Published online Aug 21, 2016. doi: 10.3748/wjg.v22.i31.6965
Peer-review started: April 7, 2016
First decision: May 12, 2016
Revised: June 2, 2016
Accepted: June 28, 2016
Article in press: June 28, 2016
Published online: August 21, 2016
Processing time: 130 Days and 9.7 Hours
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease worldwide, affecting approximately one third of the Western world. It consists of a wide spectrum of liver disorders, ranging from fatty liver to nonalcoholic steatohepatitis (NASH), which consists of steatosis, ballooning injury and inflammation. Despite an alarming growth in the statistics surrounding NAFLD, there are as yet no effective therapies for its treatment. Innate immune signaling has been thought to play a significant role in initiating and augmenting hepatic inflammation, contributing to the transition from nonalcoholic fatty liver to NASH. An immune response is triggered by countless signals called damage-associated molecular patterns (DAMPs) elicited by lipid-laden and damaged hepatocytes, which are recognized by pattern recognition receptors (PRRs) on hepatic immune cells to initiate inflammatory signaling. In this editorial, in addition to summarizing innate immune signaling in NAFLD and discussing potential therapies that target innate immune pathways, we have described a recent study that demonstrated that mitochondrial DNA serves as a DAMP activating a hepatic PRR, TLR9, in mice and in the plasma of NASH patients. In addition to identifying a new ligand for TLR9 during NASH progression, the study shows that blocking TLR9 reverses NASH, paving the way for the development of future NASH therapy.
Core tip: Nonalcoholic fatty liver disease (NAFLD) is a chronic liver disease. It has been shown that innate immune activation contributes to the progression of NAFLD by inducing hepatic inflammation. A new study recently demonstrated that hepatocyte mitochondrial DNA acts as a damage -induced molecular pattern activating hepatic TLR9 to initiate and amplify inflammatory signaling leading to nonalcoholic steatohepatitis (NASH). Inhibiting TLR9 by using a synthetic antagonist, IRS 954, reversed steatohepatitis; as did a whole body ablation of TLR9 or a myeloid-specific knockout of TLR9. This editorial summarizes the findings of the new study and describes potential novel therapeutic targets that may hold promise for NASH treatment.
- Citation: Handa P, Vemulakonda A, Kowdley KV, Uribe M, Méndez-Sánchez N. Mitochondrial DNA from hepatocytes as a ligand for TLR9: Drivers of nonalcoholic steatohepatitis? World J Gastroenterol 2016; 22(31): 6965-6971
- URL: https://www.wjgnet.com/1007-9327/full/v22/i31/6965.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i31.6965
Nonalcoholic fatty liver disease (NAFLD) is the most prominent form of liver disease in the western world, affecting 30% of the United States population[1,2]. The incidence of NAFLD is growing due to its close association with obesity, type 2 diabetes and the metabolic syndrome; but despite the alarming growth, there are currently no approved therapies[1]. NAFLD includes a spectrum of liver disorders ranging from simple steatosis or nonalcoholic fatty liver (NAFL) on the one end, to nonalcoholic steatohepatitis (NASH), characterized by steatosis, inflammation, ballooning injury and varying degrees of fibrosis, on the other. It is reported that approximately 30% of the patients with NAFL will advance to NASH, while 15%-25% of NASH patients may go on to develop cirrhosis and 30%-40% of the patients with cirrhosis will succumb to liver-related complications[1,3-5].
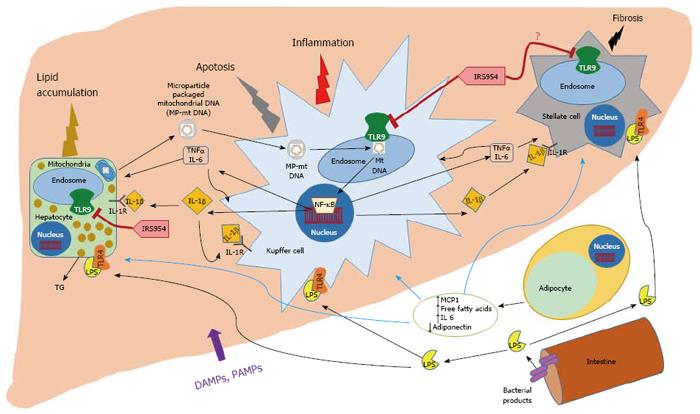
The pathophysiology of NASH is still not fully understood, but it is commonly accepted in the field that the initiating events in NAFLD are obesity and insulin resistance at the level of adipose tissue and liver, which contribute to the increased uptake of serum free fatty acids in the liver, as a result of the dysregulation of peripheral lipolysis, de novo lipogenesis and intake of dietary fats[1,3-6]. This leads to the accumulation of triglycerides or hepatic steatosis. While the transition from steatosis to steatohepatitis is not clearly understood, many complex mechanisms such as the altered production of proinflammatory cytokines and adipokines, lipotoxicity in hepatocytes, oxidative stress, mitochondrial dysfunction, hyperactivation of innate immune pathways, among others, are believed to contribute to the inflammation, hepatocyte ballooning injury and fibrosis that constitutes NASH[1-7]. Innate immune activation is thought to play a vital role in inducing and magnifying the inflammation in NAFLD/NASH, as shown in Figure 1. A greater understanding of the mechanisms by which the immune cells in the liver recognize endogenous and exogenous ligands through cognate receptors and amplify the inflammatory signaling cascades leading to liver injury will inform the design of novel effective therapeutics.
Innate immunity is the conserved and universal form of host defense mechanism against infection. It is a non-specific defense mechanism that comes to play immediately or within hours of an antigen exposure. These mechanisms include physical barriers such as skin, and the cells of the immune system[8]. The innate immune response is activated by chemical properties of the antigen. Innate immune recognition relies on conserved receptors, such as the Toll-like receptors (TLR) and the nucleotide oligomerization domain (NOD) like receptors (NLRs)[8-11] which recognize conserved products of microbial metabolism produced by pathogens (PAMPs).
Innate immune signaling is believed to play an important role in the pathogenesis of NASH. Signals derived locally from the liver or those from the adipose tissue or gut consisting of endogenous ligands or microbial products stimulate hepatic immune cells leading to inflammation, cell injury and death[9-11].
Damage associated molecular patterns (DAMPs) and Pathogen associated molecular patterns (PAMPs) activate innate immune mechanisms in the host leading to an inflammatory response[9,10]. Molecules like nuclear or mitochondrial DNA, adenosine triphosphate (ATP), uridine triphosphate (UTP), uric acid and High mobility group box 1 (HMGB1), among others, are classified as DAMPs. DAMPs are secreted or produced upon cellular injury or death and induce sterile inflammation that contributes to the hepatic inflammation that propels NAFLD. Bacterial products like LPS, peptidoglycans, lipoprotein flagellins, bacterial RNA and DNA are some of the well-characterized PAMPs. On disruption of the intestinal mucosal barrier, PAMPs reach the liver, recruit and activate hepatic innate immune cells inducing intracellular signaling cascades that result in amplification of liver injury contributing to NASH[9,10].
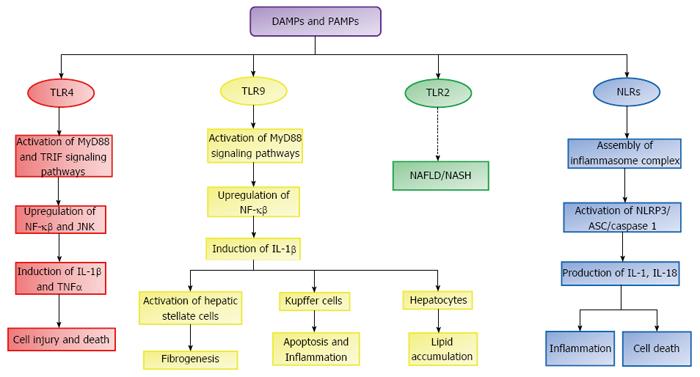
When released, DAMPs and PAMPs bind to PRRs like TLRs, which are a family of cell surface and endocytic receptors expressed on liver cells like hepatocytes, hepatic stellate cells, Kupffer cells, biliary epithelial cells and sinusoidal endothelial cells that will trigger a local inflammatory response leading to injury amplification and organ damage[9,10]. TLRs activate either myeloid differentiation factor 88 (MyD88) dependent or Toll/interleukin-1 receptor domain containing adaptor inducing interferon β (TRIF) dependent signaling pathways, which results in the activation of transcriptions factors like JNK and NF-κβ respectively[11]. These transcription factors induce the expression of various inflammatory cytokines (TNFα, IL-1β, IL-6) and chemokines, which contribute to the progression of NAFLD, as shown in the schematic Figure 2. TLRs that are thought to contribute to NAFLD pathophysiology are TLR4, TLR9 and TLR2[9,10]. LPS, in the presence of co-receptors CD14 and MD-2, activates TLR4 present on all hepatic cell types leading to production of inflammatory cytokines like TNFα and IL-1β[8-10,12]. TLR9 is a ubiquitously expressed endosomal PRR, which recognizes CpG bacterial DNA and mammalian self-DNA as antigens[7]. Upon activation TLR9 upregulates inflammatory cytokines including interleukin IL-1β, which contributes to lipid accumulation in hepatocytes, inflammation, apoptosis and fibrogenesis[9,10,13,14]. Attenuation of TLR4 and TLR9 has led to mitigation of inflammatory NASH in several experimental animal models[9,10,13]. While the role of TLR2 in NAFLD is not understood, TLR2 null mice were protected from NASH in a mouse model[9,10,15].
DAMPs and PAMPs are also recognized by nucleotide oligomerization domain (NOD) like receptors (NLRs), which belong to the PRR family. Activation of NLRs promotes assembly of inflammasome multiprotein complexes, consisting of NLR family CARD domain-containing proteins, NLRPs (NLRP3 is the most well-studied inflammasome sensor with respect to NAFLD), adaptor proteins such as the apoptosis-associated speck-like protein containing a caspase-recruitment domain (ASC), and the serine protease caspase 1 (Casp1)[9,10]. Upon sensing of DAMPs and PAMPs by the inflammasome complex, Casp1 is cleaved and activated, further inducing interleukin-1β (IL-1β) and IL-18 maturation, which contribute to inflammation, fibrosis and cell death in NASH[9,10,12,16].
In an elegant study that appeared recently as a brief report in the Journal of Clinical Investigation, Garcia-Martinez et al[7] asked whether TLR9 activation was the reason why some patients with NAFL develop NASH. The authors had previously demonstrated the involvement of TLR9 in inflammation and fibrosis using an acetaminophen-induced liver injury model[17,18]. TLR9 was also shown to be required for steatohepatitis and inflammation in a diet-induced mouse model[7,13,18].
In this study, the investigators asked what the hepatic ligand for TLR9 during NAFLD progression was? TLR9 located in which hepatic cell type was important for NASH pathogenesis? They hypothesized that hepatocyte mitochondrial DNA (mtDNA) could be a ligand for TLR9. It had previously been shown that hepatocyte mitochondrial DNA levels are elevated in NASH patients and it was proinflammatory[19]. They tested their hypothesis by isolating hepatocyte mtDNA from livers of high fat diet (HFD)-fed mice, which developed NASH in 12 wk. Relative to control mtDNA and nuclear DNA (nDNA) from HFD mice, the mtDNA from livers of HFD-fed mice showed a strong activation of TLR9 in a reporter cell line, and showed robust inflammatory response in primary mouse Kupffer cells, by strongly inducing TNFα mRNA expression.
The researchers then asked if the hepatocyte mtDNA is released extracellularly into the plasma. It is thought that cell injury, apoptosis or necrosis results in the release of mtDNA out of the cell leading to increased amount of extracellular mtDNA. It is also know that plasma extracellular mtDNA levels are elevated in mice and humans during liver injury[20]. The investigators found that the plasma mtDNA levels were elevated in mice from HFD group and that the plasma from the HFD mice caused a significant increase in TLR9 activation using the TLR9 reporter cell line, relative to the plasma from the chow-fed control mice. They next asked the all-important question: does the plasma from NAFLD patients show an increase in mtDNA and does it activate TLR9? They compared plasma from three groups: plasma from lean humans with normal ALT levels, obese humans with normal ALT levels and the third group consisting of obese individuals with elevated ALT levels. The authors discovered that it is patients from the third group that showed the highest increase of total DNA and mtDNA in plasma (but not nuclear DNA) and showed a strong activation signal from the TLR9 reporter line.
Plasma mtDNA can be free or enclosed within extracellular vesicles called microparticles (MP)[7]. To ask if the plasma of the patients had intact mitochondria which were free or in MP, the researchers performed flow cytometry. They found that the mitochondria were stainable using a dye called Mitotracker Red and that the mitochondria from the obese patients with elevated serum ALT had the highest percentage of mitochondria in the MP. The authors also discovered that plasma of obese people with elevated serum ALT had the highest levels of oxidized DNA in the MPs, by staining for 8-hydroxy-deoxyguanosine (an oxidized DNA adduct).
The investigators further asked if the TLR9 in the inflammatory cells of the liver are important. They ablated TLR9 in the lysozyme-producing cells (macrophages, monocytes, neutrophils among others). While WT mice on a HFD showed steatohepatitis in 12 wk, the whole body TLR9 knockout mice and the LysM-Cre TLR9fl/fl mice on HFD were protected from NASH, showed reduced ALT levels and had significantly reduced proinflammatory cytokines such as IL-1β, demonstrating a pivotal role for the macrophage (and other LysM+ cells) TLR9 in NASH.
Having demonstrated a key role for myeloid TLR9 in NASH pathogenesis in humans and mice, the authors finally asked if they could use an antagonist to TLR7/9, IRS954, to abrogate the ability of hepatocyte mtDNA from livers of HFD-fed mice to activate TLR9 reporter cell line and therefore mitigate TLR9-mediated NASH? The antagonist, IRS954, led to a significant reduction in histological NAFLD parameters such as steatosis, ballooning, inflammation; showed appreciably reduced serum ALT levels and reduced pro-IL-1β, IL-6 and TNFα, relative to the vehicle- treated mice on HFD. This exciting study paves the way for potential therapeutics aimed at blocking TLR9 ligand-binding.
One missing piece of the study is that the authors did not examine the effect of TLR9 ablation and TLR9 antagonism on stellate cell activation and fibrosis, as the HFD mouse model used in this study did not yield fibrosis. It would not only be useful to examine in a model that yields fibrosis as a read-out, but also assess the effect of ablation of TLR9 in stellate cells.
In summary, this novel study links the metabolic alterations in the lipid-laden hepatocytes with the hyperactivation of an innate inflammatory response by TLR9 in the macrophages, and emphasizes its importance in the progression of NASH, as shown in the Figure 1.
Targeting various components of the innate immune signaling pathways has yielded several potential pharmacologic agents, which have shown some promise in animal models and/or human trials. Some of them have been briefly described below.
Probiotics have been suggested as a potential treatment option for NASH as bacterial overgrowth is associated with 50% of NASH patients and fluctuation in intestinal bacterial content may be related to the pathophysiology of NASH such as increased intestinal permeability, increased inflammatory cytokines and absorption of endotoxins[4]. It was shown in a meta-analysis of 4 double-blind randomized trials involving 134 biopsy-proven NAFLD/NASH patients that probiotics significantly decreased total cholesterol, aminotransferases, HOMA-IR and TNFα levels[4].
It is a methylxanthine derivative and a nonselective phosphodiesterase inhibitor that attenuates the synthesis of TNFα. In addition, it has been shown to decrease oxidized lipid peroxidation levels in NASH patients[1,4,21]. A recent meta-analysis showed that Pentoxifylline significantly reduced serum aminotransferase, BMI, fasting glucose, lobular inflammation and fibrosis. Large well-designed, randomized, placebo-controlled trials are still needed to confirm these results[1,4].
Active studies are being conducted on PPAR-δ agonists[1,4,18]. It has been shown that activation of PPAR-δ led to improved hepatic fatty acid oxidation, suppression of hepatic de novo lipogenesis and glucose production. Additionally, it inhibited hepatic inflammation by suppressing the synthesis of proinflammatory cytokines like IL-1β and protected mice from liver fibrosis. These metabolic and anti-inflammatory effects of PPAR-δ agonists make them attractive therapeutic targets against NASH[1,4,18].
It is a secretory product of the pineal gland and is a powerful endogenous antioxidant[22]. It has been demonstrated that melatonin suppresses TLR4-mediated inflammation and is hepatoprotective in a murine LPS and galactosamine toxin model of liver injury. In a clinical trial in NASH patients, oral melatonin administered for 12 wk significantly reduced serum AST and GGT in patients at 12 wk follow-up, relative to controls[4,22] .
It is a novel, oral, dual CCR2/CCR5 antagonist with nanomolar potency against both chemokine receptors and a long plasma half-life[23]. Although it primarily targets inflammation, inhibiting CCR2 and CCR5 also improves fibrosis and insulin sensitivity[1]. It is currently in phase 2b trials and the results of this pharmacologic agent are eagerly anticipated.
It has been demonstrated that resveratrol improves hepatic inflammation including decreased IL-1β production, serum and liver triglyceride levels and glucose control in diet-induced obesity in mice[24] suggesting potential therapeutic activity in NAFLD. However, 8 wk of therapy with resveratrol did not improve any features of NAFLD in small patient cohort[18].
It is a monoclonal antibody against IL-1β. A large study (CANTOS study, Canakinumab anti-inflammatory thrombosis outcome study) is underway to examine the efficacy of the antibody in reducing the rates of recurrent myocardial infarction, stroke, cardiovascular death, and type 2 diabetes among stable patients with coronary disease[18,25]. Studies have not been initiated to examine its therapeutic on NASH patients but positive findings in the aforementioned trial could pave the way for such studies.
It is a novel TLR4 antagonist derived from the Chinese herb Spaganium stoloniferum which has been shown to mitigate liver inflammation in experimental NASH by modulating TLR4 trafficking in lipid rafts via NADPH oxidase activation[26]. It has been observed that mice administered with Sparstolonin B showed decreased mRNA expression of proinflammatory cytokines TNFα, IFN-γ, IL-1β, IL-23 and also significant decrease in protein levels of TNFα and IL-1β[26].
It has been shown that an omega-3 fatty acid, docosahexaenoic acid (DHA), attenuates palmitate-induced lipid accumulation through suppressing NLR family CARD domain-containing protein 4 (NLRP4) inflammasome activation, caspase-1 activation and IL-1β cleavage in HepG2 cells[27]. However, further studies are required to prove the efficacy of DHA in ameliorating NASH in humans.
Thus, continuing to elucidate the complex molecular mechanisms underlying innate immune signaling pathways and the molecular players that contribute to the pathogenesis and progression of NAFLD will yield crucial targets for the design and development of effective therapeutics aimed at halting NASH progression.
The authors wish to thank Dr. Sunil Thomas, PhD at the Department of Immunology at the University of Washington, Seattle for critical reading of the manuscript and his constructive criticism.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chen GX, Ling C, Ye J S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Rinella ME, Sanyal AJ. Management of NAFLD: a stage-based approach. Nat Rev Gastroenterol Hepatol. 2016;13:196-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 284] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 2. | López-Velázquez JA, Silva-Vidal KV, Ponciano-Rodríguez G, Chávez-Tapia NC, Arrese M, Uribe M, Méndez-Sánchez N. The prevalence of nonalcoholic fatty liver disease in the Americas. Ann Hepatol. 2014;13:166-178. [PubMed] |
| 3. | Aguilar-Olivos NE, Almeda-Valdes P, Aguilar-Salinas CA, Uribe M, Méndez-Sánchez N. The role of bariatric surgery in the management of nonalcoholic fatty liver disease and metabolic syndrome. Metabolism. 2016;65:1196-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 4. | Hossain N, Kanwar P, Mohanty SR. A Comprehensive Updated Review of Pharmaceutical and Nonpharmaceutical Treatment for NAFLD. Gastroenterol Res Pract. 2016;2016:7109270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Hardy T, Oakley F, Anstee QM, Day CP. Nonalcoholic Fatty Liver Disease: Pathogenesis and Disease Spectrum. Annu Rev Pathol. 2016;11:451-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Méndez-Sánchez N, Arrese M, Zamora-Valdés D, Uribe M. Current concepts in the pathogenesis of nonalcoholic fatty liver disease. Liver Int. 2007;27:423-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 7. | Garcia-Martinez I, Santoro N, Chen Y, Hoque R, Ouyang X, Caprio S, Shlomchik MJ, Coffman RL, Candia A, Mehal WZ. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J Clin Invest. 2016;126:859-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 375] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 8. | Janeway CA, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197-216. [PubMed] |
| 9. | Arrese M, Cabrera D, Kalergis AM, Feldstein AE. Innate Immunity and Inflammation in NAFLD/NASH. Dig Dis Sci. 2016;61:1294-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 383] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 10. | Ganz M, Szabo G. Immune and inflammatory pathways in NASH. Hepatol Int. 2013;7 Suppl 2:771-781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 11. | Liu H, Li J, Tillman B, Morgan TR, French BA, French SW. TLR3/4 signaling is mediated via the NFκB-CXCR4/7 pathway in human alcoholic hepatitis and non-alcoholic steatohepatitis which formed Mallory-Denk bodies. Exp Mol Pathol. 2014;97:234-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2861] [Cited by in RCA: 2904] [Article Influence: 121.0] [Reference Citation Analysis (0)] |
| 13. | Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, Olefsky JM, Brenner DA, Seki E. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139:323-324.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 623] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 14. | Gao B. Innate immunity and steatohepatitis: a critical role of another toll (TLR-9). Gastroenterology. 2010;139:27-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Miura K, Yang L, van Rooijen N, Brenner DA, Ohnishi H, Seki E. Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology. 2013;57:577-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 230] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 16. | Wree A, McGeough MD, Peña CA, Schlattjan M, Li H, Inzaugarat ME, Messer K, Canbay A, Hoffman HM, Feldstein AE. NLRP3 inflammasome activation is required for fibrosis development in NAFLD. J Mol Med (Berl). 2014;92:1069-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 400] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 17. | Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, Flavell RA, Mehal WZ. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 319] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 18. | Tilg H, Moschen AR, Szabo G. Interleukin-1 and inflammasomes in ALD/AAH and NAFLD/NASH. Hepatology. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 254] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 19. | Koliaki C, Szendroedi J, Kaul K, Jelenik T, Nowotny P, Jankowiak F, Herder C, Carstensen M, Krausch M, Knoefel WT. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015;21:739-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 766] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 20. | Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2990] [Cited by in RCA: 2804] [Article Influence: 186.9] [Reference Citation Analysis (0)] |
| 21. | Takahashi Y, Sugimoto K, Inui H, Fukusato T. Current pharmacological therapies for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2015;21:3777-3785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 94] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (1)] |
| 22. | Tahan V, Atug O, Akin H, Eren F, Tahan G, Tarcin O, Uzun H, Ozdogan O, Tarcin O, Imeryuz N. Melatonin ameliorates methionine- and choline-deficient diet-induced nonalcoholic steatohepatitis in rats. J Pineal Res. 2009;46:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Friedman S, Sanyal A, Goodman Z, Lefebvre E, Gottwald M, Fischer L, Ratziu V. Efficacy and safety study of cenicriviroc for the treatment of non-alcoholic steatohepatitis in adult subjects with liver fibrosis: CENTAUR Phase 2b study design. Contemp Clin Trials. 2016;47:356-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 160] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 24. | Yang SJ, Lim Y. Resveratrol ameliorates hepatic metaflammation and inhibits NLRP3 inflammasome activation. Metabolism. 2014;63:693-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 25. | Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am Heart J. 2011;162:597-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 644] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 26. | Dattaroy D, Seth RK, Das S, Alhasson F, Chandrashekaran V, Michelotti G, Fan D, Nagarkatti M, Nagarkatti P, Diehl AM. Sparstolonin B attenuates early liver inflammation in experimental NASH by modulating TLR4 trafficking in lipid rafts via NADPH oxidase activation. Am J Physiol Gastrointest Liver Physiol. 2016;310:G510-G525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Luo X, Yang Y, Shen T, Tang X, Xiao Y, Zou T, Xia M, Ling W. Docosahexaenoic acid ameliorates palmitate-induced lipid accumulation and inflammation through repressing NLRC4 inflammasome activation in HepG2 cells. Nutr Metab (Lond). 2012;9:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |