Published online Oct 7, 2016. doi: 10.3748/wjg.v22.i37.8435
Peer-review started: June 16, 2016
First decision: July 12, 2016
Revised: July 21, 2016
Accepted: August 8, 2016
Article in press: August 8, 2016
Published online: October 7, 2016
Processing time: 109 Days and 2.2 Hours
A 58-year-old woman, who had undergone total gastrectomy for early gastric cancer 9 years previously, visited the outpatient clinic complaining of progressive difficulty in walking for 15 d. Laboratory examinations showed macrocytic anemia and a decreased serum vitamin B12 concentration and increased serum concentrations of folate, vitamin E and copper. Magnetic resonance imaging showed multifocal high signal intensities along the posterior column of the cervical and thoracic spinal cord. Treatment consisted of intramuscular injections of vitamin B12 for 7 d, which increased her serum level of vitamin B12 to normal. This was followed by weekly intramuscular injections of vitamin B12 for another 2 wk and oral administration of vitamin B12 three times per day. After comprehensive rehabilitation for 4 wk, she showed sufficient improvements in strength and ataxic gait, enabling her to return to her normal daily activities.
Core tip: Subacute combined degeneration (SCD) of the spinal cord is defined as the degeneration of the posterior and lateral columns of the spinal cord due to vitamin B12 deficiency. Because vitamin B12 deficiency can occur after total gastrectomy, so can SCD. Postoperative assessments have focused on the recurrence of gastric cancer after surgery, with fewer studies focusing on vitamin B12 deficiency, especially because SCD is a long-term complication. This report describes a patient who developed SCD after total gastrectomy for gastric cancer. SCD due to vitamin B12 deficiency should be suspected in patients showing gait disturbance after gastrectomy, even as a long-term complication.
- Citation: Hwang CH, Park DJ, Kim GY. Ataxic gait following total gastrectomy for gastric cancer. World J Gastroenterol 2016; 22(37): 8435-8438
- URL: https://www.wjgnet.com/1007-9327/full/v22/i37/8435.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i37.8435
Subacute combined degeneration (SCD) of the spinal cord is defined as the degeneration of the posterior and lateral columns of the spinal cord as a result of vitamin B12[1], vitamin E, and copper deficiencies[2]. Vitamin B12 deficiency, the most frequent cause of SCD, is associated with pernicious anemia, mal-absorption[3] of vitamin B12, intrinsic factor depletion, and exposure to nitrous oxide. Mal-absorption of vitamin B12 may also occur when intrinsic factor is not produced by the stomach, including after total or sub-total gastrectomy[4,5].
Vitamin B12 deficiency can cause a wide range of hematological, gastrointestinal, psychiatric, and neurological disorders. Neurologically, vitamin B12 deficiency can result in a multi-organ disorder involving the spinal cord, especially the dorsal and lateral columns, brain, and optic and peripheral nerves[6]. Abnormalities can include weakness in both the upper and lower extremities, changes in mentality, and decreased vision[6]. Although SCD associated vitamin B12 deficiency has been believed to be well researched disease since the first report by Knox and Delamore[7] in 1960, the connection between the SCD and gastrectomy is under-recognized, primarily because SCD usually occurs about 10 years after surgery[8,9]. This report describes a patient who developed SCD 9 years after total gastrectomy.
A 58-year-old woman, who had undergone total gastrectomy for early gastric cancer 9 years previously, visited the outpatient clinic complaining of progressive difficulty in walking for 15 d. Symptoms included allodynia (by light touch and pinprick, not by heat and cold), a tingling sensation along both lower limbs, ataxic gait disturbance, and decreased joint sense of the knees. Computerized neurocognitive tests showed reductions in memory and attention. Laboratory examinations showed macrocytic anemia and a decreased serum vitamin B12 concentration (< 30 pg/mL; normal range, 197-894 pg/mL) and increased serum concentrations of folate (32 ng/ml; normal range, 2.0-19.9 ng/mL), vitamin E (32 ng/mL; normal range, 5.5-17.0 ng/mL), and copper (157.99 mg/dL; normal range, 64-134 mg/dL). Her serum concentrations of homocysteine (5.80 nmol/mL; normal range, 3.36-20.44 nmol/mL) and methylmalonic acid (3.4 mg/d; normal range, 0.00-10.0 mg/d) 15 d after starting treatment were within normal ranges (Table 1).
| Variable | Value (normal range) |
| Vitamin B12 | < 30 pg/mL (197-894 pg/mL) |
| Folate | 32 ng/mL (2.0-19.9 ng/mL) |
| Vitamin E | 32 ng/mL (5.5-17.0 ng/mL) |
| Copper | 157.99 mg/dL (64-134 mg/dL) |
| Homocysteine | 5.80 nmol/mL (3.36-20.44 nmol/mL) |
| Methylmalonic acid | 3.4 mg/d (0.00-10.0 mg/d) |
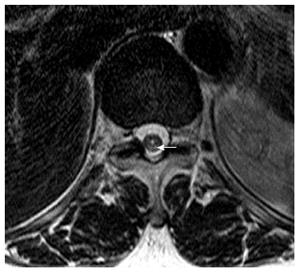
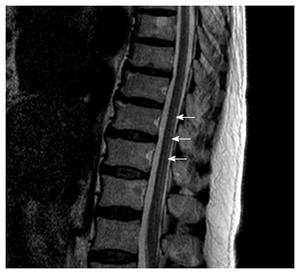
Magnetic resonance imaging (MRI) showed multifocal high signal intensities along the posterior column of the cervical and thoracic spinal cord (Figures 1 and 2)[10]. MRI of the brain showed minimal numbers of small vessel ischemic lesions in the white matter and mild ventriculomegaly. A nerve conduction study showed sensory-motor peripheral polyneuropathy, and a sensory evoked potential test showed a conduction delay from the bilateral posterior tibial sensory nerve to the somatosensory cortex. However, no definitive abnormalities were observed on the visual evoked potential test.
Treatment consisted of intramuscular injections of 5 mg/d vitamin B12 for 7 d, which increased her serum level of vitamin B12 to normal. This was followed by weekly intramuscular injections of 5 mg of vitamin B12 for another 2 wk and oral administration of 0.5 mg of vitamin B12 three times per day. After comprehensive rehabilitation for 4 wk, she showed sufficient improvements in strength and ataxic gait, enabling her to return to her normal daily activities as a housewife.
Vitamin B12 deficiency can result in a defective ability to synthesize myelin, resulting in the insidious development of demyelinating vitamin B12 deficiency neuropathy, often beginning in the peripheral nerves and progressing to involve the posterior and lateral columns of the spinal cord and the cerebellum[11]. The histo-pathological findings of SCD include patchy losses of myelin, especially in the dorsal and lateral columns of the spinal cord[1]. This can result in impaired proprioception of peripheral limbs and ataxic gait disturbance[3]. Symptoms of SCD can include abnormal sensations, including peripheral polyneuropathy, changes in mentality (i.e., nutritional encephalopathy), and decreased vision (i.e., optic neuritis).
Because SCD is caused by a vitamin B12 deficiency, it may occur after gastrectomy. However, its onset is usually slow and insidious. The likely sequence of events in the development of vitamin B12 deficiency due to its inadequate absorption includes early changes in bone marrow, occurring 1-2 years after inadequate absorption of vitamin B12 begins, followed by early myelin damage, occurring after 1.5-2 years[11]. Because severe myelin damage takes more than 3 years to develop, SCD first occurs many years after gastrectomy. Reports have described the development of neurological symptoms > 10 years[9] and even 37 years[8] after surgery.
Although postoperative follow-up focuses on the recurrence of gastric cancer after surgery, fewer studies have focused on vitamin B12 deficiency[12], especially because SCD is a long-term complication. Nutritional status after gastrectomy is important, even many years later. The increasing long-term survival of patients who undergo surgery for early gastric cancer, especially in the upper third of the stomach, emphasizes the importance of long-term metabolic consequences of total gastrectomy, especially vitamin B12 deficiency.
In conclusion, this report describes a patient who developed SCD 9 years after total gastrectomy for gastric cancer. SCD due to vitamin B12 deficiency should be suspected in patients with neurological disorders, including gait disturbance, after gastrectomy, even as a long-term complication.
We express our sincere thanks to Woo-Ram Koo, MD for his help.
A 58-year-old woman, who had undergone total gastrectomy for early gastric cancer 9 years previously, visited the outpatient clinic complaining of progressive difficulty in walking for 15 d.
Symptoms included allodynia (by light touch and pinprick, not by heat and cold), a tingling sensation along both lower limbs, ataxic gait disturbance, and decreased joint sense of the knees.
HIV-associated vacuolar myelopathy, vitamin E deficiency, copper deficiency, Friedreich’s ataxia, leukoencephalopathy
Laboratory examinations showed macrocytic anemia and a decreased serum vitamin B12 concentration and increased serum concentrations of folate, vitamin E, and copper.
Magnetic resonance imaging showed multifocal high signal intensities along the posterior column of the cervical and thoracic spinal cord.
Intramuscular injections of vitamin B12.
Vitamin B12 deficiency can result in a defective ability to synthesize myelin, resulting in the insidious development of demyelinating neuropathy, often beginning in the peripheral nerves and progressing to involve the posterior and lateral columns of the spinal cord and the cerebellum.
Subacute combined degeneration (SCD) of the spinal cord is defined as the degeneration of the posterior and lateral columns of the spinal cord due to vitamin B12 deficiency.
SCD due to vitamin B12 deficiency should be suspected in patients with neurological disorders after gastrectomy, even as a long-term complication.
This is a concise and well written paper about the possibility of SCD arising from vitamin B12 deficiency following a gastrectomy.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Fusaroli P, Kosugi S, Saglam S, Tovey FI S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
| 1. | Metz J. Cobalamin deficiency and the pathogenesis of nervous system disease. Annu Rev Nutr. 1992;12:59-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 79] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Kumar N, Gross JB, Ahlskog JE. Copper deficiency myelopathy produces a clinical picture like subacute combined degeneration. Neurology. 2004;63:33-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 193] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 3. | Reynolds E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006;5:949-960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 481] [Cited by in F6Publishing: 474] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 4. | Ardeman S, Chanarin I. Gastric intrinsic factor secretion after partial gastrectomy. Gut. 1966;7:217-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 22] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Kim HI, Hyung WJ, Song KJ, Choi SH, Kim CB, Noh SH. Oral vitamin B12 replacement: an effective treatment for vitamin B12 deficiency after total gastrectomy in gastric cancer patients. Ann Surg Oncol. 2011;18:3711-3717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Stabler SP, Allen RH, Savage DG, Lindenbaum J. Clinical spectrum and diagnosis of cobalamin deficiency. Blood. 1990;76:871-881. [PubMed] [Cited in This Article: ] |
| 7. | Knox JD, Delamore IW. Subacute combined degeneration of the cord after partial gastrectomy. Br Med J. 1960;2:1494-1496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Williams JA, Hall GS, Thompson AG, Cooke WT. Neurological disease after partial gastrectomy. Br Med J. 1969;3:210-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Olivarius Bde F, Roos D. Myelopathy following partial gastrectomy. Acta Neurol Scand. 1968;44:347-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Locatelli ER, Laureno R, Ballard P, Mark AS. MRI in vitamin B12 deficiency myelopathy. Can J Neurol Sci. 1999;26:60-63. [PubMed] [Cited in This Article: ] |
| 11. | Herbert V. Megaloblastic anemias. Lab Invest. 1985;52:3-19. [PubMed] [Cited in This Article: ] |
| 12. | Tovey FI, Hobsley M. Post-gastrectomy patients need to be followed up for 20-30 years. World J Gastroenterol. 2000;6:45-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 24] [Cited by in F6Publishing: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |