Published online Sep 7, 2016. doi: 10.3748/wjg.v22.i33.7486
Peer-review started: March 28, 2016
First decision: May 12, 2016
Revised: June 7, 2016
Accepted: July 20, 2016
Article in press: July 20, 2016
Published online: September 7, 2016
Processing time: 160 Days and 19 Hours
Hepatocellular carcinoma (HCC) is one of the most lethal malignancies in the world. Several signaling pathways, including the wingless/int-1 (Wnt) signaling pathway, have been shown to be commonly activated in HCC. The Wnt signaling pathway can be triggered via both catenin β1 (CTNNB1)-dependent (also known as “canonical”) and CTNNB1-independent (often referred to as “non-canonical”) pathways. Specifically, the canonical Wnt pathway is one of those most frequently reported in HCC. Aberrant regulation from three complexes (the cell-surface receptor complex, the cytoplasmic destruction complex and the nuclear CTNNB1/T-cell-specific transcription factor/lymphoid enhancer binding factor transcriptional complex) are all involved in HCC. Although the non-canonical Wnt pathway is rarely reported, two main non-canonical pathways, Wnt/planar cell polarity pathway and Wnt/Ca2+ pathway, participate in the regulation of hepatocarcinogenesis. Interestingly, the canonical Wnt pathway is antagonized by non-canonical Wnt signaling in HCC. Moreover, other signaling cascades have also been demonstrated to regulate the Wnt pathway through crosstalk in HCC pathogenesis. This review provides a perspective on the emerging evidence that the aberrant regulation of Wnt signaling is a critical mechanism for the development of HCC. Furthermore, crosstalk between different signaling pathways might be conducive to the development of novel molecular targets of HCC.
Core tip: The development of hepatocellular carcinoma (HCC) is regarded as a multistage process in which multiple genetic alterations are necessary. The wingless/int-1 (Wnt) pathway is a signaling mechanism that is frequently activated in HCC, especially the canonical Wnt pathway. Moreover, two main non-canonical pathways are also involved in the regulation of hepatocarcinogenesis. Interestingly, the non-canonical Wnt pathway could antagonize the canonical Wnt pathway in HCC. Crosstalk between other signaling pathways and the Wnt pathway has also been shown to promote tumorigenesis. This review highlights the details regarding the Wnt pathway in HCC, which might provide new potential targets for HCC prevention and therapy.
- Citation: Liu LJ, Xie SX, Chen YT, Xue JL, Zhang CJ, Zhu F. Aberrant regulation of Wnt signaling in hepatocellular carcinoma. World J Gastroenterol 2016; 22(33): 7486-7499
- URL: https://www.wjgnet.com/1007-9327/full/v22/i33/7486.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i33.7486
Hepatocellular carcinoma (HCC), the fifth most common malignancy in men and ninth among women worldwide, is the second leading cause of cancer deaths[1]. There are over half a million new cases diagnosed per year[1]. The pathogenesis of HCC involves a complex multistep process that derives from the accumulation of aberrant genetic and epigenetic changes and the dysregulation of certain signaling pathways[2-4], including the wingless/int-1 (Wnt) signaling pathway.
Wnt signaling plays crucial roles in the regulation of diverse processes, including cell proliferation, survival, migration and polarization, embryonic development, specification of cell fate, and self-renewal in stem cells[5]. Aberrant activation of Wnt signaling may contribute to numerous malignancies, such as colon cancer[6,7], gastric cancer[8], esophageal cancer[9], HCC[10], and others. Approximately 95% of observed HCC cases showed deregulation of the Wnt signaling cascade[11].
The Wnt signaling pathway is activated via both catenin beta 1 (CTNNB1)-dependent (also known as “canonical”) (Figure 1) and CTNNB1-independent (often referred to as “non-canonical”) pathways (Figure 2). It is suggested that abnormal regulation of the canonical Wnt signaling pathway is a major and early carcinogenic event[12]. The role of the non-canonical Wnt signaling pathway in HCC is also uncertain. Some studies have shown that non-canonical Wnt signaling is activated in HCC[11,13]. However, others have demonstrated that non-canonical Wnt ligands antagonized canonical Wnt signaling[14,15] and inhibited HCC cell proliferation and migration[15]. Here, we present the general molecular pathology of both the canonical and the non-canonical Wnt signaling pathways, and also the crosstalk between distinct signaling cascades and the Wnt signaling in HCC. This will provide potential clinical implications in finding effective therapeutic targets.
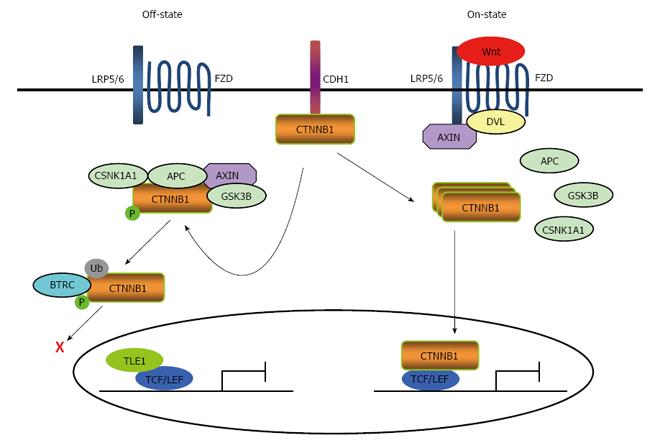
Wnt proteins, which are highly conserved in metazoan, are a family of 19 secreted glycoproteins[16]. The canonical Wnt signaling pathway is operated by stabilizing the transcriptional co-activator CTNNB1 through preventing its phosphorylation-dependent degradation. In a normal steady state, there are two pools for CTNNB1 in cells. One is known to interact with the cell adhesion molecule cadherin 1 (CDH1) at the cell-cell junction. The second is present in the destruction complex in cytoplasm, which is assembled by the scaffold proteins AXIN, the human tumor suppressor adenomatous polyposis coli (APC), glycogen synthase kinase 3 beta (GSK3B, also known as GSK3β), and casein kinase 1 alpha 1 (CSNK1A1)[17].
The second pool assembly maintains the low level of CTNNB1 in cytoplasm through phosphorylation of CTNNB1 at serine-45 (Ser45), Ser33, Ser37 and threonine-41 by CSNK1A1 and GSK3β in the destruction complex[18,19]. Phosphorylated CTNNB1 is subsequently recognized and ubiquitinated by the beta-transducin repeat containing E3 ubiquitin protein ligase (BTRC). BTRC is a component of an E3 ubiquitin ligase. This process results in the proteasomal degradation of the phosphorylated CTNNB1[20]. In the absence of nuclear CTNNB1 translocated from the cytoplasm, T-cell-specific transcription factor (TCF)/lymphoid enhancer binding factor (LEF) proteins act as transcriptional repressors by binding to Groucho/transducin-like enhancers of split 1 (TLE1) proteins. The proteins interact with histone deacetylases, leading to the transcriptional silencing of chromatin[21-23] (Figure 1). In conclusion, three complexes are involved in the dynamic activating event: (1) the cell-surface receptor complex; (2) the destruction complex in the cytoplasm; and (3) the CTNNB1/TCF/LEF transcriptional complex in the nucleus.
Functionally, the Wnt signaling cascade can be activated through several pathways via stimulation of distinct Wnt receptors[24,25]. In vertebrates, ten members of the frizzled class receptor (FZD) family of proteins comprise a series of seven-pass transmembrane receptors that have been identified as Wnt receptors[26]. In addition to FZD proteins, single-pass transmembrane proteins, such as the low density lipoprotein receptor-related protein (LRP) 5 and LRP6, have been reported to function as Wnt receptors in the canonical Wnt pathway[27,28]. The binding of Wnts to FZDs which form the cell-surface receptor complex promotes the binding of scaffold proteins, such as disheveled (DVL) proteins, to the FZD intracellular domains. This, in turn, induces the aggregation and phosphorylation of LRP6 and the translocation of AXIN[29,30].
Phosphorylated LRP6 also recruits AXIN to LRP6 on the plasma membrane. This allows AXIN to be inactivated, which then inhibits CTNNB1 phosphorylation. As a result, CTNNB1 succeeds to escape degradation, accumulate in the cytoplasm, and translocate to the nucleus[31]. In the nucleus, CTNNB1 interacts primarily with members of the TCF/LEF family of transcription factors and triggers the activation of multiple intracellular signaling cascades. This results in the regulation of various cellular functions, including gene expression, cell growth and differentiation (Figure 1).
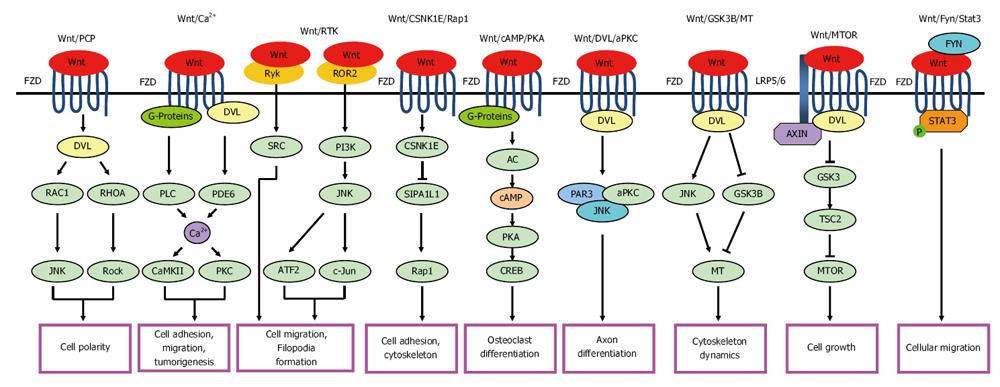
Non-canonical Wnt pathways are triggered by several possible mechanisms, which are all independent of CTNNB1-TCF/LEF transcriptional function (Figure 2). Among these non-canonical Wnt signaling pathways in vertebrates, the Wnt/planar cell polarity (PCP) pathway and the Wnt/Ca2+ pathway have been described in the most detail to date.
Wnt/PCP pathway: This pathway is often initiated by Wnt5a and Wnt11 through FZD and DVL. Next, small GTPases, such as ras-related C3 botulinum toxin substrate 2 (RAC1) and ras homolog family member A (RHOA), are activated by formation of the DVL-RAC1 complex and the DVL-dishevelled associated activator of morphogenesis 1-RHOA complex, respectively. The DVL-RAC1 complex then activates c-Jun N-terminal kinase (JNK). Finally, the triggered RHOA leads to Rho associated coiled-coil containing protein kinase (Rock) activation. This pathway regulates cell polarity in morphogenetic processes, including gastrulation and neural tube closure[32-34].
Wnt/Ca2+ pathway: In this pathway, Wnt5a/FZD2 activates phospholipase C via the heterotrimeric G proteins. This leads to the generation of dystroglycan 1 and inositol-trisphosphate 3, which increase the intracellular Ca2+ flux and levels. The Wnt/FZD complex also activates cyclic GMP-specific phosphodiesterase (PDE6), and then increases intracellular Ca2+ concentration through the depletion of cellular cGMP and the inactivation of cGMP-dependent protein kinase. Ca2+ activates calcium/calmodulin-dependent protein kinase II and protein kinase C, which in turn inhibits the canonical Wnt pathway. This leads to a wide variety of effects, such as tissue separation during gastrulation in vertebrates and ventral patterning in Xenopus species, as well as cell adhesion, migration, neurodegeneration, inflammation, and tumorigenesis[35-37].
Wnt/Receptor tyrosine kinases pathway: The receptor tyrosine kinases (RTK) of the receptor-like tyrosine kinase (RYK) and RAR related orphan receptor A (RORA/ROR2) families function as extracellular Wnt-binding domains and are implicated in Wnt signaling[38].
Wnt/RYK pathway: RYK binds to Wnt-induced repulsion of axons and mediates cell migration in Drosophila and mice. SRC kinase may act downstream of RYK in flies, wherein it was originally identified as Derailed[36].
Wnt/ROR2 pathway: Wnt5a/ROR2 activates the phosphatidylinositol 3-kinase (PI3K)-cell division cycle 42 (CDC42)-mitogen-activated protein kinase kinase 7-JNK pathway, resulting in the activation of activating transcription factor 2 and c-Jun and the expression of PAPC[39]. ROR2 also binds to the actin-binding protein filamin A and promotes filopodia formation[40,41]. The Wnt5a/ROR2 pathway inhibits the canonical Wnt pathway[36].
Wnt/casein kinase I epsilon/TERF2 interacting protein (Rap1) pathway: Wnt8 activates casein kinase I epsilon (CSNK1E), which enhances the phosphorylation and degradation of signal-induced proliferation-associated 1 like 1 (SIPA1L1), a Rap1-specific GTPase-activating protein. Rap1 is thereby activated in a CTNNB1-independent manner. Rap1 regulates actin cytoskeleton and/or cell adhesion during vertebrate gastrulation[42].
Wnt/cyclic adenosine monophosphate/protein kinase A pathway: Wnt1/Wnt7a activates the G protein and adenylyl cyclase (AC) to increase cyclic adenosine monophosphate (cAMP) levels, which in turn activates protein kinase A (PKA) and the transcription factor cAMP responsive element binding protein 1 (CREB) and myogenic gene expression[36]. Wnt3a can also trigger the cAMP/PKA pathway[43], which could suppress osteoclast differentiation by PKA-mediated phosphorylation and inactivate the nuclear factor of activated T-cells 1 (NFATC1)[44].
Wnt/DVL/atypical protein kinase C pathway: Wnt/FZD signaling induces atypical protein kinase C (aPKC) stabilization and activation via interaction with DVL. This pathway can promote axon differentiation mediated by the pulmonary adenoma resistance (PAR) 3/PAR6/aPKC complex[45].
Wnt/GSK3β/microtubule pathway: Wnt/DVL increases microtubule (MT) stability through the concomitant inhibition of GSK3β and activation of JNK. This pathway is involved in the modulation of cytoskeleton dynamics[46].
Wnt/mechanistic target of rapamycin pathway: Wnt activates mechanistic target of rapamycin (MTOR)-mediated translational regulation in tumorigenesis via inhibiting GSK3-dependent phosphorylation of tuberous sclerosis 2 (TSC2). DVL, AXIN and APC are all involved in it. Activation of the Wnt/MTOR pathway promotes cell growth and tumorigenesis[47].
Wnt/FYN (FYN proto-oncogene, Src family tyrosine kinase)/signal transducer and activator of transcription 3 pathway: Wnt5/FZD2 can be triggered by FYN through its SH2 domain. The activated complex subsequently recruits and phosphorylates signal transducer and activator of transcription 3 (STAT3) on Tyr705 and finally contributes to the epithelial-mesenchymal transition (EMT) program, cellular migration, and tumor metastasis[48].
The non-canonical Wnt pathways have also been shown to play critical roles, such as in axon differentiation, cell adhesion, cell proliferation, migration and tumorigenesis, in multi-cellular animals.
Increasing evidences have shown that the Wnt signaling pathway plays a vital role in HCC[49-51], especially the canonical Wnt pathway[52]. Additionally, two of the main non-canonical pathways (the Wnt/PCP pathway and the Wnt/Ca2+ pathway) are also involved in the development of HCC[15,53]. Interestingly, the canonical pathway is antagonized by non-canonical Wnt signaling in HCC[14,15]. Moreover, other signaling cascades have also been found to regulate the Wnt pathway through crosstalk[54-57].
Twenty percent to 90% of HCC cases exhibit CTNNB1 activation[58], which promotes cell growth and invasive capability in c-Myc/transforming growth factor alpha transgenic mice[59]. Simultaneous mutation of CTNNB1 and HRAS leads to 100% incidence of HCC in mice[60]. However, the molecular mechanism of this process is less clear. As described above, three complexes are involved in the dynamic activation of the canonical Wnt signaling pathway. We discuss this below and according to the regulation of the complexes, including the cell-surface receptor complex, the cytoplasmic destruction complex, and the nuclear CTNNB1/TCF/LEF transcriptional complex.
Dysregulation of the cell-surface receptor complex in HCC: Most of the Wnt ligands and their receptors have been reported to be highly expressed in HCC cell lines. Wnt3, Wnt9a and Wnt10b have displayed strong expression in most HCC cell lines, independent of differentiation status. Wnt2b, Wnt4, Wnt5a, Wnt5b and Wnt7b have been reported as overexpressed in poorly differentiated cell lines, while Wnt8b and Wnt9b have been reported as selectively overexpressed in well differentiated cell lines[14]. Almost all FZD receptors (except FZD9 and FZD10) and two co-receptors have also been reported as overexpressed in HCC cell lines[14]. Furthermore, LRP6 has also been found to be overexpressed in 38% of HCC[61].
It has been reported that HCV core protein correlates with increased Wnt1 and Wnt3a expression in HCC cell lines[62,63]. Interaction between Wnt3a and FZD7 could activate canonical Wnt signaling in different groups of HCC studies[64,65]. FZD7 overexpression has been shown to occur in early HCC and to contribute to enhanced tumor cell migration[65]. Overexpression of LRP6 has been shown to lead to hyperactivation of the canonical Wnt signaling pathway and to result in enhanced cell proliferation, cell migration, and invasion of human HCC[61,66].
Altered expressions of several secreted extracellular antagonists of Wnt ligands, such as secreted Frizzled-related proteins (SFRP), Wnt inhibitory factor-1 (WIF-1) and Dickkopf-related protein 3 (DKK-3), have been detected in HCC. Different SFRPs have been reported to bind with Wnt and thereby down-regulate their ability to activate FZD[67]. Numerous studies have shown that hypermethylation induces down-regulation of SFRPs (SFRP1 and SFRP5) and the subsequent activation of canonical Wnt signaling in HCC[68-71]. Down-regulation of WIF-1 and DKK-3 mediated by promoter methylation has also been reported to be a common event in HCC[72,73].
In addition, the scaffold protein DVL, which binds to the FZD intracellular domain to activate canonical Wnt signaling, has been shown to be up-regulated in a c-Myc/E2F transcription factor 1 transgenic mouse model of HCC[74]. The antagonisms of DVL, which negatively regulate the canonical Wnt signaling, including, dishevelled binding antagonist of beta catenin 2 (DACT2)[75], Prickle-1[76] and the human homologue of Dapper 1 (HDPR1)[77], are down-regulated in HCC.
Abrogation of the cytoplasmic destruction complex and CTNNB1 activation in HCC: Tumor formation is accelerated in HCC cells with active CTNNB1[78,79]. Nuclear accumulation of CTNNB1 is associated with proliferation in HCC cells, whereas CTNNB1 knockdown reduces migration and invasion of HCC cells[80]. However, the molecular mechanism for CTNNB1 activation in HCC still needs further investigation.
Researchers have reported that different degrees of mutations in CTNNB1 lead to the activation of CTNNB1. Reported mutations in exon 3 of CTNNB1 ranged from 2.8% to 44% in HCC cases[52,81-84]. The most frequently mutated site is Ser45, the principal site for phosphorylation mediated by CSNK1A1[85].
Since abnormal CTNNB1 redistribution has been reported in up to 90% of HCC cases[58], and the mutation rate of CTNNB1 in HCC is unmatched (2.8%-44%), it is implied that other mechanisms in addition to the CTNNB1 mutation are involved in the aberrant regulation of Wnt signaling in HCC. Mutations of the destruction complex members in HCC are also reported to contribute to hepatocarcinogenesis. AXIN1[52,84,86] and AXIN 2[86,87] mutations are observed in 5% to 54.2% and around 2.7%-37.5% of HCC cases, respectively. Conditional disruption of AXIN1 leads to the development of liver tumors in mice[88]. However, inactivating mutations of APC and GSK3β are quite rare in human HCC cases[86]. Nevertheless, deletion of APC showed significant connections to HCC through the activation of CTNNB1[89,90], while overexpression of wild-type APC in HCC cell lines reduces canonical Wnt signaling and results in growth suppression[91]. Elevated levels of inactive GSK3β are also observed in both human HCC tissues and mouse models of HCC harboring CTNNB1 accumulation[92-94]. Suppression of GSK3β activation by phosphorylation of Ser9 decreases CTNNB1 activity[92].
Actually, wild-type and mutated CTNNB1 transgenic mouse models indicate that abnormal CTNNB1 is not sufficient for carcinogenic transformation[95,96]. More factors are found in hepatocarcinogenesis mediated by Wnt signaling. Increasing evidences show that several etiologic factors which induce HCC might be involved in the aberrant regulation of canonical Wnt signaling, including HBV, HCV, and carcinogen exposure.
HBV-related HCC: A previous study has determined that mutations in AXIN1 were correlated with HBV-related HCC, whereas mutations in CTNNB1 were correlated with non HBV-related tumors[97]. This implies that mechanisms other than the mutation of CTNNB1 are involved in HBV-related HCC. However, a recent study has shown that genetic polymorphisms in CTNNB1 might affect tumor development and survival in HBV-related HCC[98]. The HBV x gene (HBx) up-regulates von Willebrand factor C and EGF domains (VWCE/URG11) and binds to APC to displace CTNNB1 from the destruction complex, which in turn activates CTNNB1[99]. Thereby, the canonical Wnt signaling is triggered[100].
HCV-related HCC: Inconsistent with the mechanism in HBV-related HCC, CTNNB1 mutation is shown to be approximately twice as significant in HCV-related HCC compared with other causes[101]. Additionally, more studies have proven the tumor-associated role of Wnt signaling in HCV-related HCC[102]. It has been reported that HCV up-regulates microRNA-155 (miR-155), which promotes the nuclear accumulation of CTNNB1 and an accompanying increase in downstream targets[103]. NS5A protein and core protein of HCV may increase CTNNB1 by activating PI3K and increasing the phosphorylation of GSK3β at Ser9[63,104,105].
Carcinogen exposure-induced HCC: The increased accumulation of CTNNB1 has been shown in around 45% of aflatoxin B1 (AFB1)-associated HCC cases[106]. Further studies indicate that AFB1 exposure might activate the canonical Wnt signaling pathway by down-regulating miR-34[107]. However, there is also research showing a totally distinct role of AFB1 on CTNNB1. The results suggest that AFB1 down-regulates CTNNB1 in HCC[108]. Moreover, HCC is induced in transgenic mice whose liver tumors showed conditional expression of CTNNB1 at 6 mo after diethylnitrosamine (DEN) exposure. However, no tumor is formed in wild-type mice at 6 mo after DEN exposure, indicating that overexpression of CTNNB1 accelerates tumorigenesis and progression to HCC following DEN exposure[109].
The human TCF/LEF family consists of four members: TCF-1, LEF-1, TCF-3, and TCF-4[51]. Increased LEF-1 in HCC tissues is associated with cyclin D1 overexpression in the nuclear compartment[110].
In our previous review, the role of aberrantly spliced TCF-4 variants in HCC was discussed[111]. Overexpression of TCF-4J in HCC cells up-regulates the expression level of hypoxia-inducible factor-alpha (HIF-2α) under hypoxia[112]. HIF-2α is capable of modulating TCF-4-mediated transcriptional activity by interacting with CTNNB1[113] and of up-regulating the expression of epidermal growth factor receptor (EGFR)[112]. HIF family proteins are involved in the development of HCC via promotion of angiogenesis[114]. EGFR promotes HCC cell proliferation and resistance to anti-cancer drugs[115]. In addition, a dominant-negative form of TCF-4 decreases the expression of c-Myc and cyclin D1 and suppresses the growth of BEL7402 cells[116]. Thirty-three percent of human HCC cases in which shorter survival periods are observed show c-Myc amplification[117]. Both N terminus of HCV NS5A and core protein increase TCF-4-dependent transcriptional activity and subsequently up-regulate the downstream targets, such as c-Myc and cyclin D1 in HCC[63,105].
Rare studies have demonstrated the role of non-canonical Wnt signaling in HCC. Several non-canonical Wnt signaling pathways have been proven to be involved in the regulation of hepatocarcinogenesis, such as the Wnt/PCP pathway[53] and the Wnt/Ca2+ pathway[15]. However, different factors induce distinct cell fates within the same pathways.
Cyclin-dependent kinase 14 (CDK14)[118], which is overexpressed in HCC tissues and confers cell invasive potential[119], can regulate cell cycle progression and cell proliferation by specifically interacting with members of cyclin proteins, such as cyclin D3 and cyclin Y[120,121]. Studies have demonstrated that CDK14 up-regulated DVL2 and Naked1 in non-canonical Wnt signaling in HCC by forming a direct complex with cyclin Y[53]. Exogenous overexpression of CDK14 and cyclin Y is also able to activate Rho GTPases (RHOA, RAC1, and CDC42) in HCC. The activated Rho GTPases result in the active formation of actin stress fibers[53], which lead to the modulation of cell motility[122].
Activation of non-canonical Wnt pathways, under some conditions, could suppress HCC. For instance, Wnt11 is reported to activate RHOA and Rock. Activated Rock subsequently inhibits RAC1 which contributes to decreased cell migration and motility in HCC[15].
In addition, the same Wnt ligand could also activate different non-canonical Wnt pathways in HCC. Exogenous overexpression of Wnt11 in HCC cells could also increase cytosolic free Ca2+, and subsequently activate PKC, which translocates from the cytoplasm to the plasma membrane[15].
Non-canonical Wnt pathway antagonizes the canonical pathway: It has been reported that the non-canonical Wnt pathway can inhibit canonical Wnt signaling in other cancers[123,124]. However, this phenomenon is rarely reported in HCC. Non-canonical Wnt ligand Wnt5a has been reported to inhibit TCF activation mediated by activated CTNNB1 in HCC cells[14]. Wnt11, which has been shown to inhibit HCC cell proliferation, antagonizes canonical Wnt signaling through phosphorylation of CTNNB1 and reduction of TCF-mediated transcriptional activity induced by activated PKC[15].
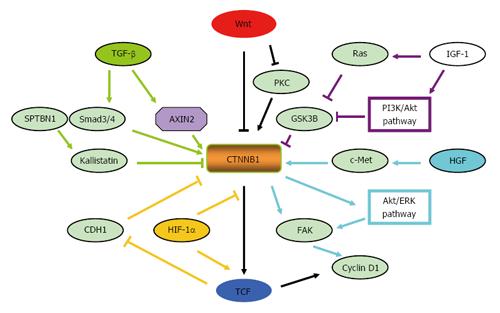
Other signaling pathways activate the Wnt signaling pathway: Accumulating evidences have demonstrated that activation of Wnt signaling can act in concert with other oncogenes, such as transforming growth factor beta (TGF-β)[54], hepatocyte growth factor (HGF)/c-Met pathway[55], HIF-1α/EMT pathway[125] and insulin/insulin-like growth factor-1 (IGF-1) pathway[57], to promote tumor progression (Figure 3).
Wnt pathway activation may be mediated by TGF-β[54,126,127]. Interactions between the TGF-β and CTNNB1 pathways are crucial for expression of CTNNB1 target genes in HCC[126]. The TGF-β effector Smad3 can promote the nuclear translocation of CTNNB1[128]. Recently, AXIN2 was reported to be up-regulated by TGF-β treatment in HCC cell lines, resulting in the activation of Wnt signaling[129]. βII-spectrin (SPTBN1), an adapter protein for Smad3/Smad4 complex formation during TGF-β signal transduction, is down-regulated in HCC cells[130]. Loss of SPTBN1 promotes tumor formation and invasion of HCC cells through suppressing Wnt inhibitor Kallistatin and subsequently promoting CTNNB1 dephosphorylation and nuclear localization[130].
Crosstalk between the HGF/c-Met pathway and the Wnt pathway might also contribute to the progression of HCC. C-Met, a tyrosine kinase receptor of HGF, which can be associated with CTNNB1 at the inner surface of the hepatocyte membrane[131], is often co-activated with CTNNB1 in HCC[132]. Co-delivery of c-Met and constitutively active CTNNB1 into mouse livers rapidly induced primary hepatic tumors[132-134]. Monga et al[131] have shown that HGF treatment could induce the dissociation of CTNNB1 from c-Met and its subsequent translocation to the nucleus via tyrosine phosphorylation. Further studies have determined that CTNNB1 enhanced c-Met-stimulated focal adhesion kinase (FAK) activation and synergistically induced the activation of the AKT/extracellular receptor kinase (ERK)-Cyclin D1 signaling pathway in a FAK kinase-dependent manner[55]. FAK is also reported to be overexpressed in HCC[135] and required for CTNNB1-induced Cyclin D1 expression in a kinase-independent way[55].
EMT is a process of phenotype shifting of cells associated with embryogenesis, inflammation, and cancer metastasis[136]. HIF-1α is reported to mediate the hypoxia-induced EMT via up-regulation of transcription effectors such as TCF-3, which suppress CDH1 expression[137]. HIF-1α can compete with TCF-4 to bind with CTNNB1 and form the HIF-1α/CTNNB1 complex. Increased HIF-1α activity, in turn, leads to decreased canonical Wnt signaling activity, and consequently enhanced hypoxia-induced EMT in HCC[56].
Studies have demonstrated that the presence of insulin/IGF-1 could result in CTNNB1 stabilization through inhibition of GSK3β activity, which stimulates TCF/LEF-dependent transcription activation[57]. The activation of PI3K/Akt and Ras might mediate the inactivation of GSK3β[57].
Development of HCC is a multistage process precipitated by multiple specific molecular alterations. Several signaling pathways take part in this process, such as the PI3K/Akt pathway, the Wnt pathway, the TGF-β pathway, the HGF/c-Met pathway, and the IGF pathway. Among these, aberrant regulation of the Wnt signaling pathway appears to be an important event leading to inappropriate transcription of various oncogenic target genes. Most importantly, Wnt signaling might play vital roles in hepatocarcinogenesis through crosstalking with several different signaling cascades (Figure 3). However, the molecular mechanisms of the crosstalk in HCC context still demand further investigation.
Considering that targeting the Wnt signaling pathway might provide potential therapeutics in the treatment of HCC, extra studies are still needed. Our recent study has shown that urolithin A, one of the intestinal metabolites of ellagic acid, exerts antiproliferative and antioxidant effects in HepG2 cells through the inhibition of canonical Wnt signaling[138]. In addition, several types of antagonisms, such as peptides, small synthetic compounds, and blocking antibodies, etc. could suppress tumor formation and metastasis by targeting different factors in the Wnt pathway. Some of them target the interaction between the Wnt ligand and the Fzd receptor; some target the destruction complex; the others could target the CTNNB1/TCF/LEF transcriptional complex[5,139-141]. Actually, some commercial medicines for other diseases have been found to modulate the Wnt signaling pathway. For instance, antipsychotic medications like dopamine D(2) receptor antagonist may treat symptoms of psychosis, at least in part, through modulation of the Wnt signaling pathway[142]. The non-steroidal anti-inflammatory drugs aspirin and indomethacin attenuate the canonical Wnt signaling pathway[143]. The cyclooxygenase-2 inhibitor celecoxib can inhibit CTNNB1-dependent transcription in colorectal cells[144] and suppress polyp formation in familial adenomatous polyposos patients[145]. However, these drugs may function through other signaling cascades either. Furthermore, there is still no inhibitor specific to the Wnt signaling pathway that have progressed to HCC clinical therapy. As a result, a better definition of the role of the Wnt pathway in the cascades network during hepatocarcinogenesis may reveal novel molecular targets which might be used for therapy of HCC.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Michalopoulos GK, Troncoso MF S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Wang CH
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20516] [Article Influence: 2051.6] [Reference Citation Analysis (20)] |
| 2. | Tornesello ML, Buonaguro L, Izzo F, Buonaguro FM. Molecular alterations in hepatocellular carcinoma associated with hepatitis B and hepatitis C infections. Oncotarget. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Kawai-Kitahata F, Asahina Y, Tanaka S, Kakinuma S, Murakawa M, Nitta S, Watanabe T, Otani S, Taniguchi M, Goto F. Comprehensive analyses of mutations and hepatitis B virus integration in hepatocellular carcinoma with clinicopathological features. J Gastroenterol. 2016;51:473-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 4. | Revill K, Wang T, Lachenmayer A, Kojima K, Harrington A, Li J, Hoshida Y, Llovet JM, Powers S. Genome-wide methylation analysis and epigenetic unmasking identify tumor suppressor genes in hepatocellular carcinoma. Gastroenterology. 2013;145:1424-35.e1-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 175] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 5. | Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13:11-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1384] [Cited by in RCA: 1528] [Article Influence: 127.3] [Reference Citation Analysis (0)] |
| 6. | Vermeulen L, De Sousa E Melo F, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1301] [Cited by in RCA: 1446] [Article Influence: 96.4] [Reference Citation Analysis (1)] |
| 7. | Dong HJ, Jang GB, Lee HY, Park SR, Kim JY, Nam JS, Hong IS. The Wnt/β-catenin signaling/Id2 cascade mediates the effects of hypoxia on the hierarchy of colorectal-cancer stem cells. Sci Rep. 2016;6:22966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Yong X, Tang B, Xiao YF, Xie R, Qin Y, Luo G, Hu CJ, Dong H, Yang SM. Helicobacter pylori upregulates Nanog and Oct4 via Wnt/β-catenin signaling pathway to promote cancer stem cell-like properties in human gastric cancer. Cancer Lett. 2016;374:292-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 9. | Zhang M, Linghu E, Zhan Q, He T, Cao B, Brock MV, Herman JG, Xiang R, Guo M. Methylation of DACT2 accelerates esophageal cancer development by activating Wnt signaling. Oncotarget. 2016;7:17957-17969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Chen J, Rajasekaran M, Xia H, Zhang X, Kong SN, Sekar K, Seshachalam VP, Deivasigamani A, Goh BK, Ooi LL. The microtubule-associated protein PRC1 promotes early recurrence of hepatocellular carcinoma in association with the Wnt/β-catenin signalling pathway. Gut. 2016;65:1522-1534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 179] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 11. | Bengochea A, de Souza MM, Lefrançois L, Le Roux E, Galy O, Chemin I, Kim M, Wands JR, Trepo C, Hainaut P. Common dysregulation of Wnt/Frizzled receptor elements in human hepatocellular carcinoma. Br J Cancer. 2008;99:143-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 12. | Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29:4989-5005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 680] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 13. | Cetin GO, Toylu A, Atabey N, Sercan Z, Sakizli M. Downregulation of VANGL1 inhibits cellular invasion rather than cell motility in hepatocellular carcinoma cells without stimulation. Genet Test Mol Biomarkers. 2015;19:283-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Yuzugullu H, Benhaj K, Ozturk N, Senturk S, Celik E, Toylu A, Tasdemir N, Yilmaz M, Erdal E, Akcali KC. Canonical Wnt signaling is antagonized by noncanonical Wnt5a in hepatocellular carcinoma cells. Mol Cancer. 2009;8:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 169] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 15. | Toyama T, Lee HC, Koga H, Wands JR, Kim M. Noncanonical Wnt11 inhibits hepatocellular carcinoma cell proliferation and migration. Mol Cancer Res. 2010;8:254-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Papkoff J, Brown AM, Varmus HE. The int-1 proto-oncogene products are glycoproteins that appear to enter the secretory pathway. Mol Cell Biol. 1987;7:3978-3984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 89] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Kimelman D, Xu W. beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene. 2006;25:7482-7491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 503] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 18. | Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M, Ben-Neriah Y, Alkalay I. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 2002;16:1066-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 576] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 19. | Behrens J, Jerchow BA, Würtele M, Grimm J, Asbrand C, Wirtz R, Kühl M, Wedlich D, Birchmeier W. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 959] [Cited by in RCA: 976] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 20. | Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797-3804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1938] [Cited by in RCA: 2036] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 21. | Jennings BH, Ish-Horowicz D. The Groucho/TLE/Grg family of transcriptional co-repressors. Genome Biol. 2008;9:205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 22. | Cadigan KM. TCFs and Wnt/β-catenin signaling: more than one way to throw the switch. Curr Top Dev Biol. 2012;98:1-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Chen G, Fernandez J, Mische S, Courey AJ. A functional interaction between the histone deacetylase Rpd3 and the corepressor groucho in Drosophila development. Genes Dev. 1999;13:2218-2230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 336] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 24. | Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 862] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 25. | James RG, Conrad WH, Moon RT. Beta-catenin-independent Wnt pathways: signals, core proteins, and effectors. Methods Mol Biol. 2008;468:131-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Wang HY, Liu T, Malbon CC. Structure-function analysis of Frizzleds. Cell Signal. 2006;18:934-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 27. | Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429-22433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 969] [Cited by in RCA: 1030] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 28. | Kikuchi A, Yamamoto H, Kishida S. Multiplicity of the interactions of Wnt proteins and their receptors. Cell Signal. 2007;19:659-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 206] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 29. | Schwarz-Romond T, Fiedler M, Shibata N, Butler PJ, Kikuchi A, Higuchi Y, Bienz M. The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization. Nat Struct Mol Biol. 2007;14:484-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 342] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 30. | Schwarz-Romond T, Metcalfe C, Bienz M. Dynamic recruitment of axin by Dishevelled protein assemblies. J Cell Sci. 2007;120:2402-2412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 183] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 31. | Kikuchi A, Yamamoto H, Sato A. Selective activation mechanisms of Wnt signaling pathways. Trends Cell Biol. 2009;19:119-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 194] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 32. | Axelrod JD. Progress and challenges in understanding planar cell polarity signaling. Semin Cell Dev Biol. 2009;20:964-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 33. | Simons M, Mlodzik M. Planar cell polarity signaling: from fly development to human disease. Annu Rev Genet. 2008;42:517-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 465] [Cited by in RCA: 431] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 34. | Gómez-Orte E, Sáenz-Narciso B, Moreno S, Cabello J. Multiple functions of the noncanonical Wnt pathway. Trends Genet. 2013;29:545-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 35. | Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol. 2012;13:767-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 1136] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 36. | Semenov MV, Habas R, Macdonald BT, He X. SnapShot: Noncanonical Wnt Signaling Pathways. Cell. 2007;131:1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 261] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 37. | Kohn AD, Moon RT. Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium. 2005;38:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 559] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 38. | Green J, Nusse R, van Amerongen R. The role of Ryk and Ror receptor tyrosine kinases in Wnt signal transduction. Cold Spring Harb Perspect Biol. 2014;6:pii: a009175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 39. | Schambony A, Wedlich D. Wnt-5A/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Dev Cell. 2007;12:779-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 227] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 40. | Nomachi A, Nishita M, Inaba D, Enomoto M, Hamasaki M, Minami Y. Receptor tyrosine kinase Ror2 mediates Wnt5a-induced polarized cell migration by activating c-Jun N-terminal kinase via actin-binding protein filamin A. J Biol Chem. 2008;283:27973-27981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 41. | Nishita M, Yoo SK, Nomachi A, Kani S, Sougawa N, Ohta Y, Takada S, Kikuchi A, Minami Y. Filopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5a-induced cell migration. J Cell Biol. 2006;175:555-562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 173] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 42. | Tsai IC, Amack JD, Gao ZH, Band V, Yost HJ, Virshup DM. A Wnt-CKIvarepsilon-Rap1 pathway regulates gastrulation by modulating SIPA1L1, a Rap GTPase activating protein. Dev Cell. 2007;12:335-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 43. | Weivoda MM, Ruan M, Hachfeld CM, Pederson L, Howe A, Davey RA, Zajac JD, Kobayashi Y, Williams BO, Westendorf JJ. Wnt Signaling Inhibits Osteoclast Differentiation by Activating Canonical and Noncanonical cAMP/PKA Pathways. J Bone Miner Res. 2016;31:65-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 44. | Yoon SH, Ryu Jy, Lee Y, Lee ZH, Kim HH. Adenylate cyclase and calmodulin-dependent kinase have opposite effects on osteoclastogenesis by regulating the PKA-NFATc1 pathway. J Bone Miner Res. 2011;26:1217-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Zhang X, Zhu J, Yang GY, Wang QJ, Qian L, Chen YM, Chen F, Tao Y, Hu HS, Wang T. Dishevelled promotes axon differentiation by regulating atypical protein kinase C. Nat Cell Biol. 2007;9:743-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 176] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 46. | Ciani L, Salinas PC. c-Jun N-terminal kinase (JNK) cooperates with Gsk3beta to regulate Dishevelled-mediated microtubule stability. BMC Cell Biol. 2007;8:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 47. | Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1111] [Cited by in RCA: 1052] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 48. | Gujral TS, Chan M, Peshkin L, Sorger PK, Kirschner MW, MacBeath G. A noncanonical Frizzled2 pathway regulates epithelial-mesenchymal transition and metastasis. Cell. 2014;159:844-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 281] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 49. | Peng YY, He YH, Chen C, Xu T, Li L, Ni MM, Meng XM, Huang C, Li J. NLRC5 regulates cell proliferation, migration and invasion in hepatocellular carcinoma by targeting the Wnt/β-catenin signaling pathway. Cancer Lett. 2016;376:10-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 50. | Bai XL, Zhang Q, Ye LY, Liang F, Sun X, Chen Y, Hu QD, Fu QH, Su W, Chen Z. Myocyte enhancer factor 2C regulation of hepatocellular carcinoma via vascular endothelial growth factor and Wnt/β-catenin signaling. Oncogene. 2015;34:4089-4097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 51. | Lee HC, Kim M, Wands JR. Wnt/Frizzled signaling in hepatocellular carcinoma. Front Biosci. 2006;11:1901-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 52. | White BD, Chien AJ, Dawson DW. Dysregulation of Wnt/β-catenin signaling in gastrointestinal cancers. Gastroenterology. 2012;142:219-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 394] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 53. | Sun T, Co NN, Wong N. PFTK1 interacts with cyclin Y to activate non-canonical Wnt signaling in hepatocellular carcinoma. Biochem Biophys Res Commun. 2014;449:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 54. | Fischer AN, Fuchs E, Mikula M, Huber H, Beug H, Mikulits W. PDGF essentially links TGF-beta signaling to nuclear beta-catenin accumulation in hepatocellular carcinoma progression. Oncogene. 2007;26:3395-3405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 113] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 55. | Shang N, Arteaga M, Zaidi A, Stauffer J, Cotler SJ, Zeleznik-Le NJ, Zhang J, Qiu W. FAK is required for c-Met/β-catenin-driven hepatocarcinogenesis. Hepatology. 2015;61:214-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 56. | Zhang Q, Bai X, Chen W, Ma T, Hu Q, Liang C, Xie S, Chen C, Hu L, Xu S. Wnt/β-catenin signaling enhances hypoxia-induced epithelial-mesenchymal transition in hepatocellular carcinoma via crosstalk with hif-1α signaling. Carcinogenesis. 2013;34:962-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 194] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 57. | Desbois-Mouthon C, Cadoret A, Blivet-Van Eggelpoël MJ, Bertrand F, Cherqui G, Perret C, Capeau J. Insulin and IGF-1 stimulate the beta-catenin pathway through two signalling cascades involving GSK-3beta inhibition and Ras activation. Oncogene. 2001;20:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 248] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 58. | Thompson MD, Monga SP. WNT/beta-catenin signaling in liver health and disease. Hepatology. 2007;45:1298-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 394] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 59. | Calvisi DF, Factor VM, Loi R, Thorgeirsson SS. Activation of beta-catenin during hepatocarcinogenesis in transgenic mouse models: relationship to phenotype and tumor grade. Cancer Res. 2001;61:2085-2091. [PubMed] |
| 60. | Harada N, Oshima H, Katoh M, Tamai Y, Oshima M, Taketo MM. Hepatocarcinogenesis in mice with beta-catenin and Ha-ras gene mutations. Cancer Res. 2004;64:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 150] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 61. | Wong Yin Chi B. Significance of LRP6 coreceptor upregulation in the aberrant activation of Wnt signaling in hepatocellular carcinoma. Pokfulam, Hong Kong: The University of Hong Kong 2008; . |
| 62. | Fukutomi T, Zhou Y, Kawai S, Eguchi H, Wands JR, Li J. Hepatitis C virus core protein stimulates hepatocyte growth: correlation with upregulation of wnt-1 expression. Hepatology. 2005;41:1096-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 63. | Liu J, Ding X, Tang J, Cao Y, Hu P, Zhou F, Shan X, Cai X, Chen Q, Ling N. Enhancement of canonical Wnt/β-catenin signaling activity by HCV core protein promotes cell growth of hepatocellular carcinoma cells. PLoS One. 2011;6:e27496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 64. | Kim M, Lee HC, Tsedensodnom O, Hartley R, Lim YS, Yu E, Merle P, Wands JR. Functional interaction between Wnt3 and Frizzled-7 leads to activation of the Wnt/beta-catenin signaling pathway in hepatocellular carcinoma cells. J Hepatol. 2008;48:780-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 65. | Merle P, de la Monte S, Kim M, Herrmann M, Tanaka S, Von Dem Bussche A, Kew MC, Trepo C, Wands JR. Functional consequences of frizzled-7 receptor overexpression in human hepatocellular carcinoma. Gastroenterology. 2004;127:1110-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 182] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 66. | Tung EK, Wong BY, Yau TO, Ng IO. Upregulation of the Wnt co-receptor LRP6 promotes hepatocarcinogenesis and enhances cell invasion. PLoS One. 2012;7:e36565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 67. | Jones SE, Jomary C. Secreted Frizzled-related proteins: searching for relationships and patterns. Bioessays. 2002;24:811-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 322] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 68. | Shih YL, Hsieh CB, Lai HC, Yan MD, Hsieh TY, Chao YC, Lin YW. SFRP1 suppressed hepatoma cells growth through Wnt canonical signaling pathway. Int J Cancer. 2007;121:1028-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 69. | Shih YL, Shyu RY, Hsieh CB, Lai HC, Liu KY, Chu TY, Lin YW. Promoter methylation of the secreted frizzled-related protein 1 gene SFRP1 is frequent in hepatocellular carcinoma. Cancer. 2006;107:579-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 70. | Xie Q, Chen L, Shan X, Shan X, Tang J, Zhou F, Chen Q, Quan H, Nie D, Zhang W. Epigenetic silencing of SFRP1 and SFRP5 by hepatitis B virus X protein enhances hepatoma cell tumorigenicity through Wnt signaling pathway. Int J Cancer. 2014;135:635-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 71. | Quan H, Zhou F, Nie D, Chen Q, Cai X, Shan X, Zhou Z, Chen K, Huang A, Li S. Hepatitis C virus core protein epigenetically silences SFRP1 and enhances HCC aggressiveness by inducing epithelial-mesenchymal transition. Oncogene. 2014;33:2826-2835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 72. | Huang L, Li MX, Wang L, Li BK, Chen GH, He LR, Xu L, Yuan YF. Prognostic value of Wnt inhibitory factor-1 expression in hepatocellular carcinoma that is independent of gene methylation. Tumour Biol. 2011;32:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 73. | Ding Z, Qian YB, Zhu LX, Xiong QR. Promoter methylation and mRNA expression of DKK-3 and WIF-1 in hepatocellular carcinoma. World J Gastroenterol. 2009;15:2595-2601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 74. | Calvisi DF, Conner EA, Ladu S, Lemmer ER, Factor VM, Thorgeirsson SS. Activation of the canonical Wnt/beta-catenin pathway confers growth advantages in c-Myc/E2F1 transgenic mouse model of liver cancer. J Hepatol. 2005;42:842-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 75. | Zhang X, Yang Y, Liu X, Herman JG, Brock MV, Licchesi JD, Yue W, Pei X, Guo M. Epigenetic regulation of the Wnt signaling inhibitor DACT2 in human hepatocellular carcinoma. Epigenetics. 2013;8:373-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 76. | Chan DW, Chan CY, Yam JW, Ching YP, Ng IO. Prickle-1 negatively regulates Wnt/beta-catenin pathway by promoting Dishevelled ubiquitination/degradation in liver cancer. Gastroenterology. 2006;131:1218-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 77. | Yau TO, Chan CY, Chan KL, Lee MF, Wong CM, Fan ST, Ng IO. HDPR1, a novel inhibitor of the WNT/beta-catenin signaling, is frequently downregulated in hepatocellular carcinoma: involvement of methylation-mediated gene silencing. Oncogene. 2005;24:1607-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 78. | Wege H, Heim D, Lütgehetmann M, Dierlamm J, Lohse AW, Brümmendorf TH. Forced activation of β-catenin signaling supports the transformation of hTERT-immortalized human fetal hepatocytes. Mol Cancer Res. 2011;9:1222-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 79. | Rebouissou S, Franconi A, Calderaro J, Letouzé E, Imbeaud S, Pilati C, Nault JC, Couchy G, Laurent A, Balabaud C. Genotype-phenotype correlation of CTNNB1 mutations reveals different ß-catenin activity associated with liver tumor progression. Hepatology. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 238] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 80. | Lai TY, Su CC, Kuo WW, Yeh YL, Kuo WH, Tsai FJ, Tsai CH, Weng YJ, Huang CY, Chen LM. β-catenin plays a key role in metastasis of human hepatocellular carcinoma. Oncol Rep. 2011;26:415-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 81. | Cui J, Zhou X, Liu Y, Tang Z, Romeih M. Wnt signaling in hepatocellular carcinoma: analysis of mutation and expression of beta-catenin, T-cell factor-4 and glycogen synthase kinase 3-beta genes. J Gastroenterol Hepatol. 2003;18:280-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 82. | Lu LC, Shao YY, Lee YH, Hsieh MS, Hsiao CH, Lin HH, Kao HF, Ma YY, Yen FC, Cheng AL. β-catenin (CTNNB1) mutations are not associated with prognosis in advanced hepatocellular carcinoma. Oncology. 2014;87:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 83. | Ding X, Yang Y, Han B, Du C, Xu N, Huang H, Cai T, Zhang A, Han ZG, Zhou W. Transcriptomic characterization of hepatocellular carcinoma with CTNNB1 mutation. PLoS One. 2014;9:e95307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 84. | Kim YD, Park CH, Kim HS, Choi SK, Rew JS, Kim DY, Koh YS, Jeung KW, Lee KH, Lee JS. Genetic alterations of Wnt signaling pathway-associated genes in hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:110-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 85. | Takigawa Y, Brown AM. Wnt signaling in liver cancer. Curr Drug Targets. 2008;9:1013-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 86. | Ishizaki Y, Ikeda S, Fujimori M, Shimizu Y, Kurihara T, Itamoto T, Kikuchi A, Okajima M, Asahara T. Immunohistochemical analysis and mutational analyses of beta-catenin, Axin family and APC genes in hepatocellular carcinomas. Int J Oncol. 2004;24:1077-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 87. | Taniguchi K, Roberts LR, Aderca IN, Dong X, Qian C, Murphy LM, Nagorney DM, Burgart LJ, Roche PC, Smith DI. Mutational spectrum of beta-catenin, AXIN1, and AXIN2 in hepatocellular carcinomas and hepatoblastomas. Oncogene. 2002;21:4863-4871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 351] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 88. | Feng GJ, Cotta W, Wei XQ, Poetz O, Evans R, Jardé T, Reed K, Meniel V, Williams GT, Clarke AR. Conditional disruption of Axin1 leads to development of liver tumors in mice. Gastroenterology. 2012;143:1650-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 89. | Colnot S, Decaens T, Niwa-Kawakita M, Godard C, Hamard G, Kahn A, Giovannini M, Perret C. Liver-targeted disruption of Apc in mice activates beta-catenin signaling and leads to hepatocellular carcinomas. Proc Natl Acad Sci USA. 2004;101:17216-17221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 253] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 90. | Haramis AP, Hurlstone A, van der Velden Y, Begthel H, van den Born M, Offerhaus GJ, Clevers HC. Adenomatous polyposis coli-deficient zebrafish are susceptible to digestive tract neoplasia. EMBO Rep. 2006;7:444-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 91. | Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet. 2000;24:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 703] [Cited by in RCA: 723] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 92. | Desbois-Mouthon C, Blivet-Van Eggelpoël MJ, Beurel E, Boissan M, Delélo R, Cadoret A, Capeau J. Dysregulation of glycogen synthase kinase-3beta signaling in hepatocellular carcinoma cells. Hepatology. 2002;36:1528-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 93. | Ban KC, Singh H, Krishnan R, Seow HF. GSK-3beta phosphorylation and alteration of beta-catenin in hepatocellular carcinoma. Cancer Lett. 2003;199:201-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 94. | Monga SP. Role of Wnt/β-catenin signaling in liver metabolism and cancer. Int J Biochem Cell Biol. 2011;43:1021-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 95. | Cadoret A, Ovejero C, Saadi-Kheddouci S, Souil E, Fabre M, Romagnolo B, Kahn A, Perret C. Hepatomegaly in transgenic mice expressing an oncogenic form of beta-catenin. Cancer Res. 2001;61:3245-3249. [PubMed] |
| 96. | Tan X, Apte U, Micsenyi A, Kotsagrelos E, Luo JH, Ranganathan S, Monga DK, Bell A, Michalopoulos GK, Monga SP. Epidermal growth factor receptor: a novel target of the Wnt/beta-catenin pathway in liver. Gastroenterology. 2005;129:285-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 176] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 97. | Laurent-Puig P, Legoix P, Bluteau O, Belghiti J, Franco D, Binot F, Monges G, Thomas G, Bioulac-Sage P, Zucman-Rossi J. Genetic alterations associated with hepatocellular carcinomas define distinct pathways of hepatocarcinogenesis. Gastroenterology. 2001;120:1763-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 429] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 98. | Kim SS, Cho HJ, Lee HY, Park JH, Noh CK, Shin SJ, Lee KM, Yoo BM, Lee KJ, Cho SW. Genetic polymorphisms in the Wnt/β-catenin pathway genes as predictors of tumor development and survival in patients with hepatitis B virus-associated hepatocellular carcinoma. Clin Biochem. 2016;49:792-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 99. | Lian Z, Liu J, Li L, Li X, Clayton M, Wu MC, Wang HY, Arbuthnot P, Kew M, Fan D. Enhanced cell survival of Hep3B cells by the hepatitis B x antigen effector, URG11, is associated with upregulation of beta-catenin. Hepatology. 2006;43:415-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 100. | Hsieh A, Kim HS, Lim SO, Yu DY, Jung G. Hepatitis B viral X protein interacts with tumor suppressor adenomatous polyposis coli to activate Wnt/β-catenin signaling. Cancer Lett. 2011;300:162-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 101. | Huang H, Fujii H, Sankila A, Mahler-Araujo BM, Matsuda M, Cathomas G, Ohgaki H. Beta-catenin mutations are frequent in human hepatocellular carcinomas associated with hepatitis C virus infection. Am J Pathol. 1999;155:1795-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 205] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 102. | Rogacki K, Kasprzak A, Stępiński A. Alterations of Wnt/β-catenin signaling pathway in hepatocellular carcinomas associated with hepatitis C virus. Pol J Pathol. 2015;66:9-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 103. | Zhang Y, Wei W, Cheng N, Wang K, Li B, Jiang X, Sun S. Hepatitis C virus-induced up-regulation of microRNA-155 promotes hepatocarcinogenesis by activating Wnt signaling. Hepatology. 2012;56:1631-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 257] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 104. | Street A, Macdonald A, McCormick C, Harris M. Hepatitis C virus NS5A-mediated activation of phosphoinositide 3-kinase results in stabilization of cellular beta-catenin and stimulation of beta-catenin-responsive transcription. J Virol. 2005;79:5006-5016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 105. | Park CY, Choi SH, Kang SM, Kang JI, Ahn BY, Kim H, Jung G, Choi KY, Hwang SB. Nonstructural 5A protein activates beta-catenin signaling cascades: implication of hepatitis C virus-induced liver pathogenesis. J Hepatol. 2009;51:853-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 106. | Devereux TR, Stern MC, Flake GP, Yu MC, Zhang ZQ, London SJ, Taylor JA. CTNNB1 mutations and beta-catenin protein accumulation in human hepatocellular carcinomas associated with high exposure to aflatoxin B1. Mol Carcinog. 2001;31:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 107. | Zhu L, Gao J, Huang K, Luo Y, Zhang B, Xu W. miR-34a screened by miRNA profiling negatively regulates Wnt/β-catenin signaling pathway in Aflatoxin B1 induced hepatotoxicity. Sci Rep. 2015;5:16732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 108. | Fang Y, Feng Y, Wu T, Srinivas S, Yang W, Fan J, Yang C, Wang S. Aflatoxin B1 negatively regulates Wnt/β-catenin signaling pathway through activating miR-33a. PLoS One. 2013;8:e73004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 109. | Nejak-Bowen KN, Thompson MD, Singh S, Bowen WC, Dar MJ, Khillan J, Dai C, Monga SP. Accelerated liver regeneration and hepatocarcinogenesis in mice overexpressing serine-45 mutant beta-catenin. Hepatology. 2010;51:1603-1613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 110. | Schmitt-Graeff A, Ertelt-Heitzmann V, Allgaier HP, Olschewski M, Nitschke R, Haxelmans S, Koelble K, Behrens J, Blum HE. Coordinated expression of cyclin D1 and LEF-1/TCF transcription factor is restricted to a subset of hepatocellular carcinoma. Liver Int. 2005;25:839-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 111. | Liu L, Xie S, Zhang C, Zhu F. Aberrant regulation of alternative pre-mRNA splicing in hepatocellular carcinoma. Crit Rev Eukaryot Gene Expr. 2014;24:133-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 112. | Koga H, Tsedensodnom O, Tomimaru Y, Walker EJ, Lee HC, Kim KM, Yano H, Wands JR, Kim M. Loss of the SxxSS motif in a human T-cell factor-4 isoform confers hypoxia resistance to liver cancer: an oncogenic switch in Wnt signaling. PLoS One. 2012;7:e39981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 113. | Choi H, Chun YS, Kim TY, Park JW. HIF-2alpha enhances beta-catenin/TCF-driven transcription by interacting with beta-catenin. Cancer Res. 2010;70:10101-10111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 114. | Nakamura K, Zen Y, Sato Y, Kozaka K, Matsui O, Harada K, Nakanuma Y. Vascular endothelial growth factor, its receptor Flk-1, and hypoxia inducible factor-1alpha are involved in malignant transformation in dysplastic nodules of the liver. Hum Pathol. 2007;38:1532-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 115. | Ezzoukhry Z, Louandre C, Trécherel E, Godin C, Chauffert B, Dupont S, Diouf M, Barbare JC, Mazière JC, Galmiche A. EGFR activation is a potential determinant of primary resistance of hepatocellular carcinoma cells to sorafenib. Int J Cancer. 2012;131:2961-2969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 116. | Zhao DH, Hong JJ, Guo SY, Yang RL, Yuan J, Wen CY, Zhou KY, Li CJ. Aberrant expression and function of TCF4 in the proliferation of hepatocellular carcinoma cell line BEL-7402. Cell Res. 2004;14:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 117. | Kawate S, Fukusato T, Ohwada S, Watanuki A, Morishita Y. Amplification of c-myc in hepatocellular carcinoma: correlation with clinicopathologic features, proliferative activity and p53 overexpression. Oncology. 1999;57:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 115] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 118. | Yang T, Chen JY. Identification and cellular localization of human PFTAIRE1. Gene. 2001;267:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 119. | Pang EY, Bai AH, To KF, Sy SM, Wong NL, Lai PB, Squire JA, Wong N. Identification of PFTAIRE protein kinase 1, a novel cell division cycle-2 related gene, in the motile phenotype of hepatocellular carcinoma cells. Hepatology. 2007;46:436-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 120. | Shu F, Lv S, Qin Y, Ma X, Wang X, Peng X, Luo Y, Xu BE, Sun X, Wu J. Functional characterization of human PFTK1 as a cyclin-dependent kinase. Proc Natl Acad Sci USA. 2007;104:9248-9253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 121. | Jiang M, Gao Y, Yang T, Zhu X, Chen J. Cyclin Y, a novel membrane-associated cyclin, interacts with PFTK1. FEBS Lett. 2009;583:2171-2178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 122. | Hotulainen P, Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J Cell Biol. 2006;173:383-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 687] [Cited by in RCA: 670] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 123. | Maye P, Zheng J, Li L, Wu D. Multiple mechanisms for Wnt11-mediated repression of the canonical Wnt signaling pathway. J Biol Chem. 2004;279:24659-24665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 124. | Kremenevskaja N, von Wasielewski R, Rao AS, Schöfl C, Andersson T, Brabant G. Wnt-5a has tumor suppressor activity in thyroid carcinoma. Oncogene. 2005;24:2144-2154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 205] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 125. | Jiang YG, Luo Y, He DL, Li X, Zhang LL, Peng T, Li MC, Lin YH. Role of Wnt/beta-catenin signaling pathway in epithelial-mesenchymal transition of human prostate cancer induced by hypoxia-inducible factor-1alpha. Int J Urol. 2007;14:1034-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 171] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 126. | Hoshida Y, Nijman SM, Kobayashi M, Chan JA, Brunet JP, Chiang DY, Villanueva A, Newell P, Ikeda K, Hashimoto M. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009;69:7385-7392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 956] [Cited by in RCA: 944] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 127. | Lachenmayer A, Alsinet C, Savic R, Cabellos L, Toffanin S, Hoshida Y, Villanueva A, Minguez B, Newell P, Tsai HW. Wnt-pathway activation in two molecular classes of hepatocellular carcinoma and experimental modulation by sorafenib. Clin Cancer Res. 2012;18:4997-5007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 244] [Article Influence: 18.8] [Reference Citation Analysis (1)] |
| 128. | Jian H, Shen X, Liu I, Semenov M, He X, Wang XF. Smad3-dependent nuclear translocation of beta-catenin is required for TGF-beta1-induced proliferation of bone marrow-derived adult human mesenchymal stem cells. Genes Dev. 2006;20:666-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 248] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 129. | Steinway SN, Zañudo JG, Ding W, Rountree CB, Feith DJ, Loughran TP, Albert R. Network modeling of TGFβ signaling in hepatocellular carcinoma epithelial-to-mesenchymal transition reveals joint sonic hedgehog and Wnt pathway activation. Cancer Res. 2014;74:5963-5977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 184] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 130. | Zhi X, Lin L, Yang S, Bhuvaneshwar K, Wang H, Gusev Y, Lee MH, Kallakury B, Shivapurkar N, Cahn K. βII-Spectrin (SPTBN1) suppresses progression of hepatocellular carcinoma and Wnt signaling by regulation of Wnt inhibitor kallistatin. Hepatology. 2015;61:598-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 131. | Monga SP, Mars WM, Pediaditakis P, Bell A, Mulé K, Bowen WC, Wang X, Zarnegar R, Michalopoulos GK. Hepatocyte growth factor induces Wnt-independent nuclear translocation of beta-catenin after Met-beta-catenin dissociation in hepatocytes. Cancer Res. 2002;62:2064-2071. [PubMed] |
| 132. | Tward AD, Jones KD, Yant S, Cheung ST, Fan ST, Chen X, Kay MA, Wang R, Bishop JM. Distinct pathways of genomic progression to benign and malignant tumors of the liver. Proc Natl Acad Sci USA. 2007;104:14771-14776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 173] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 133. | Stauffer JK, Scarzello AJ, Andersen JB, De Kluyver RL, Back TC, Weiss JM, Thorgeirsson SS, Wiltrout RH. Coactivation of AKT and β-catenin in mice rapidly induces formation of lipogenic liver tumors. Cancer Res. 2011;71:2718-2727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 134. | Patil MA, Lee SA, Macias E, Lam ET, Xu C, Jones KD, Ho C, Rodriguez-Puebla M, Chen X. Role of cyclin D1 as a mediator of c-Met- and beta-catenin-induced hepatocarcinogenesis. Cancer Res. 2009;69:253-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 135. | Fujii T, Koshikawa K, Nomoto S, Okochi O, Kaneko T, Inoue S, Yatabe Y, Takeda S, Nakao A. Focal adhesion kinase is overexpressed in hepatocellular carcinoma and can be served as an independent prognostic factor. J Hepatol. 2004;41:104-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 136. | Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6575] [Cited by in RCA: 7915] [Article Influence: 494.7] [Reference Citation Analysis (0)] |
| 137. | Lu X, Kang Y. Hypoxia and hypoxia-inducible factors: master regulators of metastasis. Clin Cancer Res. 2010;16:5928-5935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 535] [Cited by in RCA: 527] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 138. | Wang Y, Qiu Z, Zhou B, Liu C, Ruan J, Yan Q, Liao J, Zhu F. In vitro antiproliferative and antioxidant effects of urolithin A, the colonic metabolite of ellagic acid, on hepatocellular carcinomas HepG2 cells. Toxicol In Vitro. 2015;29:1107-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 139. | Kim JY, Lee HY, Park KK, Choi YK, Nam JS, Hong IS. CWP232228 targets liver cancer stem cells through Wnt/β-catenin signaling: a novel therapeutic approach for liver cancer treatment. Oncotarget. 2016;7:20395-20409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 140. | Ma L, Wang X, Jia T, Wei W, Chua MS, So S. Tankyrase inhibitors attenuate WNT/β-catenin signaling and inhibit growth of hepatocellular carcinoma cells. Oncotarget. 2015;6:25390-25401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 141. | Wei W, Chua MS, Grepper S, So SK. Soluble Frizzled-7 receptor inhibits Wnt signaling and sensitizes hepatocellular carcinoma cells towards doxorubicin. Mol Cancer. 2011;10:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 142. | Freyberg Z, Ferrando SJ, Javitch JA. Roles of the Akt/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. Am J Psychiatry. 2010;167:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 235] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 143. | Dihlmann S, Siermann A, von Knebel Doeberitz M. The nonsteroidal anti-inflammatory drugs aspirin and indomethacin attenuate beta-catenin/TCF-4 signaling. Oncogene. 2001;20:645-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 155] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 144. | Tuynman JB, Vermeulen L, Boon EM, Kemper K, Zwinderman AH, Peppelenbosch MP, Richel DJ. Cyclooxygenase-2 inhibition inhibits c-Met kinase activity and Wnt activity in colon cancer. Cancer Res. 2008;68:1213-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 145. | Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y, Fujimura T. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946-1952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1815] [Cited by in RCA: 1715] [Article Influence: 68.6] [Reference Citation Analysis (0)] |