Copyright
©The Author(s) 2016.
World J Gastroenterol. Aug 21, 2016; 22(31): 6965-6971
Published online Aug 21, 2016. doi: 10.3748/wjg.v22.i31.6965
Published online Aug 21, 2016. doi: 10.3748/wjg.v22.i31.6965
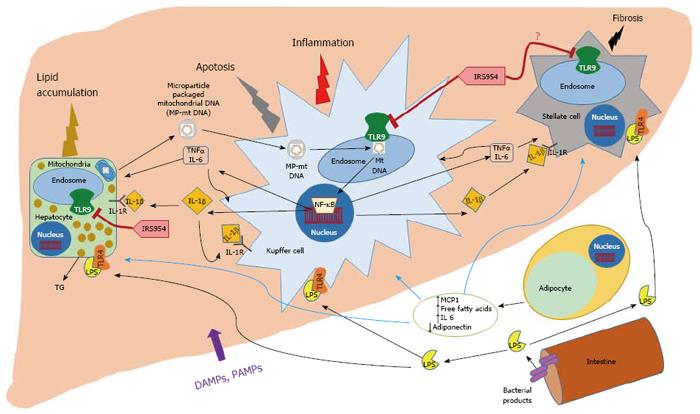
Figure 1 Hepatocyte mitochondrial DNA serves as a damage-associated molecular pattern to activate TLR9 leading to nonalcoholic steatosis progression.
Our current understanding of the pathophysiology of nonalcoholic steatosis (NASH) is that obesity and metabolic syndrome promote adipose tissue (AT) impairment to release free fatty acids (FFA) from the adipocytes into the portal circulation, which leads to accumulation of triglycerides in the hepatocytes. In addition to FFA, the adipocytes release increased levels of chemokines and cytokines, MCP-1 and IL-6, and reduced levels of beneficial adipokines such as adiponectin. In addition to the impaired AT, tissues such as the intestine contribute bacterial products such as endotoxin/LPS and other pathogen associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), which enter the liver, and activate their receptors on the Kupffer cells and other hepatic cell populations to produce cytokines such as IL-1β, TNFα and IL-6, which, in turn, promote injury and increased triglyceride accumulation in hepatocytes, inflammation and apoptosis in the hepatic immune cells and may activate fibrosis in the stellate cells, and thereby accelerate NASH progression. In addition to our current understanding, a recent study revealed that steatotic hepatocytes release mitochondrial DNA into the plasma, which once enclosed within microparticles, activates TLR9 in endosomes to undergo hyperactivation and produce inflammatory cytokines such as IL-1β, TNFα and IL-6, which, promote NASH progression by amplifying hepatic inflammation and injury. Additionally, an antagonist of TLR7/9, IRS 954, was effective in blocking TLR9 ligand binding and activation, and thereby attenuating NASH.
- Citation: Handa P, Vemulakonda A, Kowdley KV, Uribe M, Méndez-Sánchez N. Mitochondrial DNA from hepatocytes as a ligand for TLR9: Drivers of nonalcoholic steatohepatitis? World J Gastroenterol 2016; 22(31): 6965-6971
- URL: https://www.wjgnet.com/1007-9327/full/v22/i31/6965.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i31.6965