Published online Sep 7, 2016. doi: 10.3748/wjg.v22.i33.7453
Peer-review started: March 28, 2016
First decision: May 12, 2016
Revised: May 30, 2016
Accepted: June 15, 2016
Article in press: June 15, 2016
Published online: September 7, 2016
Processing time: 160 Days and 15.4 Hours
Gastrointestinal perforations, which need to be managed quickly, are associated with high morbidity and mortality. Treatments used to close these perforations range from surgery to endoscopic therapy. Nowadays, with the development of new devices and techniques, endoscopic therapy is becoming more popular. However, there are different indications and clinical efficacies between different methods, because of the diverse properties of endoscopic devices and techniques. Successful management also depends on other factors, such as the precise location of the perforation, its size and the length of time between the occurrence and diagnosis. In this study, we performed a comprehensive review of various devices and introduced the different techniques that are considered effective to treat gastrointestinal perforations. In addition, we focused on the different methods used to achieve successful closure, based on the literature and our clinical experiences.
Core tip: We introduce and discuss endoscopic devices and techniques used to treat gastrointestinal perforations, based on the literature and our clinical experiences. Endoscopists should avoid causing perforations, especially during therapeutic procedures. Sometimes, an intentional perforation is necessary for the complete removal of a tumor. However, the integrity of the mucosa should be considered, and the retained mucous membrane could contribute to the effective closure of the perforation after full-thickness resection. We also provide advice for choosing the appropriate method to close perforations effectively.
- Citation: Li Y, Wu JH, Meng Y, Zhang Q, Gong W, Liu SD. New devices and techniques for endoscopic closure of gastrointestinal perforations. World J Gastroenterol 2016; 22(33): 7453-7462
- URL: https://www.wjgnet.com/1007-9327/full/v22/i33/7453.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i33.7453
Gastrointestinal perforation is defined as the presence of gas or luminal contents outside the gastrointestinal tract. In recent years, the high incidence of gastrointestinal perforations has received more and more attentions. The absolute number of perforations is likely to increase because of the widespread implementation of endoscopic screening programs and the expansion of the indications for endoscopic therapy[1]. Gastrointestinal perforations can be caused by a number of factors, such as iatrogenic factors, spontaneity, foreign body, trauma and surgery[2-4]. Among these etiologies, iatrogenic factors contribute most to the increased incidence of perforations. These iatrogenic factors include endoscopic examination, endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), peroral endoscopic myotomy, endoscopic retrograde cholangiopancreatography (ERCP), stricture dilation, foreign body removal and malignant tumors[2,5-9]. The incidence of iatrogenic perforations varies in diagnostic and therapeutic endoscopy, ranging from 0.029% to 5%[10,11]. With the developments of new devices and techniques, endoscopic closure has been considered as the primary method and should be a priority option compared with surgery or conservative treatments[12-14]. Endoscopic closure has advantages, such as high success rate, minimally invasion, short hospitalization and low medical expense. Based on the latest studies, endoscopic devices and techniques are considered to be a safe and effective measure to close gastrointestinal perforations[15-27]. In our previous work, we used multiple-band ligators to repair successfully a lateral duodenal ERCP-related perforation[27]. The overall rate of successful endoscopic closure has been reported as approximately 89.9%, with different devices and techniques having success rates ranging from 87.5% to 100%[4]. Many studies have described various kinds of endoscopic devices and techniques. However, there is no systematic introduction to the latest developments in devices and techniques. Recently, we reported two new techniques to close a perforation and used them successfully. In this study, we introduce and summarize different endoscopic devices and techniques based on the latest research and our previous experience. We hope to provide advice for physicians that allows them to choose the appropriate method to close perforations effectively and improve the success rate of endoscopic closure.
Clipping techniques for the endoscopic closure of gastrointestinal perforations are the most common treatment methods[11,28,29]. From treating gastrointestinal bleeding to perforations, endoscopic clips have an increasingly important role. Conventional endoscopic clips, also known as through-the scope clips (TTSC), can effectively close perforations of the esophagus, stomach, duodenum and colon[5,6,9,19,30-32]. There was no failure in three patients with esophageal perforations following EMR managed by endoclips, as reported by Shimizu et al[5]. Sekiguchi et al[33] had also reported complete endoscopic closures of gastric perforations[34,35]. Attention should be paid conservatively to those patients with a medical history of laparotomy, because closure failed in one such patient[33]. Yang et al[36] demonstrated a success rate of 95.5% with effective clipping for colonoscopy-associated perforations. When the tissues around the edge of defect were inflamed or indurated, closing the perforation using endoclips may be difficult.
A combination of clips and other devices and techniques is used common to close certain special perforations. Tanaka et al[37] applied clips and a detachable snare to close a large esophageal perforation that was difficult to manage using alone[37,38]. Using a two-channel scope, clips were placed at equal distances to fix the detachable snare around the defect. When the rubber stopper was tightened, the perforation was closed successfully[38]. Endoloop and metallic clip interrupted sutures have also been used to close gastric perforations. Shi et al[39] proved that using an endoloop and metallic clip interrupted suture to repair gastric defects resulting from endoscopic full thickness resection (EFTR) was safe, easy and feasible. The endoloop was anchored to both sides of the defect using two clips. After the maneuver was repeated sufficiently around the defect, the endoloop was tightened, closing the defect. This method proved to be safe and quick, with the only side effects being slight abdominal pain and fever in the early days of recovery.
Large mucosal defect areas usually occur during ESD. A new closure device, named the loop clip, was designed to close large mucosal defect after ESD[40]. The loop clip is anchored to the edge of the mucosal defect at the distal side, and then a normal clip is inserted and attached at the proximal side, after which the nylon loop attached to the loop clip is first grasped. The first conventional endoscopic clip is placed beside the deployed loop clip to bring the distal edge to the proximal side. Next, a second clip is placed beside the loop clip on the opposite side of the first clip. This operation is repeated until the whole defect is closed.
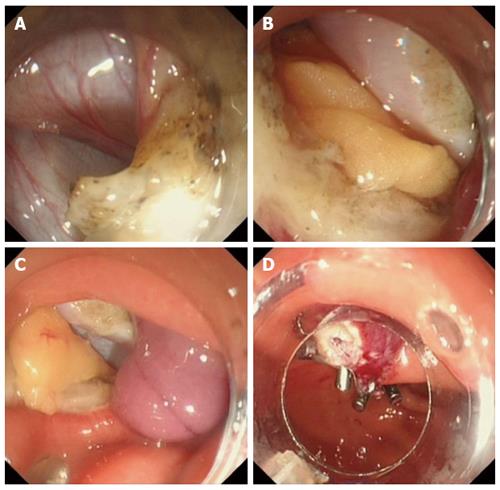
Unfortunately, gastrointestinal perforations are sometimes too difficult to manage using endoclip techniques or nylon loops. The over the scope clip (OTSC) system can be more effective than TTSC in an emergency or in a complex situation. OTSC is more suitable for larger defects and is more effective for closing a fistula. The principle of OTSC system depends on its twin grasper. When the graspers are released to grasp the sides of the lesion, they are retracted completely and the perforation is closed. The advantages of OTSC system were demonstrated in the case report by Ono et al[8]. Using the OTSC system, they closed a esophageal perforation that involved esophageal stenosis. Although the OTSC system is useful to solve difficult cases, it does have certain limitations. It is hard to remove the clips once they are placed, and the grasper is not flexible enough to rotate. We usually apply the OTSC system in complex situations, for example if the perforation is large or is difficult to manage by endoclips. Recently, we closed a large perforation successfully during ESD. The submucosal tumor (SMT) was about 20 mm and originated from the deep muscularis propria layer. To remove the tumor completely, we made a full thickness resection and a large perforation occurred. OTSC and clips were then applied to achieve a successful closure (Figure 1).
All of these new devices and techniques have good efficacy for closing different large and complicated perforations. However, whether they can be used widely in the clinic treatment or whether further clinical studies are required remains a mater of debate.
How to choose an appropriate clipping technique to treat a perforation? Normal endoclips are suggested for use when the diameter of the perforation is less than 10-20 mm. OTCS clips are suitable to close a perforation with a diameter less than 30 mm or when the edge of mucosa is swollen and invaginating. Larger perforations can be closed effectively by a combination of clips and other devices or techniques.
In a conclusion, compared with other endoscopic devices and techniques, clipping techniques are used much more commonly to close perforations. They have become the first choice for most situations that involve closing a perforation, instead of surgery or conservative treatment[41].
Recently, temporary stent placement has emerged as another endoscopic treatment for patients with perforations. There are two main types of stents with different shapes: fully covered self-expanding metal/plastic stents (fSEMS/ fSEPS) and partially covered self-expanding metal stents (pSEMS)[2,20,23,42]. Fully covered ones have the advantages of good drainage and closure of the perforation without obvious complications[23,43]. Unfortunately, they nearly all of these types of stents have a high migration rate that delays the recovery[43-45]. pSEMS have a very low migration rate compared with the fully covered ones. Nevertheless, it is a great challenge to remove them because of tissue embedding[46]. Nevertheless, they provide a further choice of method for patients and in some situations they function better than other devices and techniques when used correctly. From the literature and our clinical experiences, stents are a good choice for esophageal perforations with stenosis, where the defect’s diameter is more than 20-30 mm and there are malignant lesions around the defect. Gastric perforations near the pylorus, perforation caused by dilating an anastomotic stricture and perforation that are not close to the duodenal ampulla are suitable for stent treatment.
Kumbhari et al[20] reported that an iatrogenic pharyngoesophageal perforation was closed by fSEMS after three days. The lack of working space and risk of pulmonary aspiration made it almost impossible to apply endoscopic clips, OTSC or endoscopic suturing. This case highlighted the importance of using stents instead. Ribeiro et al[21] closed a large fistula perfectly using a combination of fibrin glue, a partially covered stent and a biological patch. Stents are much more effective for esophageal perforations, having a 100% success rate from a technical aspect. Generally speaking, when stents were used to treat upper gastrointestinal perforations, anastomotic leaks and fistulas, the success rate ranged from 65% to 88%, with different migration rates[23].
Endoscopic band ligation (EBL) is one of the first-line choices for the management of gastroesophageal varices and variceal hemorrhage in cirrhosis[47]. EBL is also safe and effective to treat dieulafoy lesions and diverticular bleeding[48-50]. Furthermore, EBL is also shows promise as an effective and safe treatment for gastric small gastrointestinal stromal tumors[51-53]. In recent years, EBL has been used to close GI perforations, such as gastric, duodenal, colonic and rectal perforations. In most cases, EBL is an alternative choice to close those perforations after failure of metal clips. According to studies worldwide, perforations could be closed with a very high success rate when EBL was applied. EBL is easy, safe, quick and effective. Lee et al[26] compared the EBL technique with endoclips for the closure of colonic perforations. Closure by EBL was faster than closure by endoclipping (3.2 ± 1.7 min vs 6.8 ± 1.3 min, P < 0.01). Our group reported the successful closure of a lateral duodenal perforation by EBL after endoscopic clipping failed because of the fragile edge of the tear[27]. Although the endoscopic management of a duodenal perforation is much more difficult than the others, the patient’s perforation was closed perfectly by the EBL technique, without any symptoms after six months. We revealed that EBL might be easier and faster than endoclipping, and could be considered as the primary repair method for duodenal perforations[27].
Han et al[54] carried out a case study to evaluate the clinical efficacy and safety of EBL in gastric perforations when endoclips closure failed. Successful closures were achieved in all cases. However, the number of patients was limited and the study was not a comparative study. Han et al[55] also reported similar case studies for colon perforations and obtained the same results. Moon et al[56] also used the EBL technique to close a rectal perforation caused by ESD, with reasonably good results.
There is no doubt that new devices and techniques have limitations. EBL may prolong the hospital stay by binding together more tissue than required. In addition, it can cause injury to adjacent organs[25,26,54]. Further studies and developments are needed to expand EBL’s clinical use.
Among numerous tissue sealants, biological glue, a mixture of fibrinogen and thrombin, is used widely[2,3,57]. Originally, the fibrin glue was used in the area of gastroduodenal ulcer bleeding, wound healing and bleeding caused by resections of the gastrointestinal tract[58]. Fibrin glue can form a clot in vivo and can be fully reabsorbed by macrophages after approximately two weeks[59,60]. The fibrin glue can promote the growth of proliferating cells and increase the number of microvessels[61]. In addition, the use of fibrin glue induces the upregulation of growth factors’ expressions, which contribute to healing defects and stopping bleeding[61,62].
Many recent studies have verified the efficacy of biological glue to close GI defects. Kotzampassi et al[57] gained a 96.8% success rate among 63 patients with anastomotic leaks. Mutignani et al[59] obtained similar results using for fibrin glue to treat GI perforations. All six patients in his study had refractory post-ERCP bleeding and were treated with fibrin glue. The study revealed that fibrin glue might provide a new therapeutic choice to cure ERCP-related type 1 perforations after the failure of clipping techniques[59]. Biological glue is mainly applied to close fistulas and leaks. However, its use to close GI perforations has been rarely reported and deserves further exploration.
To improve the efficacy of biological glue, Doyama et al[63] adopted a treatment comprising polyglycolic acid (PGA) sheets, fibrin glue and clips. This technique solved the problems of gravitational influence on PGA and the weakness of clips to close larger defects of more than 30 mm in diameter[32]. Although biological glue seems inspiring and easy to apply, it does have disadvantages. Whether biological glue is suitable for retroperitoneal perforations and how to handle emergencies involving fibrin clot infection remain unknown. In addition, the frequent use of fibrin glue might increase surgical difficulties[59,60].
At present, there are a number of innovative endoscopic devices for suturing. The Bard EndoCinch suturing device (Davol, Cranston, RI, United States)[64] is still used commonly. However, most of the suturing devices developed in last two decades are cumbersome and expensive, and more and more physicians are searching for simple but useful suturing devices[65].
Bergström et al[65] conducted a clinical study using a new, simple stitching technique. The technique relied on two threads in the tissue on each side of a defect, and then the stitching technique locked the threads and finally the defect was closed perfectly[65-67]. It was used successfully to treat three patients with a duodenal perforation, an upper-GI vessel leak and an anastomosis leak. This technique does not need multiple and complicated devices to close the perforation and is more effective[65,66]. Nevertheless, its limitations are obvious. It takes more time to finish the operation and it is clumsy to suture in the gastric fundus, suggesting that this device needs further improvement. Moran et al[68] designed a cap-type suturing device based on natural orifice transluminal endoscopic surgery (NOTES)[69,70]. This suturing device could have a great ability to close full-thickness perforations effectively and efficiently compared with most of the other endoscopic devices[68,71,72]. The device consists mainly of a dual channel, a tissue retractor or a grasping forceps, and a detachable needle tip attached with suture material, making it easy to use. After the forceps grasp one side of the defect, the reloaded needle tip is passed through the tissue surface and then the same operation is done on the other side of the defect. When the grasping forceps are retracted, the suture ends are pulled tight to close the defect. In Moran et al[68]’s study, it was effective and universally adaptable to almost all kinds of endoscopes, providing additional suture choices. This technique requires considerable technical skill, for example, having a good command of the methods for tying a knot. Mori et al[73] performed a study on 30 excised swine stomachs to investigate properties of their innovative devices termed the double-arm-bar suturing system (DBSS). The results showed that the efficacy of DBSS was nearly equal to hand-sewn sutures. Although better efficacy was achieved in the OTSC group, according to the statistical results, DBSS performed much better at closing perforations larger than 20 mm.
Although many useful and innovative endoscopic devices for suturing have been reported, large-scale clinical applications have not yet been carried out and the long-term safety and efficacy of these devices require further evaluation.
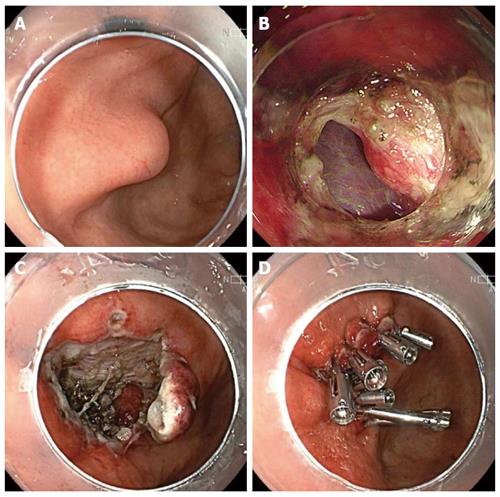
As mentioned above, most of gastrointestinal perforations are iatrogenic and therapeutic endoscopy-related perforations are the most important factors. The integrity of the gastric mucosa is not consciously emphasized in the current endoscopic resection, which usually leads to mucosal defects and perforations. Recently, we proposed a new method, termed endoscopic mucosa-sparing lateral dissection, to remove SMTs, which not only retains an intact mucous membrane, but also provides a good operating field during the procedure. In addition, the retained mucous membrane contributed to the effective closure of the perforation after full-thickness resection for a tumor that originated from the deep layer[74]. In our experience, endoscopists should avoid perforation consciously, especially during therapeutic procedures. Sometimes, intentional perforation is necessary to completely remove the tumor. However, the integrity of the mucosa should be considered because a retained mucous membrane contributes to successful closure. To date, we have applied this method to close large perforations in four patients, and all the perforations were successfully managed (Figure 2).
The European Society of Gastrointestinal Endoscopy (ESGE) suggests that endoscopic dilations, mucosal resection/submucosal dissection and foreign body removal should be considered to carry an increased risk of esophageal or gastric perforation[11]. Most esophageal perforations are iatrogenic. As mentioned above, there are many methods to close esophageal perforations. TTSC is suitable for closing defects with a diameter less than 10 mm and OTSC performs well in closing perforations with swollen and everted edges or those with a diameter less than 30 mm. In addition, esophageal stents show great advantages in handling perforations with malignant lesions or stenosis. Endoscopic devices for suturing can be applied for lesions less than 20 mm. Particular attention should be paid as follows: (1) The use of endoscopic techniques may be challenging in the proximal esophagus because of space constraints and patient intolerance; a conservative treatment should be considered in stable patients; (2) stent fixation with clip application or suturing techniques may be useful to prevent migration of the stent; and (3) fibrin glue application has been reported for the closure of esophageal perforations, but experiences are limited.
Gastric perforations are most often related to therapeutic procedures, including gastroenteric anastomosis dilation (2%)[75]; overdistension during argon plasma coagulation or cryotherapy (< 0.5%); standard snare polypectomy; EMR (0.5%) and more frequently, ESD[11,76,77]. Compared with esophageal perforations, more methods are available for closure of gastric perforations. TTSC clips alone are not recommended for perforations of more than 10 mm. In the case of perforations measuring 10-30 mm, the OTSC system has been the most evaluated and should be recommended. If the OTSC technique is unavailable, the combined technique using TTSC clips plus endoloop can be recommended. Techniques combining omental patches or nylon rope with clips are also good choices to close defect greater than 10 mm. Endoscopic suturing is required to close post-ESD defects. Stents are an option for perforations near the pylorus, or caused by dilating an anastomotic stricture. Evidence supporting the use of endoscopic band ligation for gastric perforations is scarce, and requires further exploration. Particular attention should be paid as follows: (1) most perforations of the stomach are small defects that occur during EMR, ESD procedures; (2) intentional perforation during endoscopic resection is becoming more frequent, and the integrity of the mucosa should be emphasized; (3) closing perforations in the proximal stomach might be challenging; and (4) EBL for gastric perforation closure has been reported, but experiences are limited.
In the case of the immediate recognition of a perforation, an endoscopic closure should be attempted; however, this is effective in a minority of cases only (22%)[11]. Reports about new devices and techniques for the endoscopic closure of duodenal perforations are relatively rare. TTSC clips alone are recommended for perforations less than 10 mm. In the case of perforations measuring 10-30 mm, TTSC clips combined with endoloops or the OTSC system should be considered. EBL could be attempted when clips fail, but is not recommended routinely. If the iatrogenic perforation is diagnosed several hours after endoscopy and the patient shows symptoms of generalized peritonitis and/or sepsis, the only option is surgery. Particular attention should be paid as follows: (1) the use of a transparent cap might be helpful in difficult locations; (2) closure of medial duodenal wall defects with clips may be challenging because of the risk of clipping the ampulla and anatomic location; and (3) a nasoduodenal drain to divert pancreatic and biliary secretions may be beneficial.
ESGE recommends that complex EMR, ESD and balloon dilation procedures should be considered to carry increased risk of colorectal perforation. Risk factors include female gender, presumably related to pelvic adhesions; major co-morbidities; inflammatory bowel disease; and older age[78-81]. TTSC is suitable to close small holes and the OTSC system is useful for larger ones. Clipping plus endoloops can also close large colonic perforations. EBL is verified to be useful for this type defects, but more evidence is needed. Particular attention should be paid as follows: (1) asymptomatic patients with retroperitoneal air alone require no additional treatment; (2) the success rate of endoscopic closure is higher when the perforation is recognized and managed during the same procedure; and (3) large vertical perforations should be closed from top to bottom, and horizontal perforations should be clipped from left to right.
It is sometimes difficult to decide which device or technique is the best method for endoscopic closure of gastrointestinal perforations. In general, the decision to attempt endoscopic closure of an iatrogenic perforation depends on multiple factors, including the location, size and the cause of the perforation, the endoscopist’s experience and the accessories available at the time. The devices and techniques discussed in this study may not apply in all situations and should be interpreted in the light of specific clinical situations. With the rapid development of new endoscopic devices and techniques, more and more perforations can be managed well by endoscopy. However, more attention should be paid to avoid perforation during endoscopic procedure. Occasionally, intentional perforation is necessary to completely remove a tumor. However, the integrity of the mucosa should be considered: the retained mucous membrane can contribute to successful closure. We introduced and discussed endoscopic devices and techniques in this review, with the aim of providing more information about choosing the appropriate method to close perforations effectively and perfectly. Undoubtedly, further, large, randomized, controlled trials are needed to compare the clinical efficacies of the different endoscopic techniques in every situation.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Freeman HJ, Thandassery RB S- Editor: Ma YJ L- Editor: Stewart G E- Editor: Wang CH
| 1. | Bielawska B, Day AG, Lieberman DA, Hookey LC. Risk factors for early colonoscopic perforation include non-gastroenterologist endoscopists: a multivariable analysis. Clin Gastroenterol Hepatol. 2014;12:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (2)] |
| 2. | Goenka MK, Goenka U. Endotherapy of leaks and fistula. World J Gastrointest Endosc. 2015;7:702-713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Rogalski P, Daniluk J, Baniukiewicz A, Wroblewski E, Dabrowski A. Endoscopic management of gastrointestinal perforations, leaks and fistulas. World J Gastroenterol. 2015;21:10542-10552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Verlaan T, Voermans RP, van Berge Henegouwen MI, Bemelman WA, Fockens P. Endoscopic closure of acute perforations of the GI tract: a systematic review of the literature. Gastrointest Endosc. 2015;82:618-28.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Shimizu Y, Kato M, Yamamoto J, Nakagawa S, Komatsu Y, Tsukagoshi H, Fujita M, Hosokawa M, Asaka M. Endoscopic clip application for closure of esophageal perforations caused by EMR. Gastrointest Endosc. 2004;60:636-639. [PubMed] |
| 6. | Qadeer MA, Dumot JA, Vargo JJ, Lopez AR, Rice TW. Endoscopic clips for closing esophageal perforations: case report and pooled analysis. Gastrointest Endosc. 2007;66:605-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Eroglu A, Turkyilmaz A, Aydin Y, Yekeler E, Karaoglanoglu N. Current management of esophageal perforation: 20 years experience. Dis Esophagus. 2009;22:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Ono H, Tanaka M, Takizawa K, Kakushima N, Kawata N, Imai K, Hotta K, Matsubayashi H. Utility of the over-the-scope-clip system for treating a large esophageal perforation. Esophagus. 2015;12:336-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Yılmaz B, Unlu O, Roach EC, Can G, Efe C, Korkmaz U, Kurt M. Endoscopic clips for the closure of acute iatrogenic perforations: Where do we stand? Dig Endosc. 2015;27:641-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Merchea A, Cullinane DC, Sawyer MD, Iqbal CW, Baron TH, Wigle D, Sarr MG, Zielinski MD. Esophagogastroduodenoscopy-associated gastrointestinal perforations: a single-center experience. Surgery. 2010;148:876-80; discussion 881-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Paspatis GA, Dumonceau JM, Barthet M, Meisner S, Repici A, Saunders BP, Vezakis A, Gonzalez JM, Turino SY, Tsiamoulos ZP. Diagnosis and management of iatrogenic endoscopic perforations: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2014;46:693-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 186] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 12. | von Renteln D, Schmidt A, Vassiliou MC, Rudolph HU, Gieselmann M, Caca K. Endoscopic closure of large colonic perforations using an over-the-scope clip: a randomized controlled porcine study. Endoscopy. 2009;41:481-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Lee TH, Bang BW, Jeong JI, Kim HG, Jeong S, Park SM, Lee DH, Park SH, Kim SJ. Primary endoscopic approximation suture under cap-assisted endoscopy of an ERCP-induced duodenal perforation. World J Gastroenterol. 2010;16:2305-2310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Baron TH, Wong Kee Song LM, Zielinski MD, Emura F, Fotoohi M, Kozarek RA. A comprehensive approach to the management of acute endoscopic perforations (with videos). Gastrointest Endosc. 2012;76:838-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Mangiavillano B, Viaggi P, Masci E. Endoscopic closure of acute iatrogenic perforations during diagnostic and therapeutic endoscopy in the gastrointestinal tract using metallic clips: a literature review. J Dig Dis. 2010;11:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Dişibeyaz S, Köksal AŞ, Parlak E, Torun S, Şaşmaz N. Endoscopic closure of gastrointestinal defects with an over-the-scope clip device. A case series and review of the literature. Clin Res Hepatol Gastroenterol. 2012;36:614-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Weiland T, Fehlker M, Gottwald T, Schurr MO. Performance of the OTSC System in the endoscopic closure of iatrogenic gastrointestinal perforations: a systematic review. Surg Endosc. 2013;27:2258-2274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (1)] |
| 18. | Mennigen R, Senninger N, Laukoetter MG. Novel treatment options for perforations of the upper gastrointestinal tract: endoscopic vacuum therapy and over-the-scope clips. World J Gastroenterol. 2014;20:7767-7776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 89] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 19. | Kantsevoy SV, Bitner M, Hajiyeva G, Mirovski PM, Cox ME, Swope T, Alexander K, Meenaghan N, Fitzpatrick JL, Gushchin V. Endoscopic management of colonic perforations: clips versus suturing closure (with videos). Gastrointest Endosc. 2015; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 20. | Kumbhari V, Azola AA, Tieu AH, Sachdeva R, Saxena P, Messallam AA, El Zein MH, Okolo PI, Khashab MA. Iatrogenic pharyngoesophageal perforations treated with fully covered self-expandable metallic stents (with video). Surg Endosc. 2015;29:987-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Ribeiro MS, de Barros RA, Wallace MB. Vicryl patch and fibrin glue as treatment of an esophageal leak. Gastrointest Endosc. 2015;82:402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Smallwood NR, Fleshman JW, Leeds SG, Burdick JS. The use of endoluminal vacuum (E-Vac) therapy in the management of upper gastrointestinal leaks and perforations. Surg Endosc. 2016;30:2473-2480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | van den Berg MW, Kerbert AC, van Soest EJ, Schwartz MP, Bakker CM, Gilissen LP, van Hooft JE. Safety and efficacy of a fully covered large-diameter self-expanding metal stent for the treatment of upper gastrointestinal perforations, anastomotic leaks, and fistula. Dis Esophagus. 2015; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Fan CS, Soon MS. Repair of a polypectomy-induced duodenal perforation with a combination of hemoclip and band ligation. Gastrointest Endosc. 2007;66:203-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Law R, Deters JL, Miller CA, Marler RJ, Baron TH. Endoscopic band ligation for closure of GI perforations in a porcine animal model (with video). Gastrointest Endosc. 2014;80:717-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Lee TH, Han JH, Jung Y, Lee SH, Kim DH, Shin JY, Lee TS, Kim M, Choi SH, Kim H. Comparison of endoscopic band ligation and endoclip closure of colonic perforation: technical feasibility and efficacy in an ex vivo pig model. Dig Endosc. 2014;26:659-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Li Y, Han Z, Zhang W, Wang X, Li A, Xu Y, Zhou D, Wan T, Zhong J, Mi W. Successful closure of lateral duodenal perforation by endoscopic band ligation after endoscopic clipping failure. Am J Gastroenterol. 2014;109:293-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Putcha RV, Burdick JS. Management of iatrogenic perforation. Gastroenterol Clin North Am. 2003;32:1289-1309. [PubMed] |
| 29. | Al Ghossaini N, Lucidarme D, Bulois P. Endoscopic treatment of iatrogenic gastrointestinal perforations: an overview. Dig Liver Dis. 2014;46:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Voermans RP, Le Moine O, von Renteln D, Ponchon T, Giovannini M, Bruno M, Weusten B, Seewald S, Costamagna G, Deprez P. Efficacy of endoscopic closure of acute perforations of the gastrointestinal tract. Clin Gastroenterol Hepatol. 2012;10:603-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 31. | Gomez-Esquivel R, Raju GS. Endoscopic closure of acute esophageal perforations. Curr Gastroenterol Rep. 2013;15:321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Nishiyama N, Mori H, Kobara H, Rafiq K, Fujihara S, Kobayashi M, Oryu M, Masaki T. Efficacy and safety of over-the-scope clip: including complications after endoscopic submucosal dissection. World J Gastroenterol. 2013;19:2752-2760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 101] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 33. | Sekiguchi M, Suzuki H, Oda I, Yoshinaga S, Nonaka S, Saka M, Katai H, Taniguchi H, Kushima R, Saito Y. Dehiscence following successful endoscopic closure of gastric perforation during endoscopic submucosal dissection. World J Gastroenterol. 2012;18:4224-4227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Minami S, Gotoda T, Ono H, Oda I, Hamanaka H. Complete endoscopic closure of gastric perforation induced by endoscopic resection of early gastric cancer using endoclips can prevent surgery (with video). Gastrointest Endosc. 2006;63:596-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 230] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 35. | Yokoi C, Gotoda T, Hamanaka H, Oda I. Endoscopic submucosal dissection allows curative resection of locally recurrent early gastric cancer after prior endoscopic mucosal resection. Gastrointest Endosc. 2006;64:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Yang DH, Byeon JS, Lee KH, Yoon SM, Kim KJ, Ye BD, Myung SJ, Yang SK, Kim JH. Is endoscopic closure with clips effective for both diagnostic and therapeutic colonoscopy-associated bowel perforation? Surg Endosc. 2010;24:1177-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | Tanaka S, Toyonaga T, Ohara Y, Yoshizaki T, Kawara F, Ishida T, Hoshi N, Morita Y, Azuma T. Esophageal diverticulum exposed during endoscopic submucosal dissection of superficial cancer. World J Gastroenterol. 2015;21:3121-3126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Matsuda T, Fujii T, Emura F, Kozu T, Saito Y, Ikematsu H, Saito D. Complete closure of a large defect after EMR of a lateral spreading colorectal tumor when using a two-channel colonoscope. Gastrointest Endosc. 2004;60:836-838. [PubMed] |
| 39. | Shi Q, Chen T, Zhong YS, Zhou PH, Ren Z, Xu MD, Yao LQ. Complete closure of large gastric defects after endoscopic full-thickness resection, using endoloop and metallic clip interrupted suture. Endoscopy. 2013;45:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 40. | Osada T, Sakamoto N, Ritsuno H, Murakami T, Ueyama H, Shibuya T, Matsumoto K, Nagahara A, Ogihara T, Watanabe S. Process of wound healing of large mucosal defect areas that were sutured by using a loop clip-assisted closure technique after endoscopic submucosal dissection of a colorectal tumor. Gastrointest Endosc. 2013;78:793-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Cai SL, Chen T, Yao LQ, Zhong YS. Management of iatrogenic colorectal perforation: From surgery to endoscopy. World J Gastrointest Endosc. 2015;7:819-823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 42. | Ngamruengphong S, von Rahden BH, Filser J, Tyberg A, Desai A, Sharaiha RZ, Lambroza A, Kumbhari V, El Zein M, Abdelgelil A. Intraoperative measurement of esophagogastric junction cross-sectional area by impedance planimetry correlates with clinical outcomes of peroral endoscopic myotomy for achalasia: a multicenter study. Surg Endosc. 2016;30:2886-2894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 43. | Freeman RK, Van Woerkom JM, Ascioti AJ. Esophageal stent placement for the treatment of iatrogenic intrathoracic esophageal perforation. Ann Thorac Surg. 2007;83:2003-2007; discussion 2003-2007;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 44. | van Boeckel PG, Dua KS, Weusten BL, Schmits RJ, Surapaneni N, Timmer R, Vleggaar FP, Siersema PD. Fully covered self-expandable metal stents (SEMS), partially covered SEMS and self-expandable plastic stents for the treatment of benign esophageal ruptures and anastomotic leaks. BMC Gastroenterol. 2012;12:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 45. | Leenders BJ, Stronkhorst A, Smulders FJ, Nieuwenhuijzen GA, Gilissen LP. Removable and repositionable covered metal self-expandable stents for leaks after upper gastrointestinal surgery: experiences in a tertiary referral hospital. Surg Endosc. 2013;27:2751-2759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Hirdes MM, Vleggaar FP, Van der Linde K, Willems M, Totté ER, Siersema PD. Esophageal perforation due to removal of partially covered self-expanding metal stents placed for a benign perforation or leak. Endoscopy. 2011;43:156-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol. 2007;102:2086-2102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 257] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 48. | Van Stiegmann G, Goff JS. Endoscopic esophageal varix ligation: preliminary clinical experience. Gastrointest Endosc. 1988;34:113-117. [PubMed] |
| 49. | Brown GR, Harford WV, Jones WF. Endoscopic band ligation of an actively bleeding Dieulafoy lesion. Gastrointest Endosc. 1994;40:501-503. [PubMed] |
| 50. | Marques S, Barreiro P, Chagas C. Endoscopic Band Ligation: A Safe And Effective Treatment For Active Diverticular Bleeding. ACG Case Rep J. 2016;3:77-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 51. | Sun S, Ge N, Wang C, Wang M, Lü Q. Endoscopic band ligation of small gastric stromal tumors and follow-up by endoscopic ultrasonography. Surg Endosc. 2007;21:574-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 52. | Huang WH, Feng CL, Lai HC, Yu CJ, Chou JW, Peng CY, Yang MD, Chiang IP. Endoscopic ligation and resection for the treatment of small EUS-suspected gastric GI stromal tumors. Gastrointest Endosc. 2010;71:1076-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 53. | Nan G, Siyu S, Shiwei S, Sheng W, Xiang L. Hemoclip-reinforced and EUS-assisted band ligation as an effective and safe technique to treat small GISTs in the gastric fundus. Am J Gastroenterol. 2011;106:1560-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Han JH, Lee TH, Jung Y, Lee SH, Kim H, Han HS, Chae H, Park SM, Youn S. Rescue endoscopic band ligation of iatrogenic gastric perforations following failed endoclip closure. World J Gastroenterol. 2013;19:955-959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Han JH, Park S, Youn S. Endoscopic closure of colon perforation with band ligation; salvage technique after endoclip failure. Clin Gastroenterol Hepatol. 2011;9:e54-e55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 56. | Moon SY, Park CH, Yoon SM, Chung Ys, Chung YW, Kim KO, Hahn TH, Yoo KS, Park SH, Kim JH, Park CK. Repair of an endoscopic submucosal dissection-induced rectal perforation with band ligation. Gastrointest Endosc. 2009;69:160-161; discussion 161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 57. | Kotzampassi K, Eleftheriadis E. Tissue sealants in endoscopic applications for anastomotic leakage during a 25-year period. Surgery. 2015;157:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 58. | Born P, Ott R, Rösch T. Endoscopic hemostasis using fibrin sealant for postsphincterotomy bleeding: report of two cases. Gastrointest Endosc. 2000;51:731-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 59. | Mutignani M, Iacopini F, Dokas S, Larghi A, Familiari P, Tringali A, Costamagna G. Successful endoscopic closure of a lateral duodenal perforation at ERCP with fibrin glue. Gastrointest Endosc. 2006;63:725-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | Mutignani M, Seerden T, Tringali A, Feisal D, Perri V, Familiari P, Costamagna G. Endoscopic hemostasis with fibrin glue for refractory postsphincterotomy and postpapillectomy bleeding. Gastrointest Endosc. 2010;71:856-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 61. | Becker JC, Beckbauer M, Domschke W, Herbst H, Pohle T. Fibrin glue, healing of gastric mucosal injury, and expression of growth factors: results from a human in vivo study. Gastrointest Endosc. 2005;61:560-567. [PubMed] |
| 62. | Mizuki A, Tatemichi M, Nishiya H, Fukui K, Hayashi T, Tsukada N, Nagata H, Ishii H. Mucosal concentration of basic fibroblast growth factor in the healing process in human giant gastric ulcers. J Gastroenterol Hepatol. 2004;19:528-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 63. | Doyama H, Tominaga K, Yoshida N, Takemura K, Yamada S. Endoscopic tissue shielding with polyglycolic acid sheets, fibrin glue and clips to prevent delayed perforation after duodenal endoscopic resection. Dig Endosc. 2014;26 Suppl 2:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 64. | Swain P, Park PO, Mills T. Bard EndoCinch: the device, the technique, and pre-clinical studies. Gastrointest Endosc Clin N Am. 2003;13:75-88. [PubMed] |
| 65. | Bergström M, Swain P, Park PO. Early clinical experience with a new flexible endoscopic suturing method for natural orifice transluminal endoscopic surgery and intraluminal endosurgery (with videos). Gastrointest Endosc. 2008;67:528-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 66. | Ikeda K, Fritscher-Ravens A, Mosse CA, Mills T, Tajiri H, Swain CP. Endoscopic full-thickness resection with sutured closure in a porcine model. Gastrointest Endosc. 2005;62:122-129. [PubMed] |
| 67. | Park PO, Bergström M, Ikeda K, Fritscher-Ravens A, Mosse S, Kochman M, Swain P. Endoscopic pyloroplasty with full-thickness transgastric and transduodenal myotomy with sutured closure. Gastrointest Endosc. 2007;66:116-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 68. | Moran EA, Gostout CJ, Bingener J. Preliminary performance of a flexible cap and catheter-based endoscopic suturing system. Gastrointest Endosc. 2009;69:1375-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 69. | Fritscher-Ravens A, Mosse CA, Mills TN, Mukherjee D, Park PO, Swain P. A through-the-scope device for suturing and tissue approximation under EUS control. Gastrointest Endosc. 2002;56:737-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 70. | Zorrón R, Filgueiras M, Maggioni LC, Pombo L, Lopes Carvalho G, Lacerda Oliveira A. NOTES. Transvaginal cholecystectomy: report of the first case. Surg Innov. 2007;14:279-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 166] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 71. | Sumiyama K, Gostout CJ, Rajan E, Bakken TA, Deters JL, Knipschield MA. Endoscopic full-thickness closure of large gastric perforations by use of tissue anchors. Gastrointest Endosc. 2007;65:134-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 72. | Rajan E, Gostout CJ, Lurken MS, Talley NJ, Locke GR, Szarka LA, Sumiyama K, Bakken TA, Stoltz GJ, Knipschield MA. Endoscopic “no hole” full-thickness biopsy of the stomach to detect myenteric ganglia. Gastrointest Endosc. 2008;68:301-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 73. | Mori H, Kobara H, Fujihara S, Nishiyama N, Rafiq K, Oryu M, Fujiwara M, Suzuki Y, Masaki T. Feasibility of pure EFTR using an innovative new endoscopic suturing device: the Double-arm-bar Suturing System (with video). Surg Endosc. 2014;28:683-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 74. | Zhang Q, Li Y, Meng Y, Bai Y, Cai JQ, Han ZL, Wang Z, Zhi FC, Liu SD. Should the Integrity of Mucosa Be Considered in Endoscopic Resection of Gastric Submucosal Tumors? Gastroenterology. 2016;150:822-824.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 75. | Ukleja A, Afonso BB, Pimentel R, Szomstein S, Rosenthal R. Outcome of endoscopic balloon dilation of strictures after laparoscopic gastric bypass. Surg Endosc. 2008;22:1746-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 76. | Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 476] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 77. | Yoo JH, Shin SJ, Lee KM, Choi JM, Wi JO, Kim DH, Lim SG, Hwang JC, Cheong JY, Yoo BM. Risk factors for perforations associated with endoscopic submucosal dissection in gastric lesions: emphasis on perforation type. Surg Endosc. 2012;26:2456-2464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 78. | Saunders BP, Fukumoto M, Halligan S, Jobling C, Moussa ME, Bartram CI, Williams CB. Why is colonoscopy more difficult in women? Gastrointest Endosc. 1996;43:124-126. [PubMed] |
| 79. | Korman LY, Overholt BF, Box T, Winker CK. Perforation during colonoscopy in endoscopic ambulatory surgical centers. Gastrointest Endosc. 2003;58:554-557. [PubMed] |
| 80. | Rabeneck L, Paszat LF, Hilsden RJ, Saskin R, Leddin D, Grunfeld E, Wai E, Goldwasser M, Sutradhar R, Stukel TA. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology. 2008;135:1899-1906, 1906.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 357] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 81. | Navaneethan U, Kochhar G, Phull H, Venkatesh PG, Remzi FH, Kiran RP, Shen B. Severe disease on endoscopy and steroid use increase the risk for bowel perforation during colonoscopy in inflammatory bowel disease patients. J Crohns Colitis. 2012;6:470-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |