Published online Jan 28, 2016. doi: 10.3748/wjg.v22.i4.1335
Peer-review started: May 23, 2015
First decision: September 29, 2015
Revised: October 29, 2015
Accepted: November 19, 2015
Article in press: November 19, 2015
Published online: January 28, 2016
Processing time: 243 Days and 5.6 Hours
The primary malignancies of the biliary tract, cholangiocarcinoma and gallbladder cancer, often present at an advanced stage and are marginally sensitive to radiation and chemotherapy. Accumulating evidence indicates that molecularly targeted agents may provide new hope for improving treatment response in biliary tract carcinoma (BTC). In this article, we provide a critical review of the pathogenesis and genetic abnormalities of biliary tract neoplasms, in addition to discussing the current and emerging targeted therapeutics in BTC. Genetic studies of biliary tumors have identified the growth factors and receptors as well as their downstream signaling pathways that control the growth and survival of biliary epithelia. Target-specific monoclonal antibodies and small molecules inhibitors directed against the signaling pathways that drive BTC growth and invasion have been developed. Numerous clinical trials designed to test these agents as either monotherapy or in combination with conventional chemotherapy have been completed or are currently underway. Research focusing on understanding the molecular basis of biliary tumorigenesis will continue to identify for targeted therapy the key mutations that drive growth and invasion of biliary neoplasms. Additional strategies that have emerged for treating this malignant disease include targeting the epigenetic alterations of BTC and immunotherapy. By integrating targeted therapy with molecular profiles of biliary tumor, we hope to provide precision treatment for patients with malignant diseases of the biliary tract.
Core tip: For patients with cholangiocarcinoma and gallbladder carcinoma, targeted therapeutics provide new opportunity of treatment that is potentially less toxic and more effective. These target-specific monoclonal antibodies and small molecule inhibitors are directed against the signaling pathways that drive the progression of biliary tract cancers. This article provides an updated review of the molecular pathogenesis of these malignant neoplasms as the framework for describing the mechanisms by which targeted agents work. The preclinical and clinical data from investigation of targeted therapeutics in biliary tract cancer are discussed.
- Citation: Marks EI, Yee NS. Molecular genetics and targeted therapeutics in biliary tract carcinoma. World J Gastroenterol 2016; 22(4): 1335-1347
- URL: https://www.wjgnet.com/1007-9327/full/v22/i4/1335.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i4.1335
Cholangiocarcinoma and gallbladder carcinoma are relatively rare malignancies that are collectively referred to as biliary tract carcinoma (BTC). Approximately 10000 new cases of BTC arise within the United States each year, nearly two-thirds of which are gallbladder carcinoma[1]. These tumors generally present with insidious onset of non-specific abdominal symptoms that seldom prompt patients to seek timely medical evaluation. As a result, approximately 90% of BTC is not diagnosed until the disease has become locally advanced or metastatic[2]. The relative frequency of each BTC subtype is provided in Table 1, along with the stereotypical risk factors, clinical features, and diagnostic studies that are commonly utilized.
| Type of malignancy | Incidence | Risk factors | Typical presentation | Diagnosis |
| CC | 1-2 per 100000 population[88] | Increasing age[89] | CT or MRI: Mass lesion with contrast uptake during arterial and venous phases[89] | |
| Hispanic or Asian ethnicity[89] | ||||
| PSC[89] | ||||
| Helminth infection[89] | ||||
| Choledochal cyst[89] | ||||
| Thorotrast[89] | ||||
| Metabolic syndrome[89,90] | ||||
| Hepatobiliary stones[89] | ||||
| Viral hepatitis[89,90] | ||||
| Intrahepatic | 10% of CC[89] | Constitutional symptoms (fevers, night sweats, unintended weight loss)[89] | Differentiate from hepatocellular carcinoma via timing of contrast uptake[89] | |
| Extrahepatic | 90% of CC[90] | Painless jaundice[89,90] | ERCP with brushing can obtain sample for cytology | |
| EUS with FNA of lymph nodes can assess for metastasis | ||||
| GBC | 1-2 per 100000 population[91] | Increasing age[92] | Painless jaundice[92] | EUS: Allows for FNA and is considered definitive for staging[92] |
| Female gender[92] | ||||
| Hispanic, Asian, or Eastern European heritage[92] | ||||
| Gallstones[92] | Constitutional symptoms (fevers, night sweats, unintended weight loss)[92] | CT or MRCP: Determines resectability | ||
| Salmonella[92] | ||||
| Helicobacter pylori[92] | ||||
| PSC[92] | ||||
| Heavy metal exposure[92] | ||||
| Metabolic syndrome[92] |
When patients present with a localized biliary tumor that has not macroscopically invaded the adjacent vasculature, surgical resection may be attempted with curative intent[3]. However, even patients who receive an R0 resection will often experience recurrence of their disease[4]. The management of patients with recurrent tumor, as well as those with locally advanced or metastatic disease at presentation, consists of locoregional treatments, systemic chemotherapy, and symptomatic control (Table 1).
For patients with BTC that is locally advanced or metastatic, the current standard of care involves a combination of gemcitabine and cisplatin[5]. Alternative regimens that are employed against GBC, intrahepatic cholangiocarcinoma (IHCC), and extrahepatic cholangiocarcinoma (EHCC) include gemcitabine and oxaliplatin (GEMOX), capecitabine and oxaliplatin, and monotherapy with either gemcitabine, capecitabine, or 5-fluorouracil[2,3]. Despite these interventions, the clinical outcomes of patients with BTC are generally poor. Five years after diagnosis, approximately 18% of patients with GBC or CC remain alive[6]. Patients with stage III or IV disease at initial presentation seldom survive longer than one year following diagnosis[2]. The recent development of targeted therapeutics directed against the pathways that drive biliary tumor development and growth provides additional treatment options. These targeted therapies tend to be selective for the malignant cells, thus potentially improving the efficacy and tolerability of treatment.
Genetic studies of BTC have shed new insights upon the pathogenic mechanisms of this disease and the signaling pathways that drive its progression. Multiple elements of these pathways have been identified and targeted by this new generation of therapeutics. In this article, we provide an updated review of the molecular genetics of CC and GBC. The pathogenesis and cellular patho-physiology of these malignancies are described, with emphasis on those molecular abnormalities that could be targeted for intervention. The mechanism of action of each targeted agent under investigation for treating BTC is discussed, as well as data from pre-clinical and clinical studies. Ongoing clinical trials of these molecularly targeted agents in BTC are also presented. We hope this article will help stimulate further research with the goal of developing precision treatment for patients with this malignant disease.
Understanding the pathogenetic mechanism that underlies the development of biliary tumors is important for determining the significance of the molecular alterations that occur in this disease and thereby directs the development of targeted therapeutics. Both CC and GBC arise from malignant transformation of biliary epithelium, typically occurring in the setting of chronic inflammation. These cancers may also arise from macroscopic polypous adenomas that exist within the gallbladder or bile ducts of approximately 0.3%-0.5% of the population[7]. The precise rate at which these lesions transform into BTC is not known, but is believed to be low.
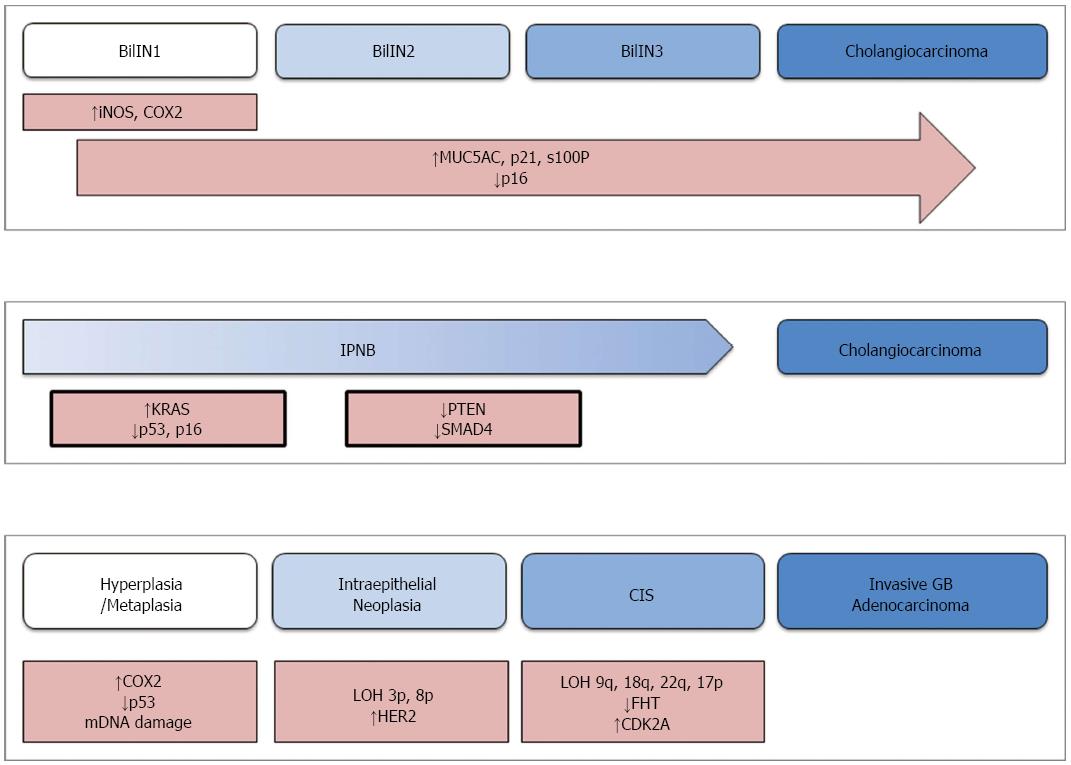
Aside from these fundamental similarities, the processes by which CC and GBC develop are disparate. The molecular and histological pathogenesis of these malignancies is described separately. A schematic diagram that represents these processes is provided in Figure 1.
CC generally develops from two distinct types of precursor lesions, biliary intraepithelial neoplasm (BilIN) and intraductal papillary neoplasm of the bile duct (IPNB)[8]. From a molecular and histologic perspective, these lesions are very similar to pancreatic intraepithelial neoplasm (PanIN) and intraductal papillary mucinous neoplasm (IPMN)-two precursors of pancreatic adenocarcinoma[8,9]. The similarities between pancreatic and biliary lesions have been exploited for developing a model of cholangiocarcinogenesis. Since CC is seldom discovered early in its development, there is limited data on its pathogenesis. Therefore, data obtained from studies of pancreatic adenocarcinoma have been used to generate working models of BTC.
The more common of the precursor lesions, BilIN, is a microscopic papillary proliferation of atypical biliary epithelia. These lesions are discovered almost exclusively in the large bile ducts and typically give rise to EHCC[8]. Histologically, BilIN is graded from I to III on the basis of cellular atypia and loss of polarity, much like PanIN lesions[10]. The initial stages of BilIN development often occur in the setting of chronic inflammation, where inflammatory cytokines stimulate expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX2)[11,12]. Nitric oxide, produced by iNOS, causes oxidative damage to DNA and limits the cellular ability to repair such damage[11]. This is further compounded by over-expression of COX2, which drives cellular growth by synthesizing prostaglandin E2 (PGE2)[13]. As the lesion advances from BilIN1 to BilIN3, additional genetic and molecular abnormalities accumulate. Increased expression of mucin core protein 5AC (MUC5AC), p21CDKN2A, and S100p is generally observed during this progression[14]. On the other hand, the tumor suppressor p16INK4A is markedly decreased in advanced BilIN and CC[14]. Similar abnormalities of p21 and p16 are observed in the progression of PanIN, with the exception of pancreatic lesions being more likely to also harbor KRAS mutations or overexpress p53[8,15,16].
The other class of precursor to cholangiocarcinoma is intraductal papillary neoplasm of the bile duct (IPNB). Arising preferentially within the intrahepatic bile ducts, IPNB is characterized as a macroscopic papillary growth of biliary epithelia that is supported by cores of fibrovascular tissue[17,18]. Immunohistochemical staining of the associated mucin further classifies IPNB lesions into pancreaticobiliary, intestinal, gastric, or oncocytic subtypes. In this working model, these lesions are assumed to develop in a sequence similar to that observed in pancreatic IPMN[8]. Activating mutations of KRAS are believed to be among the earliest changes, along with loss of function of the tumor suppressors p53 and p16INK4A[18]. This is followed by loss of SMAD4 function, with the latter being thought to drive the progression to invasive malignancy[18].
The present model of gallbladder carcinogenesis is built upon information gained from studying this malignancy, as well as colorectal cancer and in vitro studies of epithelial inflammation[19]. In this model, normal epithelium sequentially becomes metaplastic or less commonly hyperplastic, then dysplastic/intraepithelial neoplasia, and carcinoma in situ, before eventually becoming an invasive malignancy.
Much like cholangiocarcinoma, the development of gallbladder tumors is generally preceded by chronic inflammation[20]. Inflammatory cytokines cause over-expression of COX2 and epigenetic inhibition of tumor suppressor genes. Mutations of TP53 and mitochondrial DNA are also commonly observed at this early stage[21,22]. These molecular changes drive the transformation of initially normal epithelium into metaplastic cells.
The next step in malignant transformation is defined histologically by the appearance of intraepithelial neoplasia. This is generally associated with a loss of heterozygosity at loci 3p and 8p[23], as well as over-expression of the cell surface receptor HER2[24]. The lesion then develops into carcinoma in situ, at which point mutations of fragile histidine triad (FHT) and cyclin-dependent kinase inhibitor 2A (CDKN2A) arise. Loss of heterozygosity at 9q, 18q, 22q, 5q, and 17p may also be observed at this stage[19,25,26]. Subsequently, the lesion accumulates mutations of KRAS and loss of heterozygosity at 9p, 13q, and 18q[19,25]. These latter genetic aberrations are believed to drive the lesion to develop into an invasive carcinoma.
The malignant behaviors exhibited by BTC are caused largely by dysregulation of cellular signaling networks that normally control cell growth, survival, and differentiation. These involve cell-surface receptors, including epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2 (HER2), vascular endothelial growth factor receptor (VEGFR), and hepatocyte growth factor receptor (MET). Intracellular signaling cascades, namely the RAS-RAF-MEK and PI3K-AKT-mTOR axes also play important functional roles. The genetic abnormalities that arise in each subtype of BTC have been identified and the frequency of each is summarized in Table 2.
| Target | Type of alteration | GBC | EHCC | IHCC | Ref. |
| Growth factors/receptors | |||||
| EGFR | Point mutation | 6%-9% | 14%-20% | 3%-20% | [29,84] |
| EGFR | Increased expression | 12% | 5%-19% | 11%-27% | [28,93] |
| HER2 | Increased expression | 16% | 5%-8% | 0%-1% | [28,93] |
| MET | Increased expression | 5%-74% | 0% | 21%-58% | [93,94] |
| VEGF | Increased expression | 55%-63% | 59% | 53% | [28,34] |
| RAS/RAF/MEK pathway | |||||
| KRAS | Point mutation | 0-13% | 0%-23% | 5%-54% | [29,39,95-99,101] |
| BRAF | Point mutation | 0-33% | 0%-2% | 0%-21% | [29,39-41,101] |
| MEK | - | Unknown | Unknown | Unknown | - |
| PI3K/AKT/mTOR pathway | |||||
| PI3K/PIK3CA | Point mutation | 4%-12% | 0 | 0%-9% | [45,99-101] |
| AKT | Point mutation | 0 | 0 | 0-3 | [101,102] |
| mTOR | Increased activation | 47%-64% | 65% | 70% | [50,103] |
Tumors of the biliary tract often harbor activating mutations of growth factor receptors or over-produce ligand, thus effectively hijacking the receptor and its downstream signaling pathways. Such receptors include EGFR, HER2, VEGFR, and MET.
