36 | Frequency of main meals | ||||
| 37 | Frequency of additional meals | ||||
| Meals (quality) | 38 | Appetite1 | Five-point Likert scale | Quality of ingestion subscale1 (item 38-40) | |
| 39 | Hunger feeling1 | ||||
| 40 | Satiety feeling1 | ||||
| Meals (amount) 2 | 41 | Necessity for additional meals | Five-point Likert scale | - | |
| Social activity | 42 | Ability for working | Five-point Likert scale | - | |
| QOL | Dissatisfaction (QOL) | 43 | Dissatisfaction with symptoms | Five-point Likert scale | Dissatisfaction for daily life subscale (item 43-45) |
| 44 | Dissatisfaction at the meal | ||||
| 45 | Dissatisfaction at working |
| PGSAS-45 is consisting of SF-8 (item 1-8), GSRS (item 9-23) and PGSAS original items (item 24-45) | |
| 24 | Have you been bothered by bile regurgitation (having a bitter taste in your mouth) during the past month? |
| 25 | Have you been bothered by sense of foods sticking when swallowing during the past month? (Sticking food refers to uncomfortable feeling with foods piled up in the chest.) |
| 26 | Have you been bothered by postprandial fullness during the past month? (Fullness refers to uncomfortable or heavy feeling with foods piled up in the stomach.) |
| 27 | Have you been bothered by being unable to eat enough because you feel full before you finish your meal during the past month? |
| 28 | Have you been bothered by circumumbilical pains or lower abdominal pains during the past month? |
| 29 | Have you experienced following symptoms around 30 min after eating during the past month? Please encircle the number that describes your symptom. (Please check all the symptoms you have experienced.) |
| (Ans. Q29) | |
| 1. No symptoms below | |
| [You have experienced following general symptoms.] | |
| 2. Cold sweat 3. Palpitations 4. Dizziness 5. Numbness 6. Fainting 7. Facial flushing | |
| 8. Facial pallor 9. Feeling hot 10. Fatigue or weakness 11. Lassitude 12. Drowsiness | |
| 13. Headache 14. Heaviness of the head 15. Tightness in the chest | |
| [You have experienced following abdominal symptoms.] | |
| 16. Borborygmi (except after drinking milk) 17. Abdominal cramps (except after drinking milk) | |
| 18. Diarrhoea (except after drinking milk) 19. Nausea 20. Vomiting 21. Bloating | |
| 22. Abdominal discomfort | |
| 30 | For those who encircled any of the general symptom-related items in Question 29, to what extent have you been bothered by all these general symptoms during the past month? |
| 31 | For those who encircled any of the abdominal symptom-related items in Question 29, to what extent have you been bothered by all these abdominal symptoms during the past month? |
| 32 | Have you experienced following symptoms within two to three hours after eating during the past month? Please circle the number that describes your symptom. (Please check all the symptoms you have experienced.) |
| (Ans. Q32) | |
| 1. No symptoms below | |
| [You have experienced following general symptoms.] | |
| 2. Cold sweat 3. Palpitations 4. Dizziness 5. Headache 6. Fainting 7. Fatigue or weakness | |
| 8. Lassitude 9. Languor 10. Shakiness 11. Hunger 12. Shortness of breath | |
| 33 | For those who encircled any of the general symptom-related items in Question 32, to what extent have you been bothered by all these general symptoms during the past month? |
| (Ans. Q24-28, 30 ,31, 33) | |
| 1. No discomfort at all 2. Slight discomfort 3. Mild discomfort 4. Moderate discomfort | |
| 5. Moderately severe discomfort 6. Severe discomfort 7. Very severe discomfort | |
| 34 | On average what percent of preoperative food intake have you taken in single meal during the past month? |
| (Ans. Q34) | |
| About ( ) % of the preoperative single ingested amount | |
| 35 | On average, what percent of preoperative food intake have you taken per day during the past month? |
| (Ans. Q35) | |
| About ( ) % of the preoperative total daily ingested amount | |
| 36 | On average, how many main meals have you taken per day during the past month? |
| (Ans. Q36) | |
| About ( ) times per day | |
| 37 | On average, how often have you taken additional meals (light meal or snack) per day during the past month? |
| (Ans. Q37) | |
| About ( ) times per day | |
| 38 | Have you had appetite during the past month? |
| 39 | Have you felt hunger during the past month? |
| 40 | Have you felt satiety during the past month? (Satiety refers to comfortable feeling with your stomach being full.) |
| (Ans. Q38-40) | |
| 1. Never 2. Occasionally (less than once a week) 3. Often (twice to three times per week) | |
| 4. Frequently (four to six times per week) 5. Always (every day) | |
| 41 | Please encircle the number that most accurately describes the necessity for additional meals (light meal or snack) during the past month? |
| (Ans. Q41) | |
| 1. Food intake was enough with main meals; three times per day. | |
| 2. Food intake was slightly insufficient with main meals; three times per day, and you sometimes needed to take additional meals. | |
| 3. Food intake was significantly insufficient with main meals; three times per day, and you had to take additional meals. | |
| 4. Even though you had taken additional meals besides main meals; three times per day, food intake was insufficient. | |
| 5. Food intake was insufficient because you were not able to take additional meals besides breakfast, lunch and dinner. | |
| 42 | Please encircle the number which exactly describes your living status (ability for working or housekeeping) during the past month? |
| (Ans. Q42) | |
| 1. You were able to handle your work or housework sufficiently and could even manage to work overtime. You enjoyed trip, sports, leisure activities, and dining out as you used to before operation. | |
| 2. You were able to work or handle housework as usual (By work as usual we mean during normal working hours without overtime). (You felt no difficulty when avoiding excessive work) | |
| 3. You had some difficulties with working or keeping house. You were able to handle lighter duties (70 to 80 percent of the previous activities). | |
| 4. You had moderate difficulties with working or keeping house (about 50 percent of the previous activities). | |
| 5. You could scarcely work or keep house. | |
| 43 | How often have you felt dissatisfied with the chest or abdominal symptoms due to gastrectomy during the past month? |
| 44 | How often have you felt dissatisfied with being unable to eat as intended due to gastrectomy during the past month? ("being unable to eat as intended" here means that you are not able to eat what you like, with no limitation in amount and in speed.) |
| 45 | How often have you felt dissatisfied with your limited daily activities (working or housekeeping) due to gastrectomy during the past month? |
| (Ans. Q43-45) | |
| 1. Not at all 2. Slightly 3. Moderately 4. Significantly 5. Extremely | |
| PGSAS-45 original items [item 24-45] English version 1.0 © 2016 K Nakada, M Takahashi | |
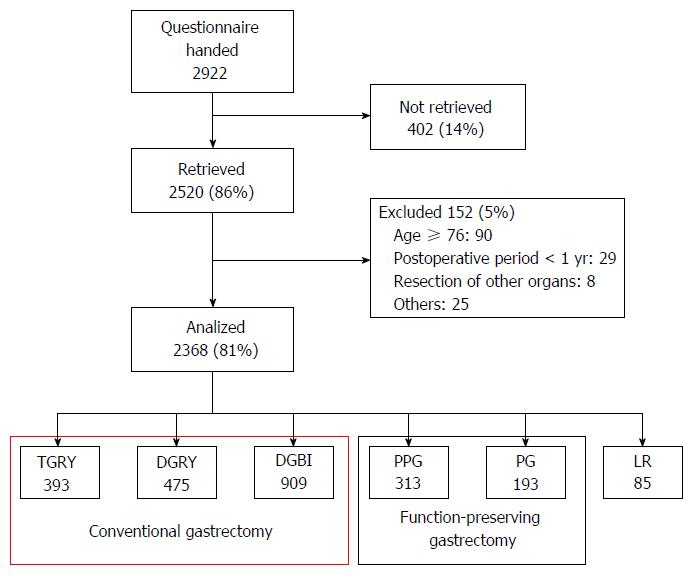
This study utilized continuous sampling from a central registration system for participant enrollment. The questionnaire was distributed to all eligible patients as they presented to participating clinics. After completing the questionnaire, patients were instructed to return forms to the data center. All QOL data from questionnaires were matched with individual patient data collected via case report forms. This study was registered with the University Hospital Medical Information Network’s Clinical Trials Registry (UMIN-CTR; registration number 000002116). This study was approved by local ethics committees at each institution. Written informed consent was obtained from all enrolled patients. Of the 2922 patients who were handed the questionnaire sheets between July 2009 and December 2010, 2520 (86%) responded and 2368 were confirmed to be eligible for the study (Figure 1). Of these, data from 1777 patients who underwent either TG or DG were used in the current study.
In comparing patients’ characteristics, living status and QOLs after TG and DG, statistical methods included the t test and χ2 test. The effects of various clinical factors such as type of gastrectomy as well as age, sex, postoperative period, the severity of symptoms, the degree of body weight loss, the necessity for additional food and the ability for working on the patients’ QOL were investigated by multiple regression analysis (MRA). Moreover, the impact of seven symptom SS on the living status and QOL of patients after gastrectomy were examined by MRA. The values of P < 0.05 were considered significant. To evaluate effect sizes, Cohen’s d, standardization coefficient of regression (β) and coefficient of determination (R2) were used. Interpretation of effect sizes were ≥ 0.2 small, ≥ 0.5 medium, and ≥ 0.8 large in Cohen’s d; ≥ 0.1 small, ≥ 0.3 medium, and ≥ 0.5 large in β; ≥ 0.02 small, ≥ 0.13 medium, and ≥ 0.26 large in R2. Statistical analyses were performed by the biostatisticians mainly using StatView for Windows Ver. 5.0 (SAS Institute Inc.).
Of the 1777 patients treated by gastrectomy who were included in this study, 393 underwent TG and 1384 underwent DG (B-I method in 909 patients and Roux-en-Y method in 475 patients). Comparison between the patients who underwent TG (TG group) and those who underwent DG (DG group) revealed that while the mean “age” was significantly higher in the TG group, there were no significant differences in the “sex” distribution and “postoperative period” between the two groups. “Symptoms”, three evaluation items; “change in body weight”, “necessity for additional meals”, and “ability for working”; which represent the living status, and another three evaluation items; “dissatisfaction for daily life SS”, “physical component summary (PCS)”, and “mental component summary (MCS)”; which represent the QOL were significantly worse in the TG group than in the DG group. Calculation of Cohen’s d effect sizes indicated that there were moderate differences in the influences of “change in body weight”, “necessity for additional meals” and “dissatisfaction for daily life SS”, and slight differences in the influence of “symptoms” and “ability for working” between the TG and DG groups. On the other hand, although there were statistically significant differences in the influence of “PCS” and “MCS” between the two groups, the Cohen’s d effect sizes were very small (< 0.2), indicating the absence of any clinically meaningful differences (Table 3).
| TG (n = 393) | DG (n = 1384) | P value | Cohen's d | ||||
| mean | SD | mean | SD | ||||
| Patients' | Age (yr) | 63.4 | 9.2 | 61.8 | 9.1 | 0.00192 | (0.18) |
| characteristics | Sex (male: n/%) | 276/71.0% | 912/66.2% | 0.07983 | - | ||
| Postoperative period (mo) | 35.0 | 24.6 | 37.9 | 27.4 | 0.09182 | (0.10) | |
| Symptoms | Total symptom score | 2.2 | 0.7 | 1.9 | 0.7 | < 0.00012 | 0.35 |
| Living status | Change in body weight (%)1 | -13.8% | 7.9% | -8.3% | 7.6% | < 0.00012 | 0.71 |
| Necessity for additional meals | 2.4 | 0.8 | 1.9 | 0.8 | < 0.00012 | 0.61 | |
| Ability for working | 2.0 | 0.9 | 1.8 | 0.9 | < 0.00012 | 0.31 | |
| QOL | Dissatisfaction for daily life SS | 2.3 | 0.9 | 1.9 | 0.8 | < 0.00012 | 0.53 |
| Physical component summary1 | 49.6 | 5.6 | 50.6 | 5.6 | 0.00202 | (0.18) | |
| Mental component summary1 | 49.2 | 6.0 | 49.9 | 5.7 | 0.04262 | (0.12) | |
| The interpretation of effect size | Cohen's d | ||||||
| None-very small | < (0.2) | ||||||
| Small | ≥ 0.2 | ||||||
| Medium | ≥ 0.5 | ||||||
| Large | ≥ 0.8 | ||||||
MRA using the type of gastrectomy, patient’s characteristics, symptom and living status as predictor variables was performed to assess the influence of each factor on the three integrated outcome measures for the QOL domain. “Symptoms” and “ability for working” significantly affected on all the QOL outcome measures, with medium effect sizes (β≥ 0.3). In addition, “necessity for additional meals” significantly affected on the “dissatisfaction for daily life SS” with a small effect size (β≥ 0.1). “Age” marginally affected on the “dissatisfaction for daily life SS” and “MCS” with β= 0.09. On the other hand, “type of gastrectomy”, “sex”, “postoperative period” and “change in body weight” had an effect size of β< 0.09 on all the QOL outcome measures, while some clinical factors had a statistically significant, but no clinically meaningful, influence. In addition, R2, which represents the degree of influence of all the predictor variables used in the analysis, was the greatest for the “dissatisfaction for daily life SS” (R2 = 0.606), followed by “PCS” (R2 = 0.368) and “MCS” (R2 = 0.333), with large effect sizes and significant influences on all the QOL outcome measures (Table 4).
| Dissatisfaction for daily life SS | Physical component summary1 | Mental component summary1 | ||||
| β | P value | β | P value | β | P value | |
| Type of gastrectomy [TG] | (0.047) | 0.0132 | (0.008) | NS | (-0.056) | 0.0238 |
| Age | (-0.091) | < 0.0001 | (-0.052) | 0.0236 | (0.090) | 0.0002 |
| Sex [Male] | (-0.016) | NS | (0.043) | 0.0576 | (0.025) | NS |
| Period after gastrectomy | (-0.026) | NS | (-0.019) | NS | (-0.004) | NS |
| Total symptoms score | 0.429 | < 0.0001 | -0.354 | < 0.0001 | -0.357 | < 0.0001 |
| Change in body weight1 | (-0.036) | 0.0551 | (0.026) | NS | (-0.008) | NS |
| Necessity for additional meals | 0.176 | < 0.0001 | (0.057) | 0.0206 | (-0.020) | NS |
| Ability for working | 0.360 | < 0.0001 | -0.377 | < 0.0001 | -0.321 | < 0.0001 |
| R2 (P value) | 0.606 | < 0.0001 | 0.368 | < 0.0001 | 0.333 | < 0.0001 |
| If βis positive, the score of the outcome measure of the patients belonging to the category in [brackets] is higher in cases when the factor is | ||||||
| a nominal scale, and the score of outcome measure of the patients with larger values is higher in cases when the factor is a numeral scale. | ||||||
| The interpretation of effect size | β | R2 | ||||
| None-very small | < (0.100) | < (0.020) | ||||
| Small | ≥ 0.100 | ≥ 0.020 | ||||
| Medium | ≥ 0.300 | ≥ 0.130 | ||||
| Large | ≥ 0.500 | ≥ 0.260 | ||||
To clarify in greater detail the clinical factors, other than the type of gastrectomy which was identified as a significant factor as shown above, that may affect the postoperative QOL of gastrectomy patients, subgroup analysis was conducted for each type of gastrectomy. Like in the analysis for the type of gastrectomy, overall, “symptoms” and “ability for working” were found to have a significant influence on all the QOL outcome measures with medium effect sizes (β≥ 0.3) in both the TG and DG groups (although only the effect size on the “MCS” in the DG group was β= 0.289). In addition, “necessity for additional meals” significantly affected on the “dissatisfaction for daily life SS” with a small effect size (β≥ 0.1) in both the TG and DG groups. “Age” also significantly affected on all the QOL outcome measures with small effect sizes (β≥ 0.1) in the TG group. However, in the DG group, “age” was not significant, or had very small effect sizes even in case it was significant, suggesting that “age” had any clinically meaningful influence. “Sex”, “postoperative period” and “change in body weight” had an effect size of β< 0.09 on all the QOL outcome measures in both groups, and while the influence was statistically significant in some cases, it was not clinically meaningful. Like in the analysis for the type of gastrectomy, overall, R2 was greatest for the “dissatisfaction for daily life SS”, followed by that for the “PCS” and “MCS”, with large effect sizes and significant influences on all the integrated QOL outcome measures (Table 5).
| Dissatisfaction for daily life SS | Physical component summary1 | Mental component summary1 | ||||
| β | P value | β | P value | β | P value | |
| TG | ||||||
| Age | -0.135 | 0.0007 | -0.160 | 0.0006 | 0.118 | 0.0141 |
| Sex (male) | (0.003) | NS | (0.065) | NS | (-0.074) | NS |
| Period after gastrectomy | (-0.036) | NS | (0.029) | NS | (-0.054) | NS |
| Total symptoms score | 0.428 | < 0.0001 | -0.441 | < 0.0001 | -0.350 | < 0.0001 |
| Change in body weight1 | (-0.001) | NS | (0.020) | NS | (-0.034) | NS |
| Necessity for additional meals | 0.281 | < 0.0001 | (0.085) | 0.0653 | (-0.028) | NS |
| Ability for working | 0.335 | < 0.0001 | -0.334 | < 0.0001 | -0.415 | < 0.0001 |
| R2 (P value) | 0.565 | < 0.0001 | 0.434 | < 0.0001 | 0.393 | < 0.0001 |
| DG | ||||||
| Age | (-0.081) | 0.0001 | (-0.018) | NS | (0.081) | 0.0029 |
| Sex (male) | (0.025) | NS | (0.036) | NS | (0.052) | 0.0502 |
| Period after gastrectomy | (-0.027) | NS | (-0.033) | NS | (0.011) | NS |
| Total symptoms score | 0.441 | < 0.0001 | -0.316 | < 0.0001 | -0.355 | < 0.0001 |
| Change in body weight1 | (-0.044) | 0.0265 | (0.024) | NS | (0.006) | NS |
| Necessity for additional meals | 0.139 | < 0.0001 | (0.043) | NS | (-0.019) | NS |
| Ability for working | 0.377 | < 0.0001 | -0.390 | < 0.0001 | -0.289 | < 0.0001 |
| R2 (P value) | 0.598 | < 0.0001 | 0.347 | < 0.0001 | 0.322 | < 0.0001 |
| If βis positive, the score of the outcome measure of the patients belonging to the category in [brackets] is higher in cases when the factor is | ||||||
| a nominal scale, and the score of outcome measure of the patients with larger values is higher in cases when the factor is a numeral scale. | ||||||
| The interpretation of effect size | β | R2 | ||||
| None-very small | < (0.100) | < (0.020) | ||||
| Small | ≥ 0.100 | ≥ 0.020 | ||||
| Medium | ≥ 0.300 | ≥ 0.130 | ||||
| Large | ≥ 0.500 | ≥ 0.260 | ||||
The influence of the seven symptoms SS often found after gastrectomy, i.e., “esophageal reflux”, “abdominal pain”, “meal-related distress”, “indigestion”, “diarrhea”, “constipation” and “dumping”, on the living status and integrated outcome measures for the QOL domain in gastrectomized patients was assessed by MRA. The results revealed that the influence on the living status and QOL outcome measures greatly differed depending on the nature of symptoms. “Meal-related distress” and “dumping” significantly affected almost all the QOL outcome measures with small effect sizes (β≥ 0.1). In addition, “abdominal pain” and “esophageal reflux” significantly affected some of the outcome measures (“PCS” and “dissatisfaction for daily life SS”) with small effect sizes (β≥ 0.1). On the other hand, “indigestion”, “diarrhea” and “constipation” had no clinically meaningful influence on any of the QOL outcome measures, with β< 0.09. The R2 was the greatest for “dissatisfaction for daily life SS” and “PCS” (significant influence with large effect sizes, R2≥ 0.26), followed by “MCS”, “ability for working” and “necessity for additional meals” (significant influence with medium effect sizes, R2≥ 0.13) and “change in body weight” (significant influence with a small effect size, R2≥ 0.02) (Table 6).
| Change in body weight1 | Necessity for additional meals | Ability for working | Dissatisfaction for daily life SS | Physical component summary1 | Mental component summary1 | |||||||
| β | P value | β | P value | β | P value | β | P value | β | P value | β | P value | |
| Esophageal reflux SS | (-0.04) | NS | (0.052) | NS | (0.081) | 0.0126 | (0.085) | 0.0011 | -0.126 | < 0.0001 | (-0.085) | 0.0062 |
| Abdominal pain SS | (0.042) | NS | (-0.004) | NS | (0.096) | 0.0046 | 0.146 | < 0.0001 | -0.261 | < 0.0001 | (-0.094) | 0.0039 |
| Meal-related distress SS | -0.170 | < 0.0001 | 0.279 | < 0.0001 | 0.116 | 0.0012 | 0.282 | < 0.0001 | (-0.074) | 0.0263 | -0.144 | < 0.0001 |
| Indigestion SS | (-0.036) | NS | (0.004) | NS | (-0.001) | NS | (0.015) | NS | (0.024) | NS | (-0.058) | 0.0699 |
| Diarrhea SS | (0.023) | NS | (-0.037) | NS | (-0.076) | 0.0061 | (0.011) | NS | (0.022) | NS | (-0.054) | 0.0441 |
| Constipation SS | (0.062) | 0.0431 | (0.007) | NS | (0.093) | 0.0008 | (0.006) | NS | (-0.037) | NS | (-0.056) | 0.0356 |
| Dumping SS | (-0.051) | NS | 0.113 | 0.0015 | 0.214 | < 0.0001 | 0.283 | < 0.0001 | -0.168 | < 0.0001 | -0.141 | < 0.0001 |
| R2 (P value) | 0.040 | < 0.0001 | 0.148 | < 0.0001 | 0.202 | < 0.0001 | 0.483 | < 0.0001 | 0.276 | < 0.0001 | 0.240 | < 0.0001 |
| If βis positive, the score of outcome measure of the patients with larger values is higher | ||||||||||||
| The interpretation of effect size | β | R2 | ||||||||||
| None-very small | < (0.100) | < (0.020) | ||||||||||
| Small | ≥ 0.100 | ≥ 0.020 | ||||||||||
| Medium | ≥ 0.300 | ≥ 0.130 | ||||||||||
| Large | ≥ 0.500 | ≥ 0.260 | ||||||||||
Multiple studies have reported the influence of different gastrectomy procedures on the postoperative QOL of the surgically treated patients[8-23], however, the influences of other clinical factors on the postoperative QOL are still unknown. The present study was conducted to clarify the influences of various clinical factors on the QOL of patients after gastrectomy using PGSAS-45; a newly developed composite questionnaire for postgastrectomy evaluation. The results of our evaluation revealed that among a variety of clinical factors, “symptoms” had the strongest influence on the postoperative QOL of gastrectomy patients, followed by “ability for working” and “necessity for additional meals”. In addition, among the symptoms, “meal-related distress” and “dumping” affected the postoperative QOL the most strongly and broadly, and “abdominal pain” and “esophageal reflux” also affected some of the outcome measures, but to a limited extent. “Indigestion”, “diarrhea” and “constipation” had the smallest influence. “Change in body weight”, which is often used as an objective evaluation index after gastrectomy, had no clinically meaningful influence on the living status or the QOL of postgastrectomy patients. This is the first study to investigate the influence of a variety of clinical factors other than type of gastrectomy on the postoperative QOL of gastrectomy patients.
PGS occurs frequently after gastrectomy that may cause significant clinical problems[2-7]. Clinical features of PGS include the occurrence of various symptoms, reduced dietary intake, weight loss, reduced physical activity, and reduced physical and mental QOL. PGS is usually the most severe after TG, and the postoperative QOL of patients is known to be better after DG, in which the proximal stomach is partially preserved, than after TG[8,10,13,18,19]. Our study also showed a significantly higher “necessity for additional meals”, greater “weight loss”, lower “ability for working” and worse “dissatisfaction for daily life SS”, “PCS” and “MCS” of SF-8 in the TG group than in the DG group. Furthermore, it has been reported that function-preserving gastrectomy, such as PPG and proximal gastrectomy, is associated with a better postoperative QOL as compared to gastrectomy[9,11,16,17,20,23]. Thus, the type of gastrectomy is a well-known factor affecting the postoperative QOL of patients who underwent gastrectomy, and improvement in the gastrectomy procedures may be expected to improve the postoperative QOL of gastrectomy patients. Accordingly, it is clinically important to make efforts to further improve the surgical procedures of gastrectomy. On the other hand, at present, we know that improvement in the gastrectomy procedures can only reduce, though not completely eliminate the development of PGS, and that its effect is limited. Therefore, it is necessary to identify other clinical factors that may affect the postoperative QOL of gastrectomy patients besides the type of gastrectomy, and to manage PGS in a multifaceted manner for further improvement of the postoperative QOL of gastrectomy patients.
It is well-known that there are considerable individual differences in the degree of interference with the daily life activities among patients who underwent the same type of gastrectomy, suggesting that clinical factors other than the type of gastrectomy also have a significant influence on the postoperative QOL of gastrectomy patients, although there is little information yet on such factors. In the present study, we investigated the influence of clinical factors such as “age”, “sex”, “postoperative period”, “symptoms”, “change in body weight”, “necessity for additional meals” and “ability for working”, on the postoperative QOL of gastrectomy patients by conducting MRA of the data of 1777 patients who underwent gastrectomy. According to the obtained results, among these clinical factors, “symptoms”, “the ability for working” and “the necessity for additional meals” had a significant influence on the integrated QOL outcome measures; “symptoms” had the strongest influence, followed by “ability for working” and “necessity for additional meals”. In particular, “symptoms” and “ability for working” significantly affected all the integrated QOL outcome measures with considerable effect sizes, suggesting that they can be counted as reliable factors adversely affecting the postoperative QOL of gastrectomy patients.
“Change in body weight” is often used as an index which objectively evaluates the physical status of postgastrectomy patients[21,22,27,28], but in this study, the influence of “change in body weight” per se on the postoperative QOL of gastrectomy patients was unexpectedly small.
In regard to the influence of “type of gastrectomy” performed, univariate analysis (Table 3) revealed that many outcome measures for living status and QOL were significantly worse in the TG group than in the DG group, with moderate to small effect sizes, indicating considerable differences in the effects among the type of gastrectomy. However, the multivariate analysis (conducted by us) revealed that the influence of “type of gastrectomy” per se on “dissatisfaction for daily life SS”, “PCS” and “MCS” was very small, based on the effect sizes. These results suggest that the differences in the living status and QOL between the TG and DG groups were caused not by the direct influence of the gastrectomy procedures, but rather, by the indirect influence to the QOL that might affect the factors such as “symptoms”, “necessity for additional meals”, and “ability for working”. Other clinical factors such as “age”, “sex” and “postoperative period” not shorter than 1 year, had little, if any, or no influence on the postoperative QOL in gastrectomy patients.
The results of subgroup analysis of the influence of these clinical factors on the postoperative QOL for each type of gastrectomy using MRA were similar to those of the analysis for all type of gastrectomy, suggesting that multiple clinical factors other than the type of gastrectomy, i.e., “symptoms”, “necessity for additional meals” and “ability for working”, have a definite influence on the QOL of postgastrectomy patients.
Identification of clinical factors affecting the QOL of gastrectomized patients and obtaining a deeper understanding of PGS is expected to be useful for the better management of PGS, besides providing clues to improve the gastrectomy procedures. Individual differences in the adaptability to gastrointestinal dysfunction caused by gastrectomy, patient food preferences, how to eat meals (e.g., overeating or eating quickly), etc., are also expected to affect these clinical factors, and it is desirable to support the reconstruction of the dietary habits according to the adaptability of each patient after gastrectomy.
MRA showed that the R2 was 0.606 for “dissatisfaction for daily life SS”, 0.368 for SF-8 “PCS” and 0.333 for SF-8 “MCS”; thus, among the integrated QOL outcome measures, R2 for “dissatisfaction for daily life SS” was exceptionally high. This indicates that “dissatisfaction for daily life SS” most appropriately reflects the influence of all the predictive variables used in the analysis, and that “dissatisfaction for daily life SS” is a valid comprehensive index to evaluate the postoperative QOL after gastrectomy.
The present study revealed that among a variety of clinical factors, “symptoms” had the greatest influence on the QOL after gastrectomy. Comparison in greater detail of the influences of the symptom SS on the living status and QOL outcome measures by MRA revealed that “dumping” and “meal-related distress” had significant and the strongest influence on almost all of the main outcome measures for the living status and QOL domains. “Abdominal pain” and “esophageal reflux” affected some of the outcome measures (“PCS” and “dissatisfaction for daily life SS”). On the other hand, “indigestion”, “diarrhea” and “constipation” scarcely affected the activities of daily life or the QOL. Thus, our results revealed that the influence on the daily life activities and QOL of postgastrectomy patients differed significantly among the various symptoms. Though it is well known that a variety of symptoms occurring after gastrectomy decrease the QOL of postoperative patients, this is the first study that weighed the size of influence by the nature of symptoms.
“Dumping” and “meal-related distress” are characteristic symptoms frequently found after gastrectomy, and are well-known as dumping syndrome and small stomach syndrome, respectively[3,29,30]. The present study revealed that these symptoms are clinically extremely important, because they have the greatest effect of interfering with the daily life activities and reducing the postoperative QOL of gastrectomy patients. Thus, improvement of the gastrectomy procedures to reduce these symptoms will contribute to the improvement of the postoperative QOL of gastrectomy patients. Namely, gastrectomy procedures that preserve the pylorus and prevent dumping (such as PPG and proximal gastrectomy) may be expected to reduce dumping symptoms and those that increase the residual gastric volume (such as a reduced extent of gastrectomy and creation of a substitute stomach) may be expected to reduce small stomach symptoms, which would be clinically useful. Therefore, it would be desirable to further improve the gastrectomy procedures with the objective of reducing these symptoms and to evaluate their efficacy using appropriate patient- reported outcome measures.
So far, various questionnaires have been used to compare the usefulness of gastrectomy procedures and to evaluate the postoperative QOL. For this purpose, existing general-purpose disease or symptom specific QOL questionnaires, such as GSRS[26,31,32], Gastrointestinal Quality of Life Index[12,33] and European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; EORTC QLQ-C30 + QLQ-STO22[10,13,18,19,21,34-36], which were established for other purposes and had verified reliability and validity, have been mainly used, because there have been no established questionnaires specified for the postgastrectomy evaluation. However, these questionnaires are likely to be inadequate for the clinical evaluation of postgastrectomy patients, because they do not contain “dumping” and/or “meal-related distress”, which are symptoms that are well-recognized as significantly affecting the postoperative QOL of gastrectomy patients. Actually, our previous study showed that comparison of the influence of the 15 symptom items of the GSRS and 8 symptom items of the original PGSAS, including dumping symptoms and meal-related distress, on the living status and QOL revealed that the effect sizes of the items on the original PGSAS on most main outcome measures were much larger than those of the GSRS items[24]. Therefore, we consider that it is necessary to use questionnaires containing both “dumping” and “meal-related distress” symptoms established for postgastrectomy evaluation (such as PGSAS-45[24]) in future studies of the QOL after gastrectomy.
A limitation of this study was that there might also be unknown clinical factors, in addition to the predictor variables used in the present analysis, which could affect the QOL of postgastrectomy patients. It is necessary to continue to try to find and manage such possible factors by closely observing the living status of gastrectomized patients. The presence, if any, of a strong correlation among the predictor variables used in MRA would cause statistical instability due to multicollinearity, leading to a reduction in the reliability of MRA. Therefore, variance inflation factor (VIF), which is an indicator of multicollinearity, was calculated for the predictor variables used in the study. The VIF values in the MRA shown in Tables 4-6 were 1.0-1.3, 1.0-1.4 and 1.4-2.3, respectively, indicating the absence of any multicollinearity.
Improvement in the gastrectomy procedures to reduce PGS is extremely important and continual efforts to improve the gastrectomy procedures are also necessary in the future. However, it is difficult to eliminate PGS only by improving the gastrectomy procedures. Therefore, attention must be paid to the other clinical factors that have been found to decrease the QOL after gastrectomy and it is necessary to try to improve the lives of postgastrectomy patients in a composite manner by, for example, sufficient surveillance and care for PGS in outpatient practice after gastrectomy. Paying attention to “symptoms” (in particular, “dumping”, “meal-related distress”, “abdominal pain” and “esophageal reflux”, which greatly affect the postoperative QOL), “ability for working” and “necessity for additional meals” to detect these abnormalities early and providing appropriate management and treatment in outpatient practice after surgery would be expected to contribute to the improvement of the postoperative QOL of gastrectomy patients.
The results of this study were presented at Digestive Disease Week 2013, Orland, United States[37].
This study was completed by 52 institutions in Japan. The authors thank all physicians who participated in this study and the patients whose cooperation made this study possible. The contributor of each institution is listed below.
Masanori Terashima (Shizuoka Cancer Center), Junya Fujita (Toyonaka Municipal Hospital), Kazuaki Tanabe (Hiroshima University), Nobuhiro Takiguchi (Chiba Cancer Center), Masazumi Takahashi (Yokohama Municipal Citizen’s Hospital), Kazunari Misawa (Aichi Cancer Center Hospital), Koji Nakada, Norio Mitsumori (The Jikei University School of Medicine), Hiroshi Kawahira (Graduate School of Medicine, Chiba University), Tsutomu Namikawa (Kochi Medical School), Takao Inada (Tochigi Cancer Center), Hiroshi Okabe (Kyoto University Graduate School of Medicine), Takashi Urushihara (Hiroshima Prefectural Hospital), Yoshiyuki Kawashima (Saitama Cancer Center), Norimasa Fukushima (Yamagata Prefectural Central Hospital), Yasuhiro Kodera (Nagoya University Graduate School of Medicine), Takeyoshi Yumiba (Osaka Kosei-Nenkin Hospital), Hideo Matsumoto (Kawasaki Medical School), Akinori Takagane (Hakodate Goryoukaku Hospital), Chikara Kunisaki (Yokohama City University Medical Center), Ryoji Fukushima (Teikyo University School of Medicine), Hiroshi Yabusaki (Niigata Cancer Center Hospital), Seshimo Akiyoshi (Tokyo Women’s Medical University), Naoki Hiki (Cancer Institute Hospital), Keisuke Koeda (Iwate Medical University), Mikihiro Kano (JA Hiroshima General Hospital), Yoichi Nakamura (Toho University Ohashi Medical Center), Makoto Yamada (Gifu Municipal Hospital), Sang-Woong Lee (Osaka Medical College), Shinnosuke Tanaka (Fukuoka University School of Medicine), Akira Miki (Kobe City Medical Center General Hospital), Masami Ikeda (Asama General Hospital, Yokosuka General Hospital Uwamachi), Satoshi Inagawa (University of Tsukuba), Shugo Ueda (Kitano Hospital), Takayuki Nobuoka (Sapporo Medical University School of Medicine), Manabu Ohta (Hamamatsu University school of Medicine), Yoshiaki Iwasaki (Tokyo Metropolitan Cancer and Infectious diseases Center Komagome Hospital), Nobuyuki Uchida (Haramachi Red-cross Hospital), Eishi Nagai (Graduate School of Medical Sciences, Kyushu University), Yoshikazu Uenosono (Kagoshima University Graduate School of Medicine), Shinichi Kinami (Kanazawa Medical University), Yasuhiro Nagata (National Hospital Organization Nagasaki Medical Center), Masashi Yoshida (International University of Health and Welfare, Mita Hospital), Keishiro Aoyagi (School of Medicine Kurume University), Shuichi Ota (Osaka Saiseikai Noe hospital), Hiroaki Hata (National Hospital Organization, Kyoto Medical Center), Hiroshi Noro (Otemae Hospital), Kentaro Yamaguchi (Tokyo Women’s Medical University Medical Center East), Hiroshi Yajima (The Jikei University Kashiwa Hospital), Toshikatsu Nitta (Shiroyama Hospital), Tsuyoshi Etoh (Oita University), Chikashi Shibata (Tohoku University Graduate School of Medicine).
Various clinical problems called postgastrectomy syndrome (PGS) occur after gastrectomy, which can interfere with the quality of life (QOL) of gastrectomized patients. To detect potential clinical factors affecting QOL after gastrectomy may improve prevention and management of PGS.
Several previous studies investigated that the type of gastrectomy procedures affect QOL after gastrectomy. However, other clinical factors affecting postgastrectomy QOL are poorly understood. The research hotspot is to detect potential clinical factors other than type of gastrectomy procedures which affecting postgastrectomy QOL in large population of gastrectomized patients by multivariate analysis using newly developed postgastrectomy syndrome assessment scale (PGSAS)-45.
Several clinical factors such as symptom severity, ability for working and necessity for additional meals had significant impact on the postoperative QOL with considerable effect sizes, while the influence of the extent of gastrectomy was unexpectedly small.
Paying attention to “symptoms”, “ability for working” and “necessity for additional meals” may help to detect PGS early and provide appropriate management and treatment in outpatient practice, which in turn would be expected to improve the QOL in patients after gastrectomy.
PGS is an organic, functinal, nutritional or metabolic problems after gastrectomy, which accompanying various symptoms, restriction of food intake, weight loss or decrease in physical activity, and can interfere with the QOL of gastrectomized patients.
This is a well-written paper to analyze the factors affecting the QOL after gastrectomy using PGSAS-45. The authors analyzed postoperative factors using newly developed scale to improve the QOL that underwent gastrectomy for gastric cancer. This paper has potentially important clinical implications.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Endo S, Garcia-Olmo D, Kim HH, Namikawa T S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
| 1. | Nashimoto A, Akazawa K, Isobe Y, Miyashiro I, Katai H, Kodera Y, Tsujitani S, Seto Y, Furukawa H, Oda I. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013;16:1-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 372] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 2. | Bolton JS, Conway WC. Postgastrectomy syndromes. Surg Clin North Am. 2011;91:1105-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Carvajal SH, Mulvihill SJ. Postgastrectomy syndromes: dumping and diarrhea. Gastroenterol Clin North Am. 1994;23:261-279. [PubMed] |
| 5. | Eagon JC, Miedema BW, Kelly KA. Postgastrectomy syndromes. Surg Clin North Am. 1992;72:445-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 104] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Jay BS, Burrell M. Iatrogenic problems following gastric surgery. Gastrointest Radiol. 1977;2:239-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Davies J, Johnston D, Sue-Ling H, Young S, May J, Griffith J, Miller G, Martin I. Total or subtotal gastrectomy for gastric carcinoma? A study of quality of life. World J Surg. 1998;22:1048-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 108] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Fujita J, Takahashi M, Urushihara T, Tanabe K, Kodera Y, Yumiba T, Matsumoto H, Takagane A, Kunisaki C, Nakada K. Assessment of postoperative quality of life following pylorus-preserving gastrectomy and Billroth-I distal gastrectomy in gastric cancer patients: results of the nationwide postgastrectomy syndrome assessment study. Gastric Cancer. 2016;19:302-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 10. | Goh YM, Gillespie C, Couper G, Paterson-Brown S. Quality of life after total and subtotal gastrectomy for gastric carcinoma. Surgeon. 2015;13:267-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Isozaki H, Matsumoto S, Murakami S, Takama T, Sho T, Ishihara K, Sakai K, Takeda M, Nakada K, Fujiwara T. Diminished Gastric Resection Preserves Better Quality of Life in Patients with Early Gastric Cancer. Acta Med Okayama. 2016;70:119-130. [PubMed] |
| 12. | Lee MS, Ahn SH, Lee JH, Park DJ, Lee HJ, Kim HH, Yang HK, Kim N, Lee WW. What is the best reconstruction method after distal gastrectomy for gastric cancer? Surg Endosc. 2012;26:1539-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 13. | Lee SS, Chung HY, Kwon OK, Yu W. Long-term Quality of Life After Distal Subtotal and Total Gastrectomy: Symptom- and Behavior-oriented Consequences. Ann Surg. 2016;263:738-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Nakamura M, Nakamori M, Ojima T, Iwahashi M, Horiuchi T, Kobayashi Y, Yamade N, Shimada K, Oka M, Yamaue H. Randomized clinical trial comparing long-term quality of life for Billroth I versus Roux-en-Y reconstruction after distal gastrectomy for gastric cancer. Br J Surg. 2016;103:337-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Nunobe S, Okaro A, Sasako M, Saka M, Fukagawa T, Katai H, Sano T. Billroth 1 versus Roux-en-Y reconstructions: a quality-of-life survey at 5 years. Int J Clin Oncol. 2007;12:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Nunobe S, Sasako M, Saka M, Fukagawa T, Katai H, Sano T. Symptom evaluation of long-term postoperative outcomes after pylorus-preserving gastrectomy for early gastric cancer. Gastric Cancer. 2007;10:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Park DJ, Lee HJ, Jung HC, Kim WH, Lee KU, Yang HK. Clinical outcome of pylorus-preserving gastrectomy in gastric cancer in comparison with conventional distal gastrectomy with Billroth I anastomosis. World J Surg. 2008;32:1029-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 18. | Park S, Chung HY, Lee SS, Kwon O, Yu W. Serial comparisons of quality of life after distal subtotal or total gastrectomy: what are the rational approaches for quality of life management? J Gastric Cancer. 2014;14:32-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Rausei S, Mangano A, Galli F, Rovera F, Boni L, Dionigi G, Dionigi R. Quality of life after gastrectomy for cancer evaluated via the EORTC QLQ-C30 and QLQ-STO22 questionnaires: surgical considerations from the analysis of 103 patients. Int J Surg. 2013;11 Suppl 1:S104-S109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Takiguchi N, Takahashi M, Ikeda M, Inagawa S, Ueda S, Nobuoka T, Ota M, Iwasaki Y, Uchida N, Kodera Y. Long-term quality-of-life comparison of total gastrectomy and proximal gastrectomy by postgastrectomy syndrome assessment scale (PGSAS-45): a nationwide multi-institutional study. Gastric Cancer. 2015;18:407-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 166] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 21. | Takiguchi S, Yamamoto K, Hirao M, Imamura H, Fujita J, Yano M, Kobayashi K, Kimura Y, Kurokawa Y, Mori M. A comparison of postoperative quality of life and dysfunction after Billroth I and Roux-en-Y reconstruction following distal gastrectomy for gastric cancer: results from a multi-institutional RCT. Gastric Cancer. 2012;15:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Terashima M, Tanabe K, Yoshida M, Kawahira H, Inada T, Okabe H, Urushihara T, Kawashima Y, Fukushima N, Nakada K. Postgastrectomy Syndrome Assessment Scale (PGSAS)-45 and changes in body weight are useful tools for evaluation of reconstruction methods following distal gastrectomy. Ann Surg Oncol. 2014;21 Suppl 3:S370-S378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Xiao XM, Gaol C, Yin W, Yu WH, Qi F, Liu T. Pylorus-Preserving versus Distal Subtotal Gastrectomy for Surgical Treatment of Early Gastric Cancer: A Meta-Analysis. Hepatogastroenterology. 2014;61:870-879. [PubMed] |
| 24. | Nakada K, Ikeda M, Takahashi M, Kinami S, Yoshida M, Uenosono Y, Kawashima Y, Oshio A, Suzukamo Y, Terashima M. Characteristics and clinical relevance of postgastrectomy syndrome assessment scale (PGSAS)-45: newly developed integrated questionnaires for assessment of living status and quality of life in postgastrectomy patients. Gastric Cancer. 2015;18:147-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 25. | Turner-Bowker DM, Bayliss MS, Ware JE, Kosinski M. Usefulness of the SF-8 Health Survey for comparing the impact of migraine and other conditions. Qual Life Res. 2003;12:1003-1012. [PubMed] |
| 26. | Svedlund J, Sjödin I, Dotevall G. GSRS--a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33:129-134. [PubMed] |
| 27. | Luu C, Arrington AK, Falor A, Kim J, Lee B, Nelson R, Singh G, Kim J. Impact of gastric cancer resection on body mass index. Am Surg. 2014;80:1022-1025. [PubMed] |
| 28. | Yoshikawa T, Hiki N, Taguri M, Sano T, Nunobe S, Taniguchi H, Fukushima R, Cho H, Morita S, Tsuburaya A. A Phase III trial to evaluate the effect of perioperative nutrition enriched with eicosapentaenoic acid on body weight loss after total gastrectomy for T2-T4a gastric cancer. Jpn J Clin Oncol. 2012;42:459-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Mine S, Sano T, Tsutsumi K, Murakami Y, Ehara K, Saka M, Hara K, Fukagawa T, Udagawa H, Katai H. Large-scale investigation into dumping syndrome after gastrectomy for gastric cancer. J Am Coll Surg. 2010;211:628-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 30. | Tanizawa Y, Tanabe K, Kawahira H, Fujita J, Takiguchi N, Takahashi M, Ito Y, Mitsumori N, Namikawa T, Oshio A. Specific Features of Dumping Syndrome after Various Types of Gastrectomy as Assessed by a Newly Developed Integrated Questionnaire, the PGSAS-45. Dig Surg. 2016;33:94-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 31. | Hayami M, Seshimo A, Miyake K, Shimizu S, Kameoka S. Effects of emptying function of remaining stomach on QOL in postgastrectomy patients. World J Surg. 2012;36:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Kono K, Iizuka H, Sekikawa T, Sugai H, Takahashi A, Fujii H, Matsumoto Y. Improved quality of life with jejunal pouch reconstruction after total gastrectomy. Am J Surg. 2003;185:150-154. [PubMed] |
| 33. | Eypasch E, Williams JI, Wood-Dauphinee S, Ure BM, Schmülling C, Neugebauer E, Troidl H. Gastrointestinal Quality of Life Index: development, validation and application of a new instrument. Br J Surg. 1995;82:216-222. [PubMed] |
| 34. | Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365-376. [PubMed] |
| 35. | Kobayashi D, Kodera Y, Fujiwara M, Koike M, Nakayama G, Nakao A. Assessment of quality of life after gastrectomy using EORTC QLQ-C30 and STO22. World J Surg. 2011;35:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 36. | Vickery CW, Blazeby JM, Conroy T, Johnson CD, Alderson D. Development of an EORTC module to improve quality of life assessment in patients with gastric cancer. Br J Surg. 2000;87:362-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Nakada K, Ikeda M, Takahashi M, Kinami S, Yoshida M, Uenosono Y, Kawashima Y, Oshio A, Suzukamo Y, Terashima M. Development and validation of PGSAS-45, an integrated questionnaire to assess postgastrectomy syndrome. Gastroenterology. 2013;144 Suppl 1:S-1111. |