Published online Aug 14, 2016. doi: 10.3748/wjg.v22.i30.6890
Peer-review started: March 26, 2016
First decision: May 12, 2016
Revised: May 22, 2016
Accepted: June 15, 2016
Article in press: June 15, 2016
Published online: August 14, 2016
Processing time: 131 Days and 7.4 Hours
Nonalcoholic fatty liver disease (NAFLD), which is characterized by excessive fat accumulation in the liver of patients who consume little or no alcohol, becomes increasingly common with rapid economic development. Long-term excess fat accumulation leads to NAFLD and represents a global health problem with no effective therapeutic approach. NAFLD is considered to be a series of complex, multifaceted pathological processes involving oxidative stress, inflammation, apoptosis, and metabolism. Over the past decades, herbal medicines have garnered growing attention as potential therapeutic agents to prevent and treat NAFLD, due to their high efficacy and low risk of side effects. In this review, we evaluate the use of herbal medicines (including traditional Chinese herbal formulas, crude extracts from medicinal plants, and pure natural products) to treat NAFLD. These herbal medicines are natural resources that can inform innovative drug research and the development of treatments for NAFLD in the future.
Core tip: Herbal medicines have gained popularity as potential therapeutic agents for the prevention and treatment of nonalcoholic fatty liver disease (NAFLD), due to their high efficacy and low side effects. This review introduces traditional Chinese herbal formulas, crude extracts from medicinal plants, and pure natural products as new treatments for NAFLD.
- Citation: Yao H, Qiao YJ, Zhao YL, Tao XF, Xu LN, Yin LH, Qi Y, Peng JY. Herbal medicines and nonalcoholic fatty liver disease. World J Gastroenterol 2016; 22(30): 6890-6905
- URL: https://www.wjgnet.com/1007-9327/full/v22/i30/6890.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i30.6890
Nonalcoholic fatty liver disease (NAFLD) is rapidly becoming a serious global health problem as the prevalence of obesity and type 2 diabetes mellitus (T2DM) rises[1,2]. The term NAFLD refers to a spectrum of liver diseases, ranging from hepatic steatosis to nonalcoholic steatohepatitis (NASH) and cirrhosis, specifically in patients who do not consume excessive amounts of alcohol[3-5]. NAFLD is present in up to one-third of the population, affects all ages and ethnicities, and is the second leading cause of death in the general population[6,7]. At present, the high prevalence and negative pathological consequences of NAFLD represent a significant economic burden for many countries. However, up to now, there is no effective procedure to treat the disease[8-10]. The primary therapeutic approach is to recommend healthy lifestyle strategies that are focused on reducing body weight and increasing insulin sensitivity, including dietary and exercise regimens. Although these strategies are effective in randomized controlled trials, they have limited impact on the incidence and severity of NAFLD at the population level, due to poor patient compliance[11-13].
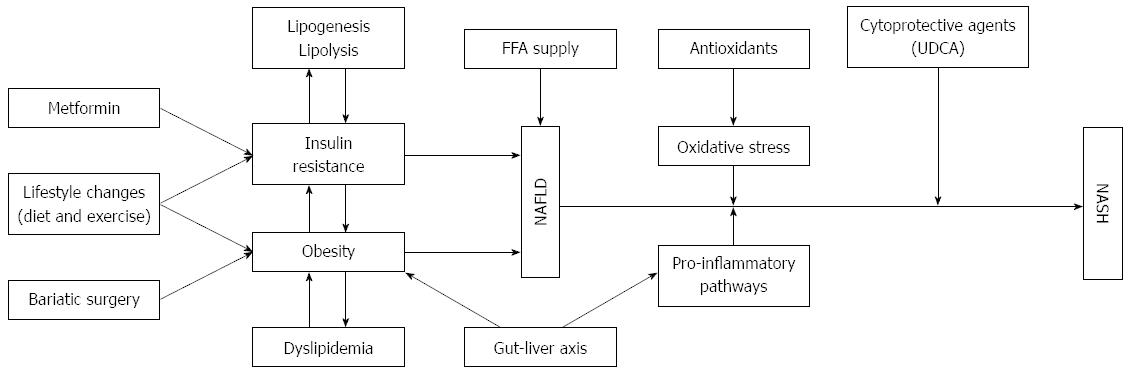
NAFLD is believed to be an essential component of the liver metabolic syndrome, including insulin resistance, obesity, hyperlipidemia, dyslipidemia, and hypertension[14-16] (Figure 1). Although the activity of plasma transaminase enzymes can serve as an early indicator of liver damage, NAFLD cannot be accurately diagnosed with routine blood tests[17,18]. Liver biopsy, accompanied by histological staining and NAFLD activity score, is the standard for NAFLD diagnosis, however, in clinical practice, its use is limited due to invasiveness[19]. In the past 10 years, the association between NAFLD and other chronic diseases, such as chronic liver disease, cardiovascular disease, and T2DM, has been a major focus of NAFLD research[20,21]. Additionally, increasing attention has also focused on NAFLD-related chronic kidney disease[22]. There is also emerging evidence that NAFLD is linked to other chronic diseases, including sleep apnea, colorectal cancer, osteoporosis, psoriasis, and various endocrinopathies[23]. Hence, there is a huge demand to explore effective approaches to NAFLD treatment.
Due to the key role of lipid accumulation in NAFLD progression, inhibition of lipid accumulation is a major focus of anti-NAFLD drug development. A variety of anti-NAFLD agents are currently in preclinical development. Additionally, metformin, statins, and fibrates, are currently being tested as NAFLD treatments in clinical trials. However, these drugs have significant adverse side effects, including enhanced risk of infection and osteoporosis[24-26]. Hence, novel treatment candidates with high efficacy and minimal side effects are urgently demanded for the treatment of NAFLD[27-30].
Traditional Chinese medicines (TCM) are abundant sources of biologically active substances that can be applied to prevent human diseases[31-34]. Currently, an increasing number of studies have focused on herbal extracts or natural products, and many of these studies have discovered herbal products with potent effects against NAFLD[35,36]. Thus, herbal medicines are promising candidate drugs for the treatment of NAFLD. The primary aim of this paper is to systematically review the available herbal medicines (including traditional Chinese herbal formulas, crude extracts from medicinal plants, and pure natural products) for the treatment of NAFLD.
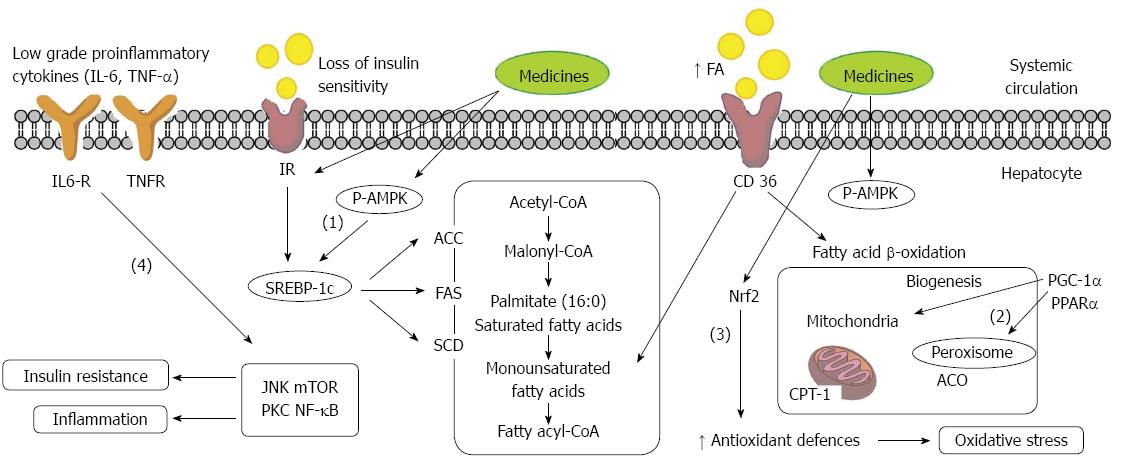
Due to the current lack of effective therapies, there is a great need to identify dietary approaches to NAFLD prevention and treatment. Evidence from cells and animal studies suggests that many drugs can protect NAFLD and its progression in steatosis. Traditional medicines can prevent NAFLD through a variety of mechanisms, including: (1) depressing lipogenesis through down-regulating sterol regulatory element-binding protein 1c (SREBP-1c); (2) increasing β-fatty acid (FA) oxidation by up-regulating peroxisome proliferator activated receptor α (PPARα); (3) increasing insulin sensitivity and depressing oxidative stress through increased antioxidant levels via nuclear factor-erythroid 2-related factor 2 (Nrf2); and (4) inhibiting activation of inflammatory pathways (Figure 2). Activation of the AMPK/SIRT-1 signaling pathway is the common trigger that regulates all of these molecular processes in recent insights. Nevertheless, more experiments are needed to verify this hypothesis. Moreover, indirect anti-inflammatory and anti-oxidative effects of TCM may also help to improve the symptoms of NAFLD.
Currently, the use of a traditional Chinese herbal formula is in a dialectical trial to assess its efficacy as an NAFLD treatment method. A traditional Chinese herbal formula consists of two or more appropriate medicinal plants for discretionary use that are selected in accordance with the composition principles of proper compatibility[37]. The formula contains complex chemical constituents with multi-level and multi-target pharmacological activity[38,39]. The traditional Chinese herbal formula prescription consists of four parts: Monarch, Minister, Assistant, and Guide. The Monarch drug, also known as the main drug, is intended to provide the major therapeutic effect to treat the main disease or principal syndrome[40]. The Minister drug, also known as the official medicine adjuvant, strengthens the effect of the auxiliary gentleman medicine drug to treat the main disease or primary syndrome. The Assistant drug either indirectly treats the primary disease by assisting the Monarch and Minister drugs, or directly treats secondary syndromes. The Guide drug acts as a messenger drug that leads other drugs to the site of disease[41,42]. Traditional Chinese herbal formulas are developed according to traditional theory, which guides the selection of appropriate medicines according to prescription principles, and determines the dosage and usage of each medicine[43].
Many traditional Chinese herbal formulas are reported to have significant anti-NAFLD effects. One famous traditional Chinese herbal formula, Yinchenhao Decoction (YCHD), first recorded in the “Shen Nong’s Herbal Classic”, has been used in treatment of gallbladder and liver diseases for centuries. YCHD consists of three medicinal plants: Artemisia capillaris (Thunb), Gardenia jasminoides (Ellis), and Rheum palmatum (L)[44]. Recent studies have reported that YCHD can reduce the accumulation of hepatic fat, enhance adiponectin secretion, increase endothelial progenitor cell proliferation, and increase PPAR-γ expression, which is probably responsible for the therapeutic effect of YCHD on NAFLD[45,46]. Another well-known traditional Chinese herbal formula, Qushi Huayu Decoction (QSHYD), consists of five kinds of medicinal plants: Artemisia capillaris (Thunb), Polygonum cuspidatum Sieb. et Zucc., Hypericum japonicum (Thunb), Curcuma Longa L, and Gardenia jasminoides (Ellis)[47]. QSHYD can effectively reverse elevated levels of free fatty acid and total triglycerides (TG), and also can improve hepatic steatosis and inflammation[48]. Furthermore, QSHYD may inhibit fat deposition and inflammation through multiple signaling pathways[49,50]. Apart from these, other traditional Chinese herbal formulas (Table 1), including Danning Tablet[51], Sini San[52], Ganzhixiao Decoction[53], Tangzhiqing Decoction[54], Hugan Qingzhi tablet[55], Cigu Xiaozhi Pill[56], BaiHuJia RenShen Decoction[57], LiGan ShiLiuBaWei San[58], Gegenqinlian Decoction[59], Lingguizhugan Decoction[60] and Huanglian Jiedu Decoction[61] are also effective treatments for NAFLD.
| Formula | Composition | Mechanisms | Ref. |
| Yinchenhao Decoction | Artemisia capillaries Thunb. | ↓PPARγ expression | [46] |
| Gardenia jasminoides Ellis | |||
| Rheum palmatum L. | |||
| Qushi Huayu Decoction | Artemisia capillaries Thunb. | ↓SCD1, ↓FAS, | [50] |
| Rhizoma polygoni Cuspidati | ↓ACAT, ↑CPT expression | ||
| Hypericum japonicum Thunb | ↓Lipid droplets and inflammatory infiltration | ||
| Rhizoma curcumae Longae | ↓TNFα | ||
| Gardenia jasminoides Ellis | |||
| Danning Tablet | Rheum palmatum L. | ↓Fat mass | [51] |
| Polygonum cuspidatum Sieb.et Zucc. | ↓ALT level | ||
| Citrus reticulata Blanco | |||
| Curcuma rcenyujin Y. | |||
| Crataegus pinnatifida Bunge | |||
| Sini San | Bupleurum scorzonerifolium Willd | ↓ALT, ↓AST level | [52] |
| Paeonia lactiflora Pall | ↓Steatosis | ||
| Fructus aurantii Immaturus | |||
| Glycyrrhiza uralensis Fisch | |||
| Ganzhixiao Decoction | Artemisia capillaries Thunb. | ↓ALT, ↓TG, ↓IHCL level | [53] |
| Rhizoma polygoni Cuspidati | ↑CT value ratio | ||
| Radix bupleuri Chinensis | |||
| Cigu Xiaozhi Pill | Sagittaria sagittifolia | ↓ALT, ↓AST level | [56] |
| Alisma plantago-aquatica Linn | ↓TC, ↓TG level | ||
| Crataegus pinnatifida Bunge | |||
| Salvia miltiorrhiza Bge | |||
| Steleophaga plancyi Boleny | |||
| Pinellia ternata Breit | |||
| Tangzhiqing Decoction | Paeonia veitchii Lynch | ↓TC, ↓TG level | [54] |
| Morus alba L. | ↓LDL-C, ↓HDL-C level | ||
| Lotus leaf Tea | ↓Fat mass | ||
| Salvia miltiorrhiza Bge. | ↓MDA level | ||
| Grataegus pinnati fida Bge. | |||
| Hugan Qingzhi Tablet | Alisma orientalis Juzep | ↓ALT, ↓AST level | [55] |
| Crataegus pinnatifida Bunge | ↓TC, ↓TG level | ||
| Typha orientalis C. Presl | ↓IL-6, ↓P65 | ||
| Nelumbo nucifera Gaertn | |||
| Panax pseudoginseng var. notoginseng | |||
| BaiHuJia RenShen Decoction | Anemarrhena asphodeloides Bunge | ↑p-AMPK level | [57] |
| Radix Glycyrrhizae Preparata | ↓SCD1, ↓FAS, | ||
| Oryza sativa L. | ↓ACAT, ↑CPT expression | ||
| Gypsum Fibrosum | |||
| Panax ginseng C. A. Mey. | |||
| LiGan ShiLiuBaWei San | Punica granatum L. | ↓ALT, ↓AST level | [58] |
| Cinnamomum tamala Nees | ↓TC, ↓TG, | ||
| Alpinia katsumadai Hayata | ↓FFA, ↓MDA level | ||
| Piper longum Linn | ↑PPARα expression | ||
| Carthamus tinctorius L. | |||
| Amomum tsao-ko Crevost et Lemaire | |||
| Zingiber oj-jicinale Rosc | |||
| Myristica fragrans Houtt. | |||
| Gegenqinlian Decoction | Pueraria omeiensis Wang | ↓LDL-C, ↓HDL-C level | [59] |
| Scutellaria baicalensis Georgi | ↓PPARγ | ||
| Coptis chinensis Franch | |||
| Glycyrrhiza uralensis Fisch | |||
| Lingguizhugan Decoction | Smilax ocreafa A. | ↓TC, ↓TG, ↓LDL-C | [60] |
| Cinnamomum tamala Nees | |||
| Rhizoma atractylodis macrocephalae | |||
| Glycyrrhiza uralensis Fisch | |||
| Huanglianjiedu Decoction | Coptis chinensis Franch | ↓TC, ↓TG, | [61] |
| Scutellaria baicalensis Georgi | ↓LDL-C, ↓HDL-C leve | ||
| Heteropogon contortus P. | |||
| Gardenia jasminoides Ellis |
Although academic journals have reported the benefits of many traditional Chinese herbal formulas in NAFLD therapy, there are several issues to note in these recent studies. The efficacy of these drugs is not clear, due to the limitations of the existing non-invasive techniques that are clinically used to assess the extent of inflammation and liver steatosis[62]. Furthermore, the impact of pharmacodynamic interactions between these formulas and other medications should be evaluated further. The molecular targets of these drugs and the signaling transduction pathways involved remain unknown, which further complicates clinicians’ ability to predict how these formulas may interact with other medicines[63,64]. Molecular targets for drug interactions are generally more difficult to predict the pharmacokinetic interactions. All of the issues mentioned above retard the scientific progress of TCM formulas in treating NAFLD.
Compared with traditional Chinese herbal formulas, the use of crude extracts from medicinal plants represents a fusion of modern pharmaceutical technology with traditional medicine. In this treatment approach, traditional medicinal materials are processed into purified bioactive compounds by leaching, clarification, filtration, evaporation, or other methods of extraction[65]. Extraction of compounds from Chinese herbal medicines is one approach to discover novel drugs. The extraction of active compounds is also important for enhancing our understanding of traditional Chinese medicine[66,67]. After extraction and separation, crude extracts have higher purity, are easy to administer, and can be subjected to quality control[68,69]. Thus, use of crude extracts from medicinal plants to treat NAFLD is a feasible approach.
Many crude extracts from medicinal plants have significant anti-NAFLD effects. Polygonum hypoleucum (Ohwi) is the dry root of leguminous plants belonging to the genus Pueraria, which is recorded in the “Treatise on Febrile Diseases”. It has been used to treat cancer, arthritis, and nephritis[70]. Extract of P. hypoleucum contains the chemicals epicatechin, emodin, epicatechin-3-O-gallate, catechin and procyanidin B2P[71]. P. hypoleucum can also inhibit acetyl-CoA carboxylase (ACC) activity, which plays a key role in FA metabolism. Inhibiting ACC expression has been demonstrated to prevent high-fat diet (HFD)-induced NAFLD and hepatic ischemia-reperfusion (IR)[72-74]. Artemisia Sacrorum Ledeb (ASL) is a TCM used to treat multiple liver diseases. Ethanol extract from ASL can attenuate hepatic lipid accumulation via activating adenosine 5'-monophosphate-activated protein kinase (AMPK) in human HepG2 cells[75]. Besides promoting AMPK and ACC phosphorylation, ethanol extract from ASL down-regulates expression of the lipogenesis gene SREBP-1c, and also decreases the expression of target genes of SREBP-1c, including FA synthase (FAS) and stearoyl-coenzyme A desaturase 1. Conversely, EE also increases the expression of lipolytic genes, including PPAR-α and cluster of differentiation 36 (CD36)[76]. Other herbal extracts (shown in Table 2) from Chinese blueberry[77], Hibiscus sabdariffa L.[78], red grapes[79], grape skin[80], coffee[81], Roiboos (Aspalathus linearis)[82], Lotus root[83], hawthorn leaf[84], araliaelata[85], rubus aleaefolius[15], neomangiferin[86] and tea[87] are also effective in treating NAFLD.
| Crude extract | Model | Mechanisms | Ref. |
| Polyphenols extract from Chinese blueberry | HepG2 cells | ↓TG | [73] |
| ↓Inflammatory | |||
| Polyphenols extract from Hibiscus sabdariffa L. | BALB/c normal liver cells | ↓Death cell | [74] |
| ↓p-JNK, ↓Bax, ↓tBid expression | |||
| ↓MDA, ↑GSH levels | |||
| ↑CAT activity | |||
| Polyphenols extract from red grapes | Male Wistar rats | ↓Steatosis, ↓TG | [75] |
| (HFD) | ↑SIRT-1, ↑p-ACC level | ||
| Polyphenols extract | C57BL/6J mice | ↓Hepatic cholesterol | [76] |
| from grape skin | (HFD) | ↓ChREBP, ↓PPARγ, ↓SCD1 ↓FAS, | |
| ↓ACAT, ↑PPARα, ↑CPT expression | |||
| ↑β-oxidation, FAS, ME activity | |||
| ↓Leptin, ↑adiponectin, ↓NEFA | |||
| Polyphenols extract from Coffee | Male Wistar rats | ↓ALT plasma, | [77] |
| (HFD) | ↑GSH/GSSG ratio, ↓MDA | ||
| ↓Lipid droplets and inflammatory Infiltration | |||
| ↓TNFα | |||
| ↑PPARα and Adipo R2 expression | |||
| ↑FRAP in serum | |||
| ↑IL-4 and IL-10 | |||
| ↓IL-1a and IL-1b | |||
| Polyphenol extract from Aspalathus linearis Roiboos | Male C57BL/6J | ↓Steatosis, ↓TG | [78] |
| (HFD) | ↓Macrophage infiltration | ||
| ↓Cholesterol and NEFA in serum | |||
| ↑p-AMPK level | |||
| ↑FAS level | |||
| Polyphenolic extract from Lotus root | Male db/db mice | ↓Liver weight | [79] |
| ↓TG level | |||
| ↓FAS and ME activity | |||
| Polyphenol extract from Hibiscus sabdariffa L. | Male C57BL/6J mice | ↓Body weight ↓TG, ↓Steatosis | [72] |
| (HFD) | ↓Adipocyte size in adipose tissue | ||
| ↓Insulin resistance | |||
| ↓miR-103, ↓miR-107 and ↑miR-122 expression in liver | |||
| ↓FAS, ↑p-AMPK levels | |||
| ↓SREBP-1c expression | |||
| Flavonoids extract from Hawthorn leaf | Male Sprague Dawley rats | ↓ALT, ↓AST, ↓TC, ↓TG, ↓FFA, ↓FAS, ↑p-AMPK levels | [80] |
| (HFD) | |||
| ↑PPARα, ↓SREBP-1c expression | |||
| Total aralosides extract from araliaelata | ApoE–/– mice | ↓IL-6, ↓TNFα, ↓P65, ↓p-JNK expression | [81] |
| (HFD) | |||
| Rubus aleaefolius | Male Sprague Dawley rats | ↓TC, ↓TG, ↓FFA, | [82] |
| (HFD) | ↓SCD1, ↓FAS, | ||
| ↓ACAT, ↑CPT expression | |||
| Neomangiferin | Male Sprague Dawley rats | ↓TC, ↓TG, | [83] |
| (HFD) | ↓MDA, ↑SOD, | ||
| ↑PPARα, ↑CPT, | |||
| ↓FATP2, ↓ACSL1 expression | |||
| Deepure Tea | C57BL/6J mice | ↓SCD1, ↓FAS, ↑p-AMPK levels | [84] |
| (HFD) | ↑p-ACC level | ||
| ↓SREBP-1c expression |
On the other hand, extracting bioactive compounds from medicinal plants can be problematic. For example, many active compounds, especially water - insoluble compounds, may be lost during extraction in organic solvents. Furthermore, extraction solvents may react with active ingredients, or high temperatures during extraction may degrade labile compounds[88,89]. However, breakthroughs in science and technology could overcome these shortcomings in the future.
The term “pure natural products” refers to clear chemical structures that are different from traditional Chinese medicine formulas and crude extracts[90,91]. Pure natural products are derived from medicinal plants through extraction, separation, and purification[92]. Many pure natural products, including flavonoids, alkaloids, polysaccharides, volatile oils, quinones, terpenes, coumarins, lignans, saponins, cardiac glycosides, phenolic acids, and amino acids, have been found to have significant therapeutic benefits against NAFLD[93].
Flavonoids are compounds with a common basic structure of 15 carbons (C6-C3-C6)[94]. Flavonoids found in plants usually combine with sugar to form glycosides, however some remain in free-state (aglycone) form. There is growing evidence that flavonoids (or related compounds) have therapeutic effects on cancer and other chronic diseases, including cardiovascular disease, T2DM, and NAFLD, at least in part through immunomodulatory, anti- inflammatory, and antioxidant properties[95].
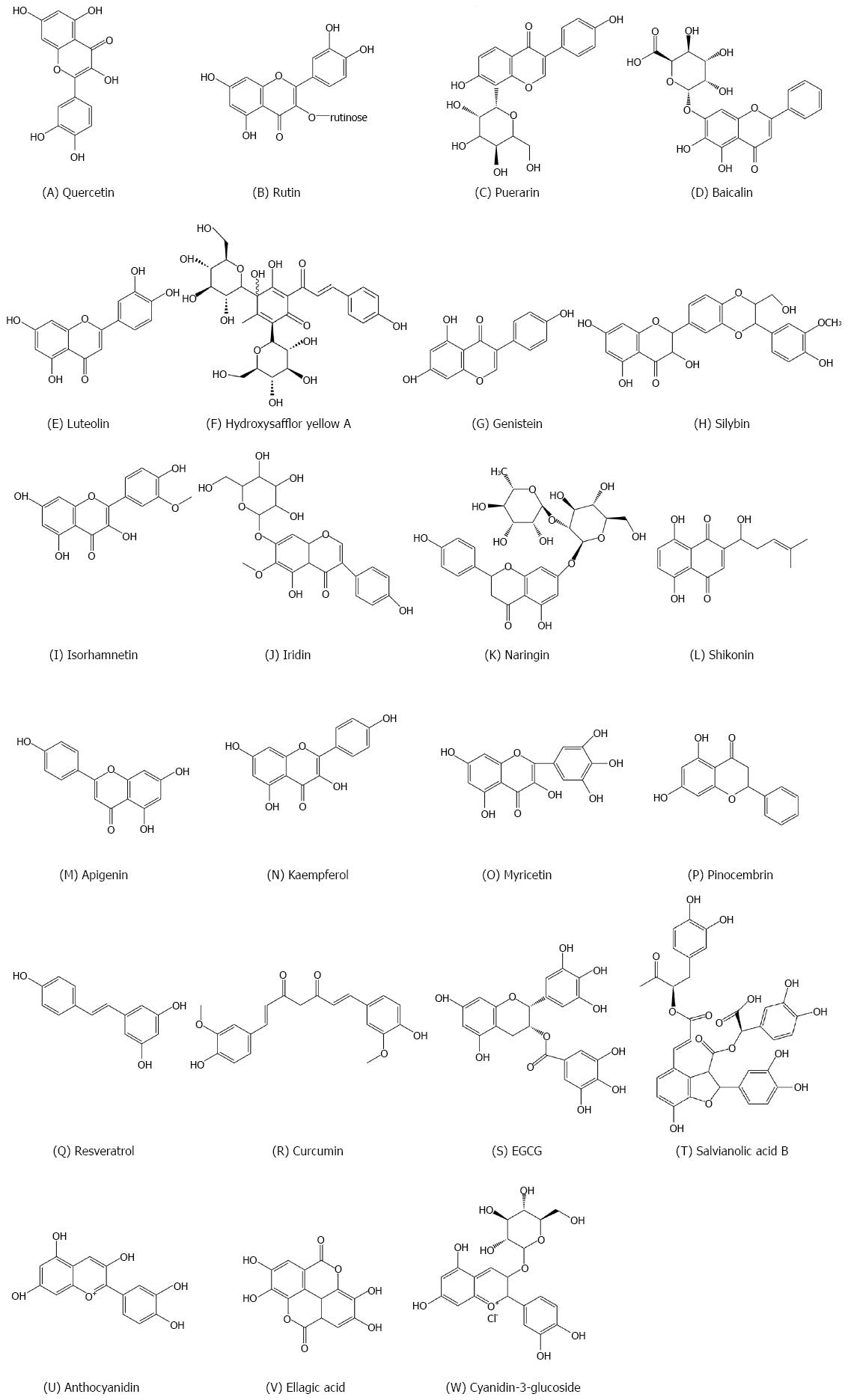
Quercetin (Figure 3A) is a well-known flavonoid that has a wide variety of biological functions. This flavonol is reported to have beneficial effects on lipid accumulation, inflammation, fibrosis, nitrosative/oxidative stress, and insulin resistance associated with NAFLD[96]. Previously, studies showed that quercetin reduces lipid accumulation in primary hepatocytes in obese mice fed a high-fat diet, through regulation of mitochondrial oxidative metabolism. Therefore, quercetin is a useful dietary additive for reducing obesity-induced hepatosteatosis[97,98].
Rutin (Figure 3B), a glycoside of quercetin, is found in many foods such as red wine, apples and onions. Panchal et al[99] first proved that rutin can decrease adiposity, improve insulin sensitivity, and reduce cardiac remodeling and liver injury in HFD rats[100]. Consistently, in a successive study, rutin effectively inhibited palmitate-induced macrophage activation and reduced liver fat by suppressing transcription of SREBP-1c and CD36 in the liver[101]. Recently, troxerutin was also shown to reduce liver steatosis and improve metabolic syndrome-related pathology in mice fed a high-fat diet, by suppressing oxidative stress-mediated NAD depletion and stimulating fat oxidation[99]. Other flavonoids, including pueraria[102], baicalein[103], luteolin[104], hydroxysafflor yellow A[105], genistein[106,107], silybin[108], isorhamnetin[109], iridin[110], naringin[111], shikonin[112], apigenin[113], kaempferol[114], myricetin[115], and pinocembrin[116] (Figure 3C-P), also play significant roles in the treatment of NAFLD.
Polyphenols are a group of phenolic compounds from plants. Phenolic compounds are present in a large amount in cereals, vegetables, fruits, and beverages including red wine, coffee and tea[117]. Polyphenols have strong antioxidant effects, and are commonly known as “the seventh kind of nutrient.” How well polyphenols exert antioxidant properties hinges on (1) the extent of their phase 1 and 2 bio-transformation; (2) the amount of conjugated products formed during the absorption of the gastrointestinal tract; and (3) the formation of conjugated products mainly absorbed in the liver[118].
Resveratrol (Figure 3Q) is contained in red grapes, Fructus Mori, Arachis hypogaea Linn. and cacao[119,120]. Two seminal studies show the positive effects of resveratrol on metabolic health and aging by activating AMPK and silent mating type information regulation 2 homolog 1 (SIRT1)[121,122]. Further studies suggest that resveratrol can reduce fat accumulation, even in the absence of weight loss. Resveratrol decreases liver fat accumulation through different mechanisms, including decreased lipogenesis and increased FA oxidation[123-126]. In addition, resveratrol has been shown to reduce lipid peroxidation by promoting the Nrf2-dependent antioxidative response in high fructose fed rats[127] and improving dysbiosis in the gut microbiome, which is induced by HFD. The proportion of resveratrol to the growth of the thick walled bacterial strain of the fungus, which was reduced by the growth of Lactobacillus and bacteria, was decreased[128]. Nevertheless, two clinical trials show that the results are contradictory. After 8 wk, the liver fat accumulation and insulin sensitivity showed no improvement compared to the men on 3000 mg of resveratrol. What's more, no change was observed in the plasma antioxidant activities. Importantly, this study reported that resveratrol supplements increased plasma liver enzyme levels, which showed hepatic stress[129,130]. However, in a trial, the signature of the liver enzyme with inflammatory cytokines was shown to improve in 50 patients with NAFLD treated with resveratrol 500 mg for 12 wk, although the antioxidant effect was not reported[131].
Curcumin (Figure 3R), responsible for the yellow colour of the plant Curcuma Longa L, is extracted from curry and spice. Its antioxidant properties are widely studied in liver metabolism[132]. Curcumin has also been studied for NASH and metabolic pathologies. Leclercq et al[133] showed that curcumin improves liver injury by inhibiting nuclear factor-kappa B (NF-κB) activation, which in turn inhibits the expression of NF-κB target genes, including intercellular cell adhesion molecule-1, cyclooxygenase-2, and monocyte chemotactic protein 1. Vizzutti et al[134] later extended that curcumin can reduce alpha-smooth muscle actin a level in the NASH mice and can reduce the production of reactive oxygen species and tissue inhibitor of metalloproteinases-1 secreting activated hepatic stellate cells. While some dietary supplements containing curcumin are commercially available, it should be emphasized that case-reports and case series provide insufficient clinical evidence to draw firm conclusions. Polyphenols including techin-3-gallate[135], salvianolic acid B[136], anthocyanidin[137], ellagic acid[138] and cyanidin-3-glucoside[139] (Figure 3S-W) also play significant roles in the treatment of NAFLD.
Terpenoids are compounds with molecular formulas containing multiple hydrocarbon isoprene units and their oxygenated derivatives. These oxygenated derivatives can be alcohols, aldehydes, ketones, carboxylic acids or esters. Terpenoids exist widely in the nature, and are the main components of some plant essence, and pigment resins[140]. Terpenoids have many physiological activities including acting as an expectorant, relieving cough, expelling wind, inducing sweating, acting as an insecticide, and reducing pain (analgesia)[141].
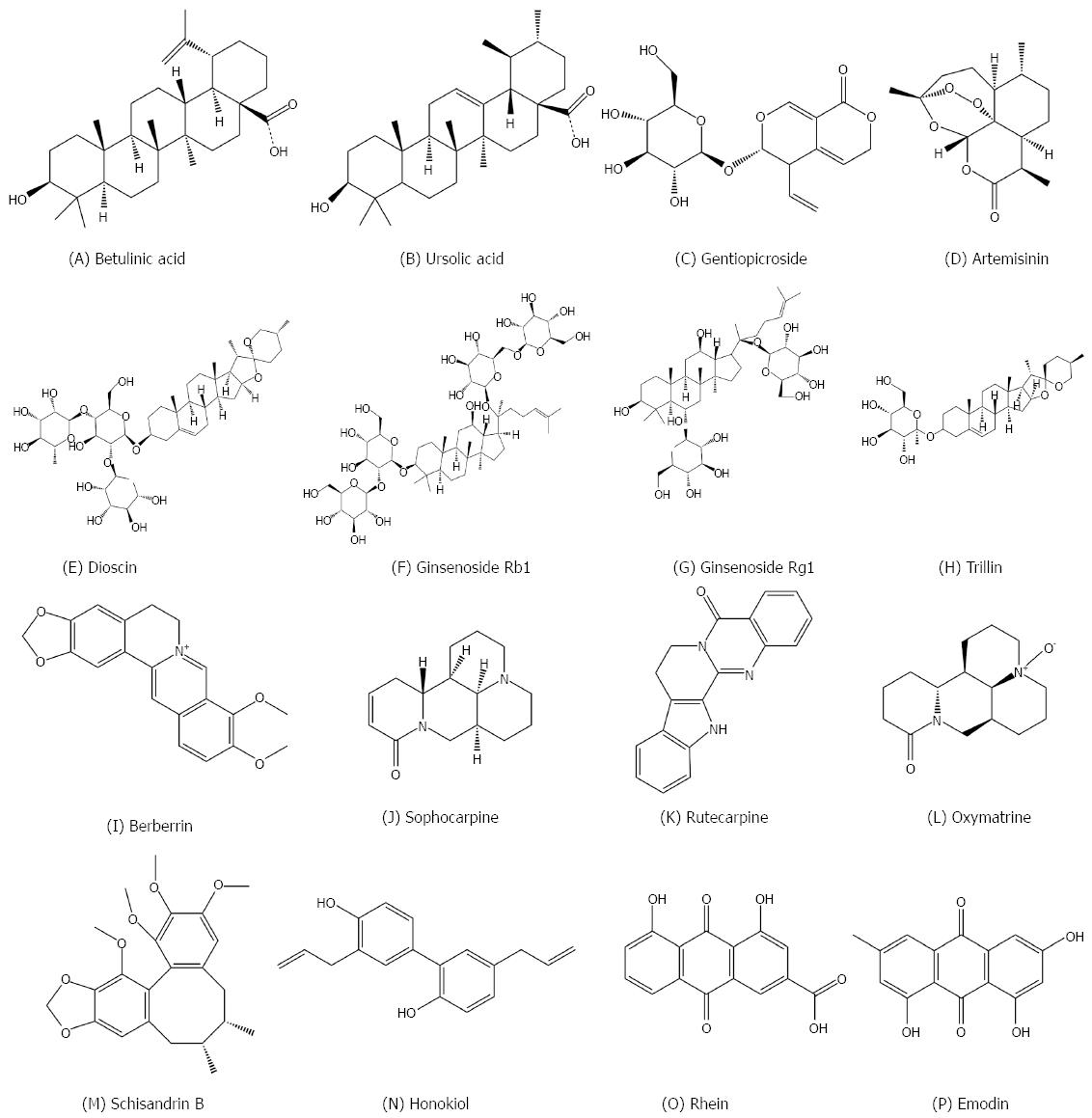
Betulinic acid (Figure 4A) is a pentacyclic triterpene found in many plants, especially Betula. Betulinic acid can be converted from its precursor, betulin. Betulinic acid plays a significant role in reducing hepatic lipid accumulation through modulation of the AMPK-SREBP signaling pathway[142]. Mice fed an HFD for a three-week period exhibit severe fat accumulation in the liver, significant reductions in hepatic AMPK phosphorylation, and increased activation of SREBP1. Betulinic acid activates AMPK by activating an upstream kinase, calmodulin-dependent protein kinase kinase. Betulinic acid also suppresses mammalian target of rapamycin and S6 kinase-mediated activation of SREBP1 in a human hepatoma cell line, primary rat hepatocytes, and liver tissue of Institute of Cancer Research mice fed an HFD. Treatment with betulinic acid inhibits HFD-induced changes in nuclear SREBP1 activation and consequent hepatic TG accumulation[143]. Other terpenoids, such as ursolic acid[144], gentiopicroside[145] and artemisinin[146] (Figure 4B-D), also play significant roles in the treatment of NAFLD.
Saponins are glycoside aglycones of three terpenoids or spirostane compounds, mainly found in terrestrial plants[147]. The primary active ingredients in many Chinese traditional herbs, such as Panax ginseng (C. A. Mey.), Polygala tenuifolia (Willd.), Glycyrrhiza uralensis (Fisch), and Platycodon grandiflorus (Jacq.) A. DC., are saponins. Some saponins also have anti-bacterial, anti-pyretic, and anti-cancer activities[148,149].
Dioscin (Figure 4E) is a natural steroid saponin widely found in various herbs[150]. Previous studies have demonstrated that dioscin has anti-tumor[151], anti-hyperlipidemic[152], and anti-fungal activities[153]. Studies have shown that dioscin can gradually reduce the weight, but not suppress appetite or increase physical activity in obese mice. Oral administration of dioscin reduces blood lipid levels, improves fat accumulation in the liver, decreases liver cholesterol and FA and triglyceride deposition through inhibition of FAS, promotes FA beta oxidation, reduces oxidative stress and inflammation, and regulates the MAPK signaling pathway and autophagy[154]. Other saponins such as ginsenoside Rb1[155], ginsenoside Rg1[156] and trillin[157] (Figure 4F-H) also play significant roles in the treatment of NAFLD.
Alkaloids are a group of nitrogenous organic compounds present in nature. They are widely found in dicotyledons. They have many pharmacological activities, such as anti-bacterial, anti-inflammatory, analgesic, anti-tumor, and anti-fungal actions[158,159]. A large number of studies have indicated that alkaloids have significant effects on NAFLD.
Berberine (Figure 4I) is isolated from the herb Coptis chinensis Franch. and widely used to treat diarrhea and other inflammatory diseases in China[160]. Recent studies have proved a new therapeutic function of berberine in metabolic disorders, including obesity and diabetes[161,162]. Berberine can be used as a cholesterol lowering drug, through a unique mechanism distinct from statins[163]. These studies suggested a potential therapeutic activity of berberine for NAFLD. Liver gene expression profile analysis showed that high fat diet induced hepatic steatosis in rats led to global changes in gene expression, and treatment with berberine reversed this process. Several modules of berberine-regulated genes, including abundant long non-coding RNAs (lncRNAs), were identified by bioinformatics analysis. Among these berberine-regulated genes, we found that the lncRNA MRAK052686 and its associated gene Nrf2 are implicated in the pathogenesis of NAFLD[164]. Hence, the study provides a new insight into the mechanism of the pharmacological action of berberine in the prevention and treatment of NAFLD. Other alkaloids such as sophocarpine[165], rutecarpine[166] and oxymatrine[167] (Figure 4J-L) also play significant roles in protecting against NAFLD.
Other pure products have been showed to be effective in the treatment of NAFLD, including schisandrin B[168], honokiol[169], rhein[170] and emodin[171] (Figure 4M-P). TCM are worthy of further study. This review only summarizes a drop in the bucket, and more Chinese medicines that are useful for the treatment of NAFLD will come to light in the future.
NAFLD, the main cause of chronic hepatic disease, is essentially a condition of over-nutrition, and the effective treatments are limited. Thus, it is very important to search ways to prevent and treat NAFLD. In this review, the experimental evidence has suggested that a number of herbal medicines can prevent steatosis and NAFLD through various underlying mechanisms. However, more convincing experiments are needed to confirm this hypothesis. What’s more, the indirect anti-inflammatory and antioxidant effects of TCM also play an important role in the treatment of NAFLD. But so far, the results of clinical studies are limited and tend to show a subtle influence in comparison with animal models. Further studies on the use of dietary doses of Chinese herbal medicines in rodents and human subjects are necessary.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Balaban YH, Fierbinteanu-Braticevici C, Grieco A, Wisse E S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Ma S
| 1. | Schuppan D, Gorrell MD, Klein T, Mark M, Afdhal NH. The challenge of developing novel pharmacological therapies for non-alcoholic steatohepatitis. Liver Int. 2010;30:795-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Stojsavljević S, Gomerčić Palčić M, Virović Jukić L, Smirčić Duvnjak L, Duvnjak M. Adipokines and proinflammatory cytokines, the key mediators in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:18070-18091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 252] [Cited by in RCA: 252] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 3. | Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47-S64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1516] [Cited by in RCA: 2140] [Article Influence: 214.0] [Reference Citation Analysis (0)] |
| 4. | Bhatia LS, Curzen NP, Calder PC, Byrne CD. Non-alcoholic fatty liver disease: a new and important cardiovascular risk factor? Eur Heart J. 2012;33:1190-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 345] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 5. | Fan JG, Farrell GC. Epidemiology of non-alcoholic fatty liver disease in China. J Hepatol. 2009;50:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 437] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 6. | Vajro P, Lenta S, Pignata C, Salerno M, D’Aniello R, De Micco I, Paolella G, Parenti G. Therapeutic options in pediatric non alcoholic fatty liver disease: current status and future directions. Ital J Pediatr. 2012;38:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Tziomalos K, Athyros VG, Paschos P, Karagiannis A. Nonalcoholic fatty liver disease and statins. Metabolism. 2015;64:1215-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Gu Y, Lambert JD. Modulation of metabolic syndrome-related inflammation by cocoa. Mol Nutr Food Res. 2013;57:948-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Dahlhoff C, Worsch S, Sailer M, Hummel BA, Fiamoncini J, Uebel K, Obeid R, Scherling C, Geisel J, Bader BL. Methyl-donor supplementation in obese mice prevents the progression of NAFLD, activates AMPK and decreases acyl-carnitine levels. Mol Metab. 2014;3:565-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Day CP. Non-alcoholic fatty liver disease: a massive problem. Clin Med (Lond). 2011;11:176-178. [PubMed] |
| 11. | Zelber-Sagi S, Lotan R, Shlomai A, Webb M, Harrari G, Buch A, Nitzan Kaluski D, Halpern Z, Oren R. Predictors for incidence and remission of NAFLD in the general population during a seven-year prospective follow-up. J Hepatol. 2012;56:1145-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 194] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 12. | Haga Y, Kanda T, Sasaki R, Nakamura M, Nakamoto S, Yokosuka O. Nonalcoholic fatty liver disease and hepatic cirrhosis: Comparison with viral hepatitis-associated steatosis. World J Gastroenterol. 2015;21:12989-12995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Jacome-Sosa MM, Borthwick F, Mangat R, Uwiera R, Reaney MJ, Shen J, Quiroga AD, Jacobs RL, Lehner R, Proctor SD. Diets enriched in trans-11 vaccenic acid alleviate ectopic lipid accumulation in a rat model of NAFLD and metabolic syndrome. J Nutr Biochem. 2014;25:692-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Karlas T, Wiegand J, Berg T. Gastrointestinal complications of obesity: non-alcoholic fatty liver disease (NAFLD) and its sequelae. Best Pract Res Clin Endocrinol Metab. 2013;27:195-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Li Y, Zhao J, Zheng H, Zhong X, Zhou J, Hong Z. Treatment of Nonalcoholic Fatty Liver Disease with Total Alkaloids in Rubus aleaefolius Poir through Regulation of Fat Metabolism. Evid Based Complement Alternat Med. 2014;2014:768540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Liu X, Gao Y, Li M, Geng C, Xu H, Yang Y, Guo Y, Jiao T, Fang F, Chang Y. Sirt1 mediates the effect of the heme oxygenase inducer, cobalt protoporphyrin, on ameliorating liver metabolic damage caused by a high-fat diet. J Hepatol. 2015;63:713-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Martínez-Uña M, Varela-Rey M, Mestre D, Fernández-Ares L, Fresnedo O, Fernandez-Ramos D, Gutiérrez-de Juan V, Martin-Guerrero I, García-Orad A, Luka Z. S-Adenosylmethionine increases circulating very-low density lipoprotein clearance in non-alcoholic fatty liver disease. J Hepatol. 2015;62:673-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Maslak E, Gregorius A, Chlopicki S. Liver sinusoidal endothelial cells (LSECs) function and NAFLD; NO-based therapy targeted to the liver. Pharmacol Rep. 2015;67:689-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 19. | McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62:1148-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 793] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 20. | Muñoz M, Sánchez A, Pilar Martínez M, Benedito S, López-Oliva ME, García-Sacristán A, Hernández M, Prieto D. COX-2 is involved in vascular oxidative stress and endothelial dysfunction of renal interlobar arteries from obese Zucker rats. Free Radic Biol Med. 2015;84:77-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Nobili V, Svegliati-Baroni G, Alisi A, Miele L, Valenti L, Vajro P. A 360-degree overview of paediatric NAFLD: recent insights. J Hepatol. 2013;58:1218-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 22. | Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 2015;62:720-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 1145] [Article Influence: 114.5] [Reference Citation Analysis (0)] |
| 23. | Popov VB, Lim JK. Treatment of Nonalcoholic Fatty Liver Disease: The Role of Medical, Surgical, and Endoscopic Weight Loss. J Clin Transl Hepatol. 2015;3:230-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Ford RJ, Fullerton MD, Pinkosky SL, Day EA, Scott JW, Oakhill JS, Bujak AL, Smith BK, Crane JD, Blümer RM. Metformin and salicylate synergistically activate liver AMPK, inhibit lipogenesis and improve insulin sensitivity. Biochem J. 2015;468:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 25. | Choi Y, Yanagawa Y, Kim S, Park T. Involvement of SIRT1-AMPK signaling in the protective action of indole-3-carbinol against hepatic steatosis in mice fed a high-fat diet. J Nutr Biochem. 2013;24:1393-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Yan F, Wang Q, Xu C, Cao M, Zhou X, Wang T, Yu C, Jing F, Chen W, Gao L. Peroxisome proliferator-activated receptor α activation induces hepatic steatosis, suggesting an adverse effect. PLoS One. 2014;9:e99245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Rodriguez-Ramiro I, Vauzour D, Minihane AM. Polyphenols and non-alcoholic fatty liver disease: impact and mechanisms. Proc Nutr Soc. 2016;75:47-60. [PubMed] |
| 28. | Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1682] [Article Influence: 140.2] [Reference Citation Analysis (0)] |
| 29. | Shin SY, Kim TH, Wu H, Choi YH, Kim SG. SIRT1 activation by methylene blue, a repurposed drug, leads to AMPK-mediated inhibition of steatosis and steatohepatitis. Eur J Pharmacol. 2014;727:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Wang X, Hai C. Redox modulation of adipocyte differentiation: hypothesis of “Redox Chain” and novel insights into intervention of adipogenesis and obesity. Free Radic Biol Med. 2015;89:99-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | Woo S, Yoon M, Kim J, Hong Y, Kim MY, Shin SS, Yoon M. The anti-angiogenic herbal extract from Melissa officinalis inhibits adipogenesis in 3T3-L1 adipocytes and suppresses adipocyte hypertrophy in high fat diet-induced obese C57BL/6J mice. J Ethnopharmacol. 2016;178:238-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Zhang JG, Liu Q, Liu ZL, Li L, Yi LT. Antihyperglycemic activity of Anoectochilus roxburghii polysaccharose in diabetic mice induced by high-fat diet and streptozotocin. J Ethnopharmacol. 2015;164:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Xia W, Sun C, Zhao Y, Wu L. Hypolipidemic and antioxidant activities of sanchi (radix notoginseng) in rats fed with a high fat diet. Phytomedicine. 2011;18:516-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Yuan L, Bambha K. Bile acid receptors and nonalcoholic fatty liver disease. World J Hepatol. 2015;7:2811-2818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 35. | Xu X, Lu L, Dong Q, Li X, Zhang N, Xin Y, Xuan S. Research advances in the relationship between nonalcoholic fatty liver disease and atherosclerosis. Lipids Health Dis. 2015;14:158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Yang Y, Li W, Liu Y, Sun Y, Li Y, Yao Q, Li J, Zhang Q, Gao Y, Gao L. Alpha-lipoic acid improves high-fat diet-induced hepatic steatosis by modulating the transcription factors SREBP-1, FoxO1 and Nrf2 via the SIRT1/LKB1/AMPK pathway. J Nutr Biochem. 2014;25:1207-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 37. | Dong SH, Zhang JF, Tang YM, Li J, Xiang YR, Liang QL. Chemical constituents from the tubers of Scirpus yagara and their anti-inflammatory activities. J Asian Nat Prod Res. 2016;18:791-797. [PubMed] |
| 38. | Li Y, Wang X, He H, Zhang D, Jiang Y, Yang X, Wang F, Tang Z, Song X, Yue Z. Steroidal Saponins from the Roots and Rhizomes of Tupistra chinensis. Molecules. 2015;20:13659-13669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Deng X, Zhang Y, Jiang F, Chen R, Peng P, Wen B, Liang J. The Chinese herb-derived Sparstolonin B suppresses HIV-1 transcription. Virol J. 2015;12:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | He Y, Gai Y, Wu X, Wan H. Quantitatively analyze composition principle of Ma Huang Tang by structural equation modeling. J Ethnopharmacol. 2012;143:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Gao R, Li S, Chen XJ, Wang XF, Wang SX, Fang MF. [Pharmacokinetic effect of combined administration on spinosin and ferulic acid in monarch drug Ziziphi Spinosae Semen kernel]. Zhongguo Zhong Yao Zazhi. 2015;40:3293-3297. [PubMed] |
| 42. | Zhao L, Li W, Li Y, Xu H, Lv L, Wang X, Chai Y, Zhang G. Simultaneous Determination of Oleanolic and Ursolic Acids in Rat Plasma by HPLC-MS: Application to a Pharmacokinetic Study After Oral Administration of Different Combinations of QingGanSanJie Decoction Extracts. J Chromatogr Sci. 2015;53:1185-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Yu JC, Han YY, Cheng HY, Zhang XZ, Zhao L, Kan BH, Jia YJ, Han JX. [Combination of acupoints based on the theory of qi tonification, blood regulation and strengthening the primary source]. Zhongguo Zhen Jiu. 2011;31:814-816. [PubMed] |
| 44. | Wu L, Zhou PQ, Xie JW, Zhu R, Zhou SC, Wang G, Wu ZX, Hao S. Effects of Yinchenhao decoction on self-regulation of renin-angiotensin system by targeting angiotensin converting enzyme 2 in bile duct-ligated rat liver. J Huazhong Univ Sci Technolog Med Sci. 2015;35:519-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Lee TY, Chang HH, Lo WC, Lin HC. Alleviation of hepatic oxidative stress by Chinese herbal medicine Yin-Chen-Hao-Tang in obese mice with steatosis. Int J Mol Med. 2010;25:837-844. [PubMed] |
| 46. | Zhou HB, Chen JM, Shao LM, Chen ZG. Apoptosis of human pancreatic carcinoma cell-1 cells induced by Yin Chen Hao Decoction. World J Gastroenterol. 2015;21:8352-8357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Li HS, Feng Q, Hu YY. [Effect of qushl huayu decoction on high-fat diet induced hepatic lipid deposition in rate]. Zhongguo Zhong Xi Yi Jie He Zazhi. 2009;29:1092-1095. [PubMed] |
| 48. | Zhang H, Feng Q, Li HS, Chen SD, Wang XN, Peng JH, Zhang N, Hu YY. [Effects of Qushi Huayu Decoction on cathepsin B and tumor necrosis factor-alpha expression in rats with non-alcoholic steatohepatitis]. Zhong Xi Yi Jie He Xuebao. 2008;6:928-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 49. | Li HS, Feng Q, Xu LL, Chen SD, Li XM, Hu YY. [Effects of Qushi Huayu Decoction in prevention and treatment of fatty liver in rats based on adiponectin-free fatty acid pathway]. Zhong Xi Yi Jie He Xuebao. 2009;7:546-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 50. | Zhang H, Hu YY, Feng Q. [Inhibitory effects of Qushi Huayu Decoction on fatty deposition and tumor necrosis factor alpha secretion in HepG2 cells induced by free fatty acid]. Zhongguo Zhong Xi Yi Jie He Zazhi. 2007;27:1105-1109. [PubMed] |
| 51. | Fan JG. Evaluating the efficacy and safety of Danning Pian in the short-term treatment of patients with non-alcoholic fatty liver disease: a multicenter clinical trial. Hepatobiliary Pancreat Dis Int. 2004;3:375-380. [PubMed] |
| 52. | Zhang Q, Zhao Y, Zhang DB, Sun LJ. Effect of Sinai san decoction on the development of non-alcoholic steatohepatitis in rats. World J Gastroenterol. 2005;11:1392-1395. [PubMed] |
| 53. | Wang N, Dong H, Wei S, Lu F. Application of proton magnetic resonance spectroscopy and computerized tomography in the diagnosis and treatment of nonalcoholic fatty liver disease. J Huazhong Univ Sci Technolog Med Sci. 2008;28:295-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 54. | Tao W, Deqin Z, Yuhong L, Hong L, Zhanbiao L, Chunfeng Z, Limin H, Xiumei G. Regulation effects on abnormal glucose and lipid metabolism of TZQ-F, a new kind of Traditional Chinese Medicine. J Ethnopharmacol. 2010;128:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 55. | Tang W, Zeng L, Yin J, Yao Y, Feng L, Yao X, Sun X, Zhou B. Hugan Qingzhi Exerts Anti-Inflammatory Effects in a Rat Model of Nonalcoholic Fatty Liver Disease. Evid Based Complement Alternat Med. 2015;2015:810369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 56. | Ma Y, Zhao J, Yang S, Jia Y. [Cigu Xiaozhi pills’s influence on lipid peroxidation and TNF-alpha expression in liver tissues of rats with nonalcoholic steatohepatitis]. Zhongguo Zhong Yao Zazhi. 2010;35:1292-1297. [PubMed] |
| 57. | Liu HK, Hung TM, Huang HC, Lee IJ, Chang CC, Cheng JJ, Lin LC, Huang C. Bai-Hu-Jia-Ren-Shen-Tang Decoction Reduces Fatty Liver by Activating AMP-Activated Protein Kinase In Vitro and In Vivo. Evid Based Complement Alternat Med. 2015;2015:651734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 58. | Jiang Y, Chen L, Wang H, Narisi B, Chen B. Li-Gan-Shi-Liu-Ba-Wei-San improves non-alcoholic fatty liver disease through enhancing lipid oxidation and alleviating oxidation stress. J Ethnopharmacol. 2015;176:499-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 59. | Wang YL, Liu LJ, Zhao WH, Li JX. Intervening TNF-α via PPARγ with Gegenqinlian Decoction in Experimental Nonalcoholic Fatty Liver Disease. Evid Based Complement Alternat Med. 2015;2015:715638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Yuanyuan W, Minghua J, Lina Z, Suhua L, Jiayu Z, Yongzhi S, Chunyu C, Jian Q. Effect of a combination of calorie-restriction therapy and Lingguizhugan decoction on levels of fasting blood lipid and inflammatory cytokines in a high-fat diet induced hyperlipidemia rat model. J Tradit Chin Med. 2015;35:218-221. [PubMed] |
| 61. | Li T, Han JY, Wang BB, Chen B, Li YM, Yu ZJ, Xue X, Zhang JP, Wang XB, Zeng H. [Huanglian jiedu decoction regulated and controlled differentiation of monocytes, macrophages, and foam cells: an experimental study]. Zhongguo Zhong Xi Yi Jie He Zazhi. 2014;34:1096-1102. [PubMed] |
| 62. | Xie H, Zhang H, Cao K, He P, Dai H, He S. Analysis of anti-asthmatic drug patents published in China between 2004 and 2013. Expert Opin Ther Pat. 2016;26:363-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 63. | Hu SC, Lee IT, Yen MH, Lin CC, Lee CW, Yen FL. Anti-melanoma activity of Bupleurum chinense, Bupleurum kaoi and nanoparticle formulation of their major bioactive compound saikosaponin-d. J Ethnopharmacol. 2016;179:432-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 64. | Wu H, She S, Liu Y, Xiong W, Guo Y, Fang H, Chen H, Li J. Protective effect of Sijunzi decoction on neuromuscular junction ultrastructure in autoimmune myasthenia gravis rats. J Tradit Chin Med. 2013;33:669-673. [PubMed] |
| 65. | Liang B, Wei W, Wang J, Zhang M, Xu R, Wu F, Xiao H, Tang L. Protective effects of Semiaquilegia adoxoides n-butanol extract against hydrogen peroxide-induced oxidative stress in human lens epithelial cells. Pharm Biol. 2016; Epub ahead of print. [PubMed] |
| 66. | Chen Y, Xian Y, Lai Z, Loo S, Chan WY, Lin ZX. Anti-inflammatory and anti-allergic effects and underlying mechanisms of Huang-Lian-Jie-Du extract: Implication for atopic dermatitis treatment. J Ethnopharmacol. 2016;185:41-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 67. | Dong J, Liu S, Zhu XL, Zhang XT, Jiang Y. [Ti-AI intermetallic compound membrane refining Chinese medicinal materials extract]. Zhong Yao Cai. 2014;37:1673-1675. [PubMed] |
| 68. | Santamarina AB, Oliveira JL, Silva FP, Carnier J, Mennitti LV, Santana AA, de Souza GH, Ribeiro EB, Oller do Nascimento CM, Lira FS. Green Tea Extract Rich in Epigallocatechin-3-Gallate Prevents Fatty Liver by AMPK Activation via LKB1 in Mice Fed a High-Fat Diet. PLoS One. 2015;10:e0141227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 69. | Nair VY, Balakrishanan N, Antony Santiago JV. Petroselinum crispum extract attenuates hepatic steatosis in rats fed with fructose enriched diet. Bratisl Lek Listy. 2015;116:547-553. [PubMed] |
| 70. | Kuo YC, Sun CM, Ou JC, Tsai WJ. A tumor cell growth inhibitor from Polygonum hypoleucum Ohwi. Life Sci. 1997;61:2335-2344. [PubMed] |
| 71. | Chen CH, Chang MY, Lin YS, Lin DG, Chen SW, Chao PM. A herbal extract with acetyl-coenzyme A carboxylase inhibitory activity and its potential for treating metabolic syndrome. Metabolism. 2009;58:1297-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 72. | Mao J, DeMayo FJ, Li H, Abu-Elheiga L, Gu Z, Shaikenov TE, Kordari P, Chirala SS, Heird WC, Wakil SJ. Liver-specific deletion of acetyl-CoA carboxylase 1 reduces hepatic triglyceride accumulation without affecting glucose homeostasis. Proc Natl Acad Sci USA. 2006;103:8552-8557. [PubMed] |
| 73. | Savage DB, Choi CS, Samuel VT, Liu ZX, Zhang D, Wang A, Zhang XM, Cline GW, Yu XX, Geisler JG. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J Clin Invest. 2006;116:817-824. [PubMed] |
| 74. | Chao PM, Kuo YH, Lin YS, Chen CH, Chen SW, Kuo YH. The metabolic benefits of Polygonum hypoleucum Ohwi in HepG2 cells and Wistar rats under lipogenic stress. J Agric Food Chem. 2010;58:5174-5180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 75. | Fintini D, Chinali M, Cafiero G, Esposito C, Giordano U, Turchetta A, Pescosolido S, Pongiglione G, Nobili V. Early left ventricular abnormality/dysfunction in obese children affected by NAFLD. Nutr Metab Cardiovasc Dis. 2014;24:72-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 76. | Yuan HD, Yuan HY, Chung SH, Jin GZ, Piao GC. An active part of Artemisia sacrorum Ledeb. attenuates hepatic lipid accumulation through activating AMP-activated protein kinase in human HepG2 cells. Biosci Biotechnol Biochem. 2010;74:322-328. [PubMed] |
| 77. | Liu Y, Wang D, Zhang D, Lv Y, Wei Y, Wu W, Zhou F, Tang M, Mao T, Li M. Inhibitory effect of blueberry polyphenolic compounds on oleic acid-induced hepatic steatosis in vitro. J Agric Food Chem. 2011;59:12254-12263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 78. | Lee CH, Kuo CY, Wang CJ, Wang CP, Lee YR, Hung CN, Lee HJ. A polyphenol extract of Hibiscus sabdariffa L. ameliorates acetaminophen-induced hepatic steatosis by attenuating the mitochondrial dysfunction in vivo and in vitro. Biosci Biotechnol Biochem. 2012;76:646-651. [PubMed] |
| 79. | Aoun M, Michel F, Fouret G, Casas F, Jullien M, Wrutniak-Cabello C, Ramos J, Cristol JP, Coudray C, Carbonneau MA. A polyphenol extract modifies quantity but not quality of liver fatty acid content in high-fat-high-sucrose diet-fed rats: possible implication of the sirtuin pathway. Br J Nutr. 2010;104:1760-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 80. | Park HJ, Jung UJ, Lee MK, Cho SJ, Jung HK, Hong JH, Park YB, Kim SR, Shim S, Jung J. Modulation of lipid metabolism by polyphenol-rich grape skin extract improves liver steatosis and adiposity in high fat fed mice. Mol Nutr Food Res. 2013;57:360-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 81. | Vitaglione P, Morisco F, Mazzone G, Amoruso DC, Ribecco MT, Romano A, Fogliano V, Caporaso N, D’Argenio G. Coffee reduces liver damage in a rat model of steatohepatitis: the underlying mechanisms and the role of polyphenols and melanoidins. Hepatology. 2010;52:1652-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 82. | Beltrán-Debón R, Rull A, Rodríguez-Sanabria F, Iswaldi I, Herranz-López M, Aragonès G, Camps J, Alonso-Villaverde C, Menéndez JA, Micol V. Continuous administration of polyphenols from aqueous rooibos (Aspalathus linearis) extract ameliorates dietary-induced metabolic disturbances in hyperlipidemic mice. Phytomedicine. 2011;18:414-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 83. | Tsuruta Y, Nagao K, Kai S, Tsuge K, Yoshimura T, Koganemaru K, Yanagita T. Polyphenolic extract of lotus root (edible rhizome of Nelumbo nucifera) alleviates hepatic steatosis in obese diabetic db/db mice. Lipids Health Dis. 2011;10:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 84. | Li Z, Xu J, Zheng P, Xing L, Shen H, Yang L, Zhang L, Ji G. Hawthorn leaf flavonoids alleviate nonalcoholic fatty liver disease by enhancing the adiponectin/AMPK pathway. Int J Clin Exp Med. 2015;8:17295-17307. [PubMed] |
| 85. | Luo Y, Dong X, Yu Y, Sun G, Sun X. Total aralosides of aralia elata (Miq) seem (TASAES) ameliorate nonalcoholic steatohepatitis by modulating IRE1α-mediated JNK and NF-κB pathways in ApoE-/- mice. J Ethnopharmacol. 2015;163:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 86. | Zhou C, Zhou J, Han N, Liu Z, Xiao B, Yin J. Beneficial effects of neomangiferin on high fat diet-induced nonalcoholic fatty liver disease in rats. Int Immunopharmacol. 2015;25:218-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 87. | Deng JN, Li J, Mu HN, Liu YY, Wang MX, Pan CS, Fan JY, Ye F, Han JY. Deepure Tea Improves High Fat Diet-Induced Insulin Resistance and Nonalcoholic Fatty Liver Disease. Evid Based Complement Alternat Med. 2015;2015:980345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 88. | Zhai L, Shi J, Xu W, Heinrich M, Wang J, Deng W. Ex Vivo and In Situ Evaluation of ‘Dispelling-Wind’ Chinese Medicine Herb-Drugs on Intestinal Absorption of Chlorogenic Acid. Phytother Res. 2015;29:1974-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 89. | Sun S, Li H, Zhou W, Liu A, Zhu H. Bacterial Quorum Sensing Inhibition Activity of the Traditional Chinese Herbs