The prototypical member of the ErbB family of receptors, EGFR, consists of an extracellular ligand-binding domain and an intracellular domain with tyrosine kinase activity. Although EGFR is normally present within the plasma membrane of many types of normal cells, it is commonly over-expressed or over-activated in malignant cells. When active, it stimulates intracellular pathways that promote cell proliferation and angiogenesis, in addition to evasion of apoptosis[27]. Increased activation of EGFR is associated with impaired overall survival in patients with IHCC[28] and suggests the presence of lymph node metastases in IHCC[28]. Pooling together all subtypes of BTC, EGFR mutation is strongly predictive of increased mortality[29] (Table 2). Some of these mutations alter the tyrosine kinase domain in a manner that is known to confer responsivity to small molecule inhibitors[30].
Another receptor that is often implicated in biliary tract malignancies is HER2, a member of the ErbB family. Similar to EGFR, stimulation of HER2 activates intracellular pathways that promote cell growth, survival, and motility. Indeed, HER2 is a more potent activator of these pathways than other receptors, stimulating prolonged and more effective signaling[31]. While it would appear that HER2 mutation should translate into poorer outcomes, clinical studies indicate quite the opposite. Patients with EHCC tumors that over-express HER2 are less likely to have metastatic disease[28], suggesting that it may indicate a relatively favorable prognosis. Increased expression of this receptor is quite common in biliary tumors (Table 2).
Many biliary tract malignancies exploit VEGFR through increased production of its endogenous ligand, VEGF (Table 2)[28,32]. A potent stimulator of angiogenesis, VEGF signaling fuels the production of vascular endothelia and directs their migration to the developing vessels[33]. The new vasculature provides the tumor with more oxygen and nutrients than could be supplied by the pre-existing vascular supply, thereby promoting tumor growth. This expanded access to vasculature also facilitates the hematogenous spread of disease[28] and suggests increased probability of metastatic disease in patients with GBC[34]. Over-production of VEGF is observed in various subtypes of BTC (Table 2).
Recently, MET has been recognized for its role in biliary tract malignancies. Also referred to as the scatter receptor, its activation promotes the invasion (or scatter) of tumor cells by degrading intercellular junctions[35]. This is further underscored by MET expression being most concentrated at the invasive front of biliary tumors (Table 2)[36,37]. MET signaling also promotes angiogenesis by acting directly on the vascular endothelia and provokes the synthesis of VEGF and interleukin-8 (IL-8)[35]. Increased expression of c-MET within GBC generally indicates a poor prognosis[36].
In biliary cancers, the RAS-RAF-MEK-MAPK signaling axis is a crucial driver of growth of invasive carcinoma. Through a complex web of interactions with cell-cycle regulatory proteins such as p53, p16INK4A, p21CDKN2A, this pathway can propel progression through the cell cycle[38]. Crosstalk between this pathway and the PI3K-AKT signaling axis also enables these cells to evade apoptosis, often via BCL-2 signaling. The first component of this pathway, KRAS, is of particular importance due to its ability to cross-stimulate the PI3K-AKT pathway in addition to propagating a signal down the RAS-RAF-MEK-MAPK sequence. Despite its theoretical importance and relatively high frequency in biliary tumors (Table 2), KRAS mutation status has not been found to correlate with clinical outcomes in either CC or BTC as a whole[29,39]. The protein kinase BRAF, the direct downstream target of KRAS, is also mutated in a subset of biliary tract cancers (Table 2). The frequency of BRAF mutation demonstrates considerable geographic variability, with about 20% of biliary tumors harboring such mutations in Germany[39] and Greece[40] compared to less than 1% in Taiwan[29] and 0% in Chile[41]. Similar to KRAS, the mutation status of BRAF is not considered to be prognostic of clinical outcomes in BTC[39]. The third element of this pathway is mitogen-activated protein kinase-kinase (MEK). Although the frequency of MEK mutation has not been established, it is likely to be quite low.
The PI3K-AKT-mTOR signaling cascade demonstrates over-activity in nearly half of human cancers, including tumors of the biliary tract[42] (Table 2). Like RAS-RAF-MEK, this pathway is stimulated by receptors for EGF, VEGF, and hepatocyte growth factor. When active, it functions to protect neoplastic cells from apoptosis by stimulating BCL-2 and blocking the activity of caspase-9, the “death protease”[43]. This pathway also drives progression through the cell cycle and facilitates angiogenesis[39]. There is also evidence that PI3K-AKT-mTOR signaling regulates the production of matrix metalloproteinases, which are instrumental in local invasion[44].
The first component of this pathway is phosphoinositide-3-kinase (PI3K), which propagates its signal downstream through the activity of a phosphotidylinositol-3-kinase catalytic alpha (PIK3CA) domain. Activating mutations of this kinase in BTC and other human malignancies typically occur at “hotspots” located within exons 9 and 20[45,46]. PI3K acts directly to stimulate AKT, which is strictly conserved in all subtypes of BTC[47]. Downstream of AKT is the mammalian target of rapamycin (mTOR). In its activated state, mTOR works through a number of intermediates to facilitate key aspects of malignant behavior. It drives progression through the cell cycle by inhibiting the regulatory protein 4E-BP1[48]. Its other functions include accelerating cell proliferation and promoting angiogenesis via production of hypoxia inducible factor[48,49]. Although it is rarely mutated in BTC, hyperactivity of mTOR is associated with shortened overall survival in CC[50].
As described earlier, inflammation is often a key element in the pathogenesis of GBC, IHCC, and EHCC. The inflammatory cytokine, IL-6, is produced by malignant cholangiocytes and acts as a potent stimulator of CC growth and survival[51,52]. IL-6 functions predominantly through activation of STAT3, a transcription factor that promotes expression of the anti-apoptotic protein Mcl-1[52]. This induces resistance to TNF-related apoptosis-inducing ligand (TRAIL) mediated apoptosis, a major mechanism by which apoptosis is induced in normal cells. In a study using human cholangiocarcinoma cell lines, the multi-kinase inhibitor sorafenib was found to induce STAT3 dephosphorylation and reduce expression of Mcl-1[53]. Increased serum concentration of IL-6 is present in nearly all patients with cholangiocarcinoma[54], whereas 62% display constitutive activation of STAT3[55].
Another mediator of inflammation, COX2, is normally involved in the synthesis of inflammatory cytokines and growth factors. In neoplastic cells, however, it also promotes angiogenesis by increasing the expression of VEGF[56]. COX2 also contributes to local invasion via the synthesis of matrix metalloproteinases[57]. Clinically, COX2 over-expression is commonly observed in GBC and it suggests shortened overall survival as well as increased likelihood of metastases[57,58]. Research in animal models of CC has shown that COX2 inhibition suppresses tumor growth and induces apoptosis[59].
Many of the growth factors receptors and signaling pathways have become targeted for therapy in BTC. This targeted therapeutics, typically monoclonal antibodies or tyrosine kinase inhibitors, seek to block the signaling networks that promote tumor survival, growth, and invasion. The mechanism of action, efficacy, and safety of each targeted agent is discussed as follows. The sites of action for the targeted drugs are illustrated in Figure 2. In addition, the outcome data for the combination regimens of targeted agents investigated in clinical trials of BTC are described in Table 3.
| Targeted therapeutics | Targets | BTC Subtypes | Treatment regimen | TTP (mo) | PFS (mo) | OS (mo) | Ref. |
| Erlotinib | EGFR | GBC, IHCC, EHCC | Erlotinib | 2.6 | - | 7.5 | [60] |
| Erlotinib | EGFR | GBC, IHCC, EHCC | Erlotinib + GEMOX | - | 5.8 | 9.5 | [61] |
| Erlotinib | EGFR | GBC, IHCC, EHCC | Erlotinib + Bevacizumab | 4.4 | - | 9.9 | [62] |
| Cetuximab | EGFR | GBC, IHCC, EHCC | Cetuximab + GEMOX | - | 6.1 | 11.0 | [63] |
| Panitumumab | EGFR | IHCC, EHCC | Panitumumab + Gemcitabine + Irinotecan | - | 9.7 | 12.9 | [64] |
| Panitumumab | EGFR | IHCC, EHCC | Panitumumab + GEMOX + Capecitabine | - | 8.3 | 10.0 | [65] |
| Lapatinib | HER2, EGFR | GBC, IHCC, EHCC | Lapatinib | - | 1.8 | 5.2 | [67] |
| Lapatinib | HER2, EGFR | GBC, IHCC, EHCC | Lapatinib | - | 2.6 | 5.1 | [68] |
| Bevacizumab | VEGF | GBC, IHCC, EHCC | Bevacizumab + GEMOX | - | 7.0 | 12.7 | [71] |
| Selumetininb | MEK | GBC, IHCC, EHCC | Selumetinib | - | 3.7 | 9.8 | [73] |
| Sorafenib | Multiple TKI | GBC, IHCC, EHCC | Sorafenib | - | 2.3 | 4.4 | [76] |
| Sorafenib | Multiple TKI | GBC, IHCC, EHCC | Sorafenib + Gemcitabine + Cisplatin | - | 6.5 | 14.4 | [77] |
Erlotinib is a tyrosine kinase inhibitor (TKI) that prevents activation of EGFR through reversible blockade of the receptor’s ATP binding site. This results in the receptor being unable to activate its effector pathways such as RAS-RAF-MEK and PI3K-AKT-mTOR. Initial studies of erlotinib as a monotherapy demonstrated activity against BTC in general[60], but subsequent investigations of this agent in combination with other drugs have thus far been disappointing. Erlotinib has been studied as an adjunct to either GEMOX (gemcitabine and oxaliplatin)[61], or the VEGF inhibitor bevacizumab[62]. Aside from a slight improvement in progression free survival (PFS) when added to GEMOX in the treatment of CC, the addition of erlotinib fails to prolong survival beyond that which would be expected from GEMOX or bevacizumab alone in patients with CC or GBC. Despite this, erlotinib displayed activity as a monotherapy and is consistently well tolerated, thus suggesting that it may carve out a niche in treating BTC. At the present time, there are several studies of erlotinib in combination with both traditional chemotherapy and targeted agents underway.
Evidence regarding the use of monoclonal antibodies against EGFR has been similarly mixed. Cetuximab and panitumumab are monoclonal antibodies that selectively block the extracellular ligand-binding domain of the receptor, thereby preventing its activation (Figure 2). In combination with GEMOX, cetuximab was found to increase the rate of 4 mo progression-free survival in patients with BTC without significant increase in toxicity[63]. This response was short lived, however, and the final analysis failed to demonstrate a benefit of progression-free or overall survival. Panitumumab, on the other hand, has consistently produced a survival benefit in patients with BTC. A single arm study of 35 patients with CC that received treatment with gemcitabine, irinotecan, and panitumumab boasted a median PFS and overall survival (OS) of 9.7 mo and 12.9 mo, respectively-the strongest survival data of targeted agents in treating BTC to date[64]. A separate single arm trial of patients with IHCC or EHCC demonstrated similarly encouraging survival data when panitumumab was administered in tandem with GEMOX followed by capecitabine[65]. The results of these trials, while promising, were demonstrated in relatively small studies that both lacked a control group for comparison. As such, further confirmation is certainly necessary. In addition, future studies should seek to identify biomarkers, such as mutations in EGFR, KRAS, and BRAF, which predict response to cetuximab and panitumumab. Similar studies in colorectal adenocarcinoma discovered that KRAS mutational analysis allowed identification of a subset of patients who derive real benefit from these agents-a relationship that has not been well studied in BTC. Currently, clinical trials are ongoing that seek to evaluate panitumumab in combination with either GEMOX (NCT01206049, NCT01389414) or cisplatin plus gemcitabine (NCT01320254).
Despite the successes of HER2 inhibition in treating other malignancies such as breast and gastric carcinoma, blockade of this receptor seems to be of little benefit in biliary tract cancers. Lapatinib is a reversible TKI that prevents the activation of both EGFR and HER2[66] (Figure 2). To date, there have been two separate phase II trials of lapatinib monotherapy in patients with biliary malignancies, both of which failed to register a single objective response[67,68]. Although preclinical evidence suggests that HER2 blockade may enhance the efficacy of gemcitabine[69], this has yet to be investigated in a clinical setting. Given the lack of success of lapatinib thus far, it is quite possible that this will remain unexamined, as there are not currently any clinic trials using lapatinib in BTC. There has not yet been a clinical trial of the anti-HER2 monoclonal antibody trastuzumab in treating biliary tract tumors, although a single case report suggests that it may have activity against GBC[70].
Bevacizumab is a humanized monoclonal antibody that binds VEGF, preventing it from stimulating the VEGF receptor (Figure 2). This agent has been studied as an adjunct to combination chemotherapy with promising results. A single arm phase II study of bevacizumab with GEMOX demonstrated good efficacy against BTC, with median PFS of 7 mo and OS of 12.7 mo[71]. This regimen was well tolerated by the majority of patients, although one patient did suffer grade IV cardiac ischemia during the study. Among other patients, the most common grade III or IV events were neutropenia (20%), neuropathy (14%), and hypertension (14%). These results, while encouraging, should be approached with some skepticism as the known efficacy of GEMOX and absence of an internal control group makes it difficult to estimate the true benefit conferred by bevacizumab. In addition, it is necessary to further characterize the risks of such treatment. Further study of bevacizumab in combination with GEMOX and other chemotherapy regimens is certainly warranted.
One of the newer targeted therapeutics being clinically used is selumetinib, a small molecule inhibitor of the protein kinase MEK (Figure 2). Selumetinib binds selectively to an allosteric regulatory site on MEK, locking the protein into a configuration that renders it unable to utilize ATP[72]. In a clinical trial of selumetinib monotherapy, this drug demonstrated moderate anti-tumor activity against BTC in general, producing stable disease in approximately two-thirds of patients with a highly favorable toxicity profile[73]. The only grade IV toxicity observed was a single report of fatigue. This favorable balance of risk and benefit could be further modified by identifying subsets of patients who are most likely to benefit from selumetinib. Previous in vitro studies of human melanoma and colorectal cancer cell lines have suggested that tumors with activating mutations of BRAF are uniquely sensitive to MEK inhibition[74]. This association has not yet been investigated in BTC. At the present time, there are several additional trials of selumetinib, further investigating its activity when given in combination with cisplatin/gemcitabine (NCT01242605, NCT01949870).
Sorafenib is a multi-kinase inhibitor that blocks several targets both within malignant cells and their microenvironment (Figure 2). Targets of sorafenib include the pro-angiogenic receptors VEGFR and PDGFR, in addition to BRAF[75]. Despite inhibiting several important mediators of malignant behavior, sorafenib has failed to consistently improve outcomes in BTC. Previous trials have investigated sorafenib as a monotherapy[76], as well as in combination with gemcitabine plus cisplatin[77], and capecitabine plus oxaliplatin[78]. In these studies, the addition of sorafenib did not produce a survival benefit over the backbone regimen. Despite its shortcomings as a systemic therapy, sorafenib may fill a niche as a locoregional treatment. Preclinical studies of sorafenib-loaded biliary stents have shown strong activity against human cholangiocarcinoma cell lines[79] - a finding that should be further investigated in a clinical study.
A number of clinical trials of targeted therapeutics in the treatment of BTC are currently underway. These studies seek to investigate the use of targeted agents as a monotherapy, in combination with chemotherapy, and in tandem with other targeted drugs. Some of the ongoing clinical trials of targeted agents in BTC are listed in Table 4.
| Treatment | Phase | Date of completion | Sponsoring institutions | ClinicalTrials.gov identifier |
| GEMOX + Erlotinib | II | Dec., 2014 | New Mexico Cancer Care Alliance | NCT00832637 |
| Sorafenib + Erlotinib | II | May, 2014 | National Cancer Institute | NCT01093222 |
| GEMOX vs GEMOX + Cetuximab | II | Dec., 2014 | National Health Research Institutes, Taiwan | NCT01267344 |
| GEMOX + Panitumumab vs GEMOX + Bevacizumab | II | Aug., 2015 | Vejle Hospital | NCT01206049 |
| Cisplatin + Gemcitabine + Panitumumab vs Cisplatin + Gemcitabine | II | June, 2014 | Hannover Medical School | NCT01320254 |
| GEMOX + Panitumumab vs GEMOX alone | II | March, 2015 | Fondazione del Piemonte per l'Oncologia | NCT01389414 |
| Gemcitabine + Capecitabine + Bevacizumab | II | May, 2014 | Roswell Park Cancer Institute | NCT01007552 |
| Everolimus | II | June, 2014 | Ratchavithi Hospital | NCT01525719 |
| GEMOX + Sorafenib | I/II | Aug., 2014 | University of Miami Sylvester Comprehensive Cancer Center | NCT00955721 |
| Gemcitabine + Cisplatin + Sorafenib | II | March, 2014 | Memorial Sloan-Kettering Cancer Center | NCT00919061 |
| Gemcitabine + Cisplatin + Selumetinib | I/II | Jan., 2015 | AstraZeneca | NCT01242605 |
| Gemcitabine + Cisplatin + Selumetinib | I | Dec., 2014 | AstraZeneca | NCT01949870 |
| Trametinib | II | Feb., 2015 | GlaxoSmithKline | NCT01943864 |
| Trametinib vs 5-FU + Leucovorin vs Capecitabine | II | July, 2016 | National Cancer Institute | NCT02042443 |
| Celecoxib | IV | Dec., 2015 | Seoul National University Hospital | NCT01111591 |
| Regorafenib | II | Feb., 2018 | University of Pittsburgh | NCT02053376 |
| Regorafenib | II | Oct., 2018 | H. Lee Moffitt Cancer Center and Research Institute | NCT02115542 |
| Regorafenib + mGEMOX | I/II | April, 2019 | Institut du Cancer de Montpellier-Val d'Aurelle | NCT02386397 |
Cancers of the biliary tract are relatively uncommon malignancies associated with high mortality rate, and the options of systemic treatment are very limited. Though the molecular basis of biliary tract tumorigenesis is poorly understood, working models have been established to help elucidate the pathogenetic mechanisms underlying development and progression of BTC. The growth factors and receptors, signaling pathways, and transcription factors that promote biliary tumor cell survival, proliferation, and invasion have been identified. Targeted therapeutics that selectively inhibit these mediators of malignant neoplasia have demonstrated potential benefits in BTC. Further investigations regarding their efficacy and safety in treating this malignant disease are ongoing.
Despite the considerable progress that has been made towards molecularly profiling BTC, there remain considerable gaps in our understanding. The cause-and-effect relationship between the molecular changes and transformation of normal biliary epithelium to invasive malignancy is lacking. There remains an incomplete understanding of the interactions among the various signaling pathways and components that produce the cancer cell phenotypes. It is likely that these complex interactions hold the key to deepening our understanding of the basis of cancer heterogeneity and predicting susceptibility of individual tumors to specific treatments. This notion is supported by the evidence that combining multiple targeted agents may delay the development of resistance and improve efficacy[80].
In addition, a variety of therapeutic strategies have emerged that focus upon the epigenetic alterations that commonly occur in CC, such as hypermethylation of particular genes and altered microRNA expression[81]. Improved understanding of the epigenetic mechanisms in biliary tumorigenesis is expected to generate novel targets for therapy. Recent advances in immunotherapy may also provide new opportunities for treating BTC. Data from clinical trials of peptide-based and dendritic cell-based vaccines against BTC suggest that this treatment approach may confer therapeutic benefit[82,83]. Antibodies that block immune checkpoint molecules such as programmed death receptor (PD-1) or ligand (PD-L1) may enhance treatment response when combined with chemotherapy, targeted therapy, or vaccines in BTC. Furthermore, recent advances in molecular profiling of BTC may enable prediction of treatment response of individual patients to particular therapeutic agents[84-87]. Ultimately, by integrating targeted therapy with the molecular profiles of tumor, we hope to accomplish the goal of precision treatment of patients with malignant diseases of the biliary tract.
P- Reviewer: Kuramitsu Y, Tu H, Verbeke CS, Zhou SF S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | American Cancer Society. What are the key statistics about gallbladder cancer? Available from: http://www.cancer.org/cancer/gallbladdercancer/detailedguide/gallbladder-key-statistics. |
| 2. | Hezel AF, Zhu AX. Systemic therapy for biliary tract cancers. Oncologist. 2008;13:415-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 3. | Benson A, D’Angelica M, Abrams T, Are C, Bloomston PM, Chang D, Clary BM, Covey AM, Ensminger WD, Iyer R. Hepatobiliary Cancers: National Comprehensive Cancer Network. Available from: http://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. |
| 4. | Silva MA, Tekin K, Aytekin F, Bramhall SR, Buckels JA, Mirza DF. Surgery for hilar cholangiocarcinoma; a 10 year experience of a tertiary referral centre in the UK. Eur J Surg Oncol. 2005;31:533-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 3164] [Article Influence: 210.9] [Reference Citation Analysis (1)] |
| 6. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9957] [Article Influence: 995.7] [Reference Citation Analysis (0)] |
| 7. | Hamilton SR, Aaltonen LA. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System. 2000; Available from: http://www.iarc.fr/en/publications/pdfs-online/pat-gen/bb2/BB2.pdf. |
| 8. | Aishima S, Kubo Y, Tanaka Y, Oda Y. Histological features of precancerous and early cancerous lesions of biliary tract carcinoma. J Hepatobiliary Pancreat Sci. 2014;21:448-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Hezel AF, Deshpande V, Zhu AX. Genetics of biliary tract cancers and emerging targeted therapies. J Clin Oncol. 2010;28:3531-3540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 171] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 10. | Zen Y, Adsay NV, Bardadin K, Colombari R, Ferrell L, Haga H, Hong SM, Hytiroglou P, Klöppel G, Lauwers GY. Biliary intraepithelial neoplasia: an international interobserver agreement study and proposal for diagnostic criteria. Mod Pathol. 2007;20:701-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 198] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 11. | Kawanishi S, Hiraku Y, Pinlaor S, Ma N. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol Chem. 2006;387:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 330] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 12. | Aishima S, Mano Y, Tanaka Y, Kubo Y, Shirabe K, Maehara Y, Oda Y. Different roles of inducible nitric oxide synthase and cyclooxygenase-2 in carcinogenesis and metastasis of intrahepatic cholangiocarcinoma. Hum Pathol. 2013;44:1031-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Sia D, Tovar V, Moeini A, Llovet JM. Intrahepatic cholangiocarcinoma: pathogenesis and rationale for molecular therapies. Oncogene. 2013;32:4861-4870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 183] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 14. | Aishima S, Iguchi T, Fujita N, Taketomi A, Maehara Y, Tsuneyoshi M, Oda Y. Histological and immunohistological findings in biliary intraepithelial neoplasia arising from a background of chronic biliary disease compared with liver cirrhosis of non-biliary aetiology. Histopathology. 2011;59:867-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Hsu M, Sasaki M, Igarashi S, Sato Y, Nakanuma Y. KRAS and GNAS mutations and p53 overexpression in biliary intraepithelial neoplasia and intrahepatic cholangiocarcinomas. Cancer. 2013;119:1669-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Nakanishi Y, Zen Y, Kondo S, Itoh T, Itatsu K, Nakanuma Y. Expression of cell cycle-related molecules in biliary premalignant lesions: biliary intraepithelial neoplasia and biliary intraductal papillary neoplasm. Hum Pathol. 2008;39:1153-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Furukawa T, Klöppel G, Volkan Adsay N, Albores-Saavedra J, Fukushima N, Horii A, Hruban RH, Kato Y, Klimstra DS, Longnecker DS. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. 2005;447:794-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 474] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 18. | Schlitter AM, Born D, Bettstetter M, Specht K, Kim-Fuchs C, Riener MO, Jeliazkova P, Sipos B, Siveke JT, Terris B. Intraductal papillary neoplasms of the bile duct: stepwise progression to carcinoma involves common molecular pathways. Mod Pathol. 2014;27:73-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 19. | Barreto SG, Dutt A, Chaudhary A. A genetic model for gallbladder carcinogenesis and its dissemination. Ann Oncol. 2014;25:1086-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Tazuma S, Kajiyama G. Carcinogenesis of malignant lesions of the gall bladder. The impact of chronic inflammation and gallstones. Langenbecks Arch Surg. 2001;386:224-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Legan M, Luzar B, Marolt VF, Cor A. Expression of cyclooxygenase-2 is associated with p53 accumulation in premalignant and malignant gallbladder lesions. World J Gastroenterol. 2006;12:3425-3429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Wistuba II, Albores-Saavedra J. Genetic abnormalities involved in the pathogenesis of gallbladder carcinoma. J Hepatobiliary Pancreat Surg. 1999;6:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 61] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Wistuba II, Maitra A, Carrasco R, Tang M, Troncoso P, Minna JD, Gazdar AF. High resolution chromosome 3p, 8p, 9q and 22q allelotyping analysis in the pathogenesis of gallbladder carcinoma. Br J Cancer. 2002;87:432-440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Chaube A, Tewari M, Garbyal RS, Singh U, Shukla HS. Preliminary study of p53 and c-erbB-2 expression in gallbladder cancer in Indian patients manuscript id: 8962091628764582. BMC Cancer. 2006;6:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Chang HJ, Kim SW, Kim YT, Kim WH. Loss of heterozygosity in dysplasia and carcinoma of the gallbladder. Mod Pathol. 1999;12:763-769. [PubMed] |
| 26. | Wistuba II, Ashfaq R, Maitra A, Alvarez H, Riquelme E, Gazdar AF. Fragile histidine triad gene abnormalities in the pathogenesis of gallbladder carcinoma. Am J Pathol. 2002;160:2073-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Baselga J. Why the epidermal growth factor receptor? The rationale for cancer therapy. Oncologist. 2002;7 Suppl 4:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 344] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 28. | Yoshikawa D, Ojima H, Iwasaki M, Hiraoka N, Kosuge T, Kasai S, Hirohashi S, Shibata T. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer. 2008;98:418-425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 317] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 29. | Chang YT, Chang MC, Huang KW, Tung CC, Hsu C, Wong JM. Clinicopathological and prognostic significances of EGFR, KRAS and BRAF mutations in biliary tract carcinomas in Taiwan. J Gastroenterol Hepatol. 2014;29:1119-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Gwak GY, Yoon JH, Shin CM, Ahn YJ, Chung JK, Kim YA, Kim TY, Lee HS. Detection of response-predicting mutations in the kinase domain of the epidermal growth factor receptor gene in cholangiocarcinomas. J Cancer Res Clin Oncol. 2005;131:649-652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | Zaczek A, Brandt B, Bielawski KP. The diverse signaling network of EGFR, HER2, HER3 and HER4 tyrosine kinase receptors and the consequences for therapeutic approaches. Histol Histopathol. 2005;20:1005-1015. [PubMed] |
| 32. | Giatromanolaki A, Sivridis E, Simopoulos C, Polychronidis A, Gatter KC, Harris AL, Koukourakis MI. Hypoxia inducible factors 1alpha and 2alpha are associated with VEGF expression and angiogenesis in gallbladder carcinomas. J Surg Oncol. 2006;94:242-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | McMahon G. VEGF receptor signaling in tumor angiogenesis. Oncologist. 2000;5 Suppl 1:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 353] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 34. | Tian Y, Ding RY, Zhi YH, Guo RX, Wu SD. Analysis of p53 and vascular endothelial growth factor expression in human gallbladder carcinoma for the determination of tumor vascularity. World J Gastroenterol. 2006;12:415-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Maulik G, Shrikhande A, Kijima T, Ma PC, Morrison PT, Salgia R. Role of the hepatocyte growth factor receptor, c-Met, in oncogenesis and potential for therapeutic inhibition. Cytokine Growth Factor Rev. 2002;13:41-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 305] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 36. | Yang L, Guo T, Jiang S, Yang Z. Expression of ezrin, HGF and c-met and its clinicopathological significance in the benign and malignant lesions of the gallbladder. Hepatogastroenterology. 2012;59:1769-1775. [PubMed] |
| 37. | Moon WS, Park HS, Lee H, Pai R, Tarnawski AS, Kim KR, Jang KY. Co-expression of cox-2, C-met and beta-catenin in cells forming invasive front of gallbladder cancer. Cancer Res Treat. 2005;37:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263-1284. [PubMed] |
| 39. | Tannapfel A, Sommerer F, Benicke M, Katalinic A, Uhlmann D, Witzigmann H, Hauss J, Wittekind C. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut. 2003;52:706-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 287] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 40. | Saetta AA, Papanastasiou P, Michalopoulos NV, Gigelou F, Korkolopoulou P, Bei T, Patsouris E. Mutational analysis of BRAF in gallbladder carcinomas in association with K-ras and p53 mutations and microsatellite instability. Virchows Arch. 2004;445:179-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Goldenberg D, Rosenbaum E, Argani P, Wistuba II, Sidransky D, Thuluvath PJ, Hidalgo M, Califano J, Maitra A. The V599E BRAF mutation is uncommon in biliary tract cancers. Mod Pathol. 2004;17:1386-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Hansel DE, Rahman A, Hidalgo M, Thuluvath PJ, Lillemoe KD, Shulick R, Ku JL, Park JG, Miyazaki K, Ashfaq R. Identification of novel cellular targets in biliary tract cancers using global gene expression technology. Am J Pathol. 2003;163:217-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2297] [Cited by in RCA: 2291] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 44. | Thant AA, Nawa A, Kikkawa F, Ichigotani Y, Zhang Y, Sein TT, Amin AR, Hamaguchi M. Fibronectin activates matrix metalloproteinase-9 secretion via the MEK1-MAPK and the PI3K-Akt pathways in ovarian cancer cells. Clin Exp Metastasis. 2000;18:423-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 62] [Reference Citation Analysis (1)] |
| 45. | Riener MO, Bawohl M, Clavien PA, Jochum W. Rare PIK3CA hotspot mutations in carcinomas of the biliary tract. Genes Chromosomes Cancer. 2008;47:363-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 46. | Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497-5510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1330] [Cited by in RCA: 1448] [Article Influence: 85.2] [Reference Citation Analysis (0)] |
| 47. | Geynisman DM, Catenacci DV. Toward personalized treatment of advanced biliary tract cancers. Discov Med. 2012;14:41-57. [PubMed] |
| 48. | Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2855] [Cited by in RCA: 3175] [Article Influence: 211.7] [Reference Citation Analysis (0)] |
| 49. | Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1925] [Cited by in RCA: 2203] [Article Influence: 122.4] [Reference Citation Analysis (0)] |
| 50. | Herberger B, Puhalla H, Lehnert M, Wrba F, Novak S, Brandstetter A, Gruenberger B, Gruenberger T, Pirker R, Filipits M. Activated mammalian target of rapamycin is an adverse prognostic factor in patients with biliary tract adenocarcinoma. Clin Cancer Res. 2007;13:4795-4799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 51. | Okada K, Shimizu Y, Nambu S, Higuchi K, Watanabe A. Interleukin-6 functions as an autocrine growth factor in a cholangiocarcinoma cell line. J Gastroenterol Hepatol. 1994;9:462-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 52. | Kobayashi S, Werneburg NW, Bronk SF, Kaufmann SH, Gores GJ. Interleukin-6 contributes to Mcl-1 up-regulation and TRAIL resistance via an Akt-signaling pathway in cholangiocarcinoma cells. Gastroenterology. 2005;128:2054-2065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 184] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 53. | Sugiyama H, Onuki K, Ishige K, Baba N, Ueda T, Matsuda S, Takeuchi K, Onodera M, Nakanuma Y, Yamato M. Potent in vitro and in vivo antitumor activity of sorafenib against human intrahepatic cholangiocarcinoma cells. J Gastroenterol. 2011;46:779-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Goydos JS, Brumfield AM, Frezza E, Booth A, Lotze MT, Carty SE. Marked elevation of serum interleukin-6 in patients with cholangiocarcinoma: validation of utility as a clinical marker. Ann Surg. 1998;227:398-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 141] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 55. | Isomoto H, Mott JL, Kobayashi S, Werneburg NW, Bronk SF, Haan S, Gores GJ. Sustained IL-6/STAT-3 signaling in cholangiocarcinoma cells due to SOCS-3 epigenetic silencing. Gastroenterology. 2007;132:384-396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 56. | Zhi YH, Liu RS, Song MM, Tian Y, Long J, Tu W, Guo RX. Cyclooxygenase-2 promotes angiogenesis by increasing vascular endothelial growth factor and predicts prognosis in gallbladder carcinoma. World J Gastroenterol. 2005;11:3724-3728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 57. | Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997;94:3336-3340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 997] [Cited by in RCA: 1039] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 58. | Kim H, Song JY, Cho JY, Yoon YS, Han HS, Lee HS, Ryu HS, Choe G. Strong cytoplasmic expression of COX2 at the invasive fronts of gallbladder cancer is associated with a poor prognosis. J Clin Pathol. 2010;63:1048-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 59. | Zhang Z, Lai GH, Sirica AE. Celecoxib-induced apoptosis in rat cholangiocarcinoma cells mediated by Akt inactivation and Bax translocation. Hepatology. 2004;39:1028-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 60. | Philip PA, Mahoney MR, Allmer C, Thomas J, Pitot HC, Kim G, Donehower RC, Fitch T, Picus J, Erlichman C. Phase II study of erlotinib in patients with advanced biliary cancer. J Clin Oncol. 2006;24:3069-3074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 243] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 61. | Lee J, Park SH, Chang HM, Kim JS, Choi HJ, Lee MA, Jang JS, Jeung HC, Kang JH, Lee HW. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2012;13:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 346] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 62. | Lubner SJ, Mahoney MR, Kolesar JL, Loconte NK, Kim GP, Pitot HC, Philip PA, Picus J, Yong WP, Horvath L. Report of a multicenter phase II trial testing a combination of biweekly bevacizumab and daily erlotinib in patients with unresectable biliary cancer: a phase II Consortium study. J Clin Oncol. 2010;28:3491-3497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 207] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 63. | Malka D, Cervera P, Foulon S, Trarbach T, de la Fouchardière C, Boucher E, Fartoux L, Faivre S, Blanc JF, Viret F. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. Lancet Oncol. 2014;15:819-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 301] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 64. | Sohal DP, Mykulowycz K, Uehara T, Teitelbaum UR, Damjanov N, Giantonio BJ, Carberry M, Wissel P, Jacobs-Small M, O’Dwyer PJ. A phase II trial of gemcitabine, irinotecan and panitumumab in advanced cholangiocarcinoma. Ann Oncol. 2013;24:3061-3065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 65. | Jensen LH, Lindebjerg J, Ploen J, Hansen TF, Jakobsen A. Phase II marker-driven trial of panitumumab and chemotherapy in KRAS wild-type biliary tract cancer. Ann Oncol. 2012;23:2341-2346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 66. | Paul B, Trovato JA, Thompson J. Lapatinib: a dual tyrosine kinase inhibitor for metastatic breast cancer. Am J Health Syst Pharm. 2008;65:1703-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 67. | Ramanathan RK, Belani CP, Singh DA, Tanaka M, Lenz HJ, Yen Y, Kindler HL, Iqbal S, Longmate J, Mack PC. A phase II study of lapatinib in patients with advanced biliary tree and hepatocellular cancer. Cancer Chemother Pharmacol. 2009;64:777-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 228] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 68. | Peck J, Wei L, Zalupski M, O’Neil B, Villalona Calero M, Bekaii-Saab T. HER2/neu may not be an interesting target in biliary cancers: results of an early phase II study with lapatinib. Oncology. 2012;82:175-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 69. | Pignochino Y, Sarotto I, Peraldo-Neia C, Penachioni JY, Cavalloni G, Migliardi G, Casorzo L, Chiorino G, Risio M, Bardelli A. Targeting EGFR/HER2 pathways enhances the antiproliferative effect of gemcitabine in biliary tract and gallbladder carcinomas. BMC Cancer. 2010;10:631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 70. | Sorscher S. Marked radiographic response of a HER-2-overexpressing biliary cancer to trastuzumab. Cancer Manag Res. 2013;9:1-3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 71. | Zhu AX, Meyerhardt JA, Blaszkowsky LS, Kambadakone AR, Muzikansky A, Zheng H, Clark JW, Abrams TA, Chan JA, Enzinger PC. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: a phase 2 study. Lancet Oncol. 2010;11:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 224] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 72. | Yeh TC, Marsh V, Bernat BA, Ballard J, Colwell H, Evans RJ, Parry J, Smith D, Brandhuber BJ, Gross S. Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin Cancer Res. 2007;13:1576-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 450] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 73. | Bekaii-Saab T, Phelps MA, Li X, Saji M, Goff L, Kauh JS, O’Neil BH, Balsom S, Balint C, Liersemann R. Multi-institutional phase II study of selumetinib in patients with metastatic biliary cancers. J Clin Oncol. 2011;29:2357-2363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 239] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 74. | Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, Ye Q, Lobo JM, She Y, Osman I. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1117] [Cited by in RCA: 1052] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 75. | Adnane L, Trail PA, Taylor I, Wilhelm SM. Sorafenib (BAY 43-9006, Nexavar), a dual-action inhibitor that targets RAF/MEK/ERK pathway in tumor cells and tyrosine kinases VEGFR/PDGFR in tumor vasculature. Methods Enzymol. 2006;407:597-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 344] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 76. | Bengala C, Bertolini F, Malavasi N, Boni C, Aitini E, Dealis C, Zironi S, Depenni R, Fontana A, Del Giovane C. Sorafenib in patients with advanced biliary tract carcinoma: a phase II trial. Br J Cancer. 2010;102:68-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 176] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 77. | Lee JK, Capanu M, O’Reilly EM, Ma J, Chou JF, Shia J, Katz SS, Gansukh B, Reidy-Lagunes D, Segal NH. A phase II study of gemcitabine and cisplatin plus sorafenib in patients with advanced biliary adenocarcinomas. Br J Cancer. 2013;109:915-919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 78. | LoConte NK, Holen KD, Schelman WR, Mulkerin DL, Deming DA, Hernan HR, Traynor AM, Goggins T, Groteluschen D, Oettel K. A phase I study of sorafenib, oxaliplatin and 2 days of high dose capecitabine in advanced pancreatic and biliary tract cancer: a Wisconsin oncology network study. Invest New Drugs. 2013;31:943-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 79. | Kim do H, Jeong YI, Chung CW, Kim CH, Kwak TW, Lee HM, Kang DH. Preclinical evaluation of sorafenib-eluting stent for suppression of human cholangiocarcinoma cells. Int J Nanomedicine. 2013;8:1697-1711. [PubMed] |
| 80. | Bozic I, Reiter JG, Allen B, Antal T, Chatterjee K, Shah P, Moon YS, Yaqubie A, Kelly N, Le DT. Evolutionary dynamics of cancer in response to targeted combination therapy. Elife. 2013;2:e00747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 430] [Cited by in RCA: 445] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 81. | Isomoto H. Epigenetic alterations associated with cholangiocarcinoma (review). Oncol Rep. 2009;22:227-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 82. | Takahashi R, Yoshitomi M, Yutani S, Shirahama T, Noguchi M, Yamada A, Itoh K, Sasada T. Current status of immunotherapy for the treatment of biliary tract cancer. Hum Vaccin Immunother. 2013;9:1069-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 83. | Aruga A, Takeshita N, Kotera Y, Okuyama R, Matsushita N, Ohta T, Takeda K, Yamamoto M. Phase I clinical trial of multiple-peptide vaccination for patients with advanced biliary tract cancer. J Transl Med. 2014;12:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 84. | Leone F, Cavalloni G, Pignochino Y, Sarotto I, Ferraris R, Piacibello W, Venesio T, Capussotti L, Risio M, Aglietta M. Somatic mutations of epidermal growth factor receptor in bile duct and gallbladder carcinoma. Clin Cancer Res. 2006;12:1680-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 138] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 85. | Voss JS, Holtegaard LM, Kerr SE, Fritcher EG, Roberts LR, Gores GJ, Zhang J, Highsmith WE, Halling KC, Kipp BR. Molecular profiling of cholangiocarcinoma shows potential for targeted therapy treatment decisions. Hum Pathol. 2013;44:1216-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 86. | Rizvi S, Borad MJ, Patel T, Gores GJ. Cholangiocarcinoma: molecular pathways and therapeutic opportunities. Semin Liver Dis. 2014;34:456-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 87. | Sia D, Hoshida Y, Villanueva A, Roayaie S, Ferrer J, Tabak B, Peix J, Sole M, Tovar V, Alsinet C. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology. 2013;144:829-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 433] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 88. | Vauthey JN, Blumgart LH. Recent advances in the management of cholangiocarcinomas. Semin Liver Dis. 1994;14:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 210] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 89. | Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 923] [Cited by in RCA: 966] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 90. | Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1072] [Cited by in RCA: 1378] [Article Influence: 125.3] [Reference Citation Analysis (1)] |
| 91. | Carriaga MT, Henson DE. Liver, gallbladder, extrahepatic bile ducts, and pancreas. Cancer. 1995;75:171-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 92. | Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99-109. [PubMed] |
| 93. | Nakazawa K, Dobashi Y, Suzuki S, Fujii H, Takeda Y, Ooi A. Amplification and overexpression of c-erbB-2, epidermal growth factor receptor, and c-met in biliary tract cancers. J Pathol. 2005;206:356-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 190] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 94. | Terada T, Nakanuma Y, Sirica AE. Immunohistochemical demonstration of MET overexpression in human intrahepatic cholangiocarcinoma and in hepatolithiasis. Hum Pathol. 1998;29:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 112] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 95. | Rashid A, Ueki T, Gao YT, Houlihan PS, Wallace C, Wang BS, Shen MC, Deng J, Hsing AW. K-ras mutation, p53 overexpression, and microsatellite instability in biliary tract cancers: a population-based study in China. Clin Cancer Res. 2002;8:3156-3163. [PubMed] |
| 96. | Hanada K, Tsuchida A, Iwao T, Eguchi N, Sasaki T, Morinaka K, Matsubara K, Kawasaki Y, Yamamoto S, Kajiyama G. Gene mutations of K-ras in gallbladder mucosae and gallbladder carcinoma with an anomalous junction of the pancreaticobiliary duct. Am J Gastroenterol. 1999;94:1638-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 97. | Suto T, Habano W, Sugai T, Uesugi N, Funato O, Kanno S, Saito K, Nakamura Si. Aberrations of the K-ras, p53, and APC genes in extrahepatic bile duct cancer. J Surg Oncol. 2000;73:158-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 98. | Tannapfel A, Benicke M, Katalinic A, Uhlmann D, Köckerling F, Hauss J, Wittekind C. Frequency of p16(INK4A) alterations and K-ras mutations in intrahepatic cholangiocarcinoma of the liver. Gut. 2000;47:721-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 149] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 99. | Deshpande V, Nduaguba A, Zimmerman SM, Kehoe SM, Macconaill LE, Lauwers GY, Ferrone C, Bardeesy N, Zhu AX, Hezel AF. Mutational profiling reveals PIK3CA mutations in gallbladder carcinoma. BMC Cancer. 2011;11:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 100. | Weiss GA, Rossi MR, Khushalani NI, Lo K, Gibbs JF, Bharthuar A, Cowell JK, Iyer R. Evaluation of phosphatidylinositol-3-kinase catalytic subunit (PIK3CA) and epidermal growth factor receptor (EGFR) gene mutations in pancreaticobiliary adenocarcinoma. J Gastrointest Oncol. 2013;4:20-29. [PubMed] |
| 101. | Borger DR, Tanabe KK, Fan KC, Lopez HU, Fantin VR, Straley KS, Schenkein DP, Hezel AF, Ancukiewicz M, Liebman HM. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist. 2012;17:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 598] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 102. | Riener MO, Bawohl M, Clavien PA, Jochum W. Analysis of oncogenic AKT1 p.E17K mutation in carcinomas of the biliary tract and liver. Br J Cancer. 2008;99:836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 103. | Leal P, García P, Sandoval A, Letelier P, Brebi P, Ili C, Álvarez H, Tapia O, Roa JC. Immunohistochemical expression of phospho-mTOR is associated with poor prognosis in patients with gallbladder adenocarcinoma. Arch Pathol Lab Med. 2013;137:552-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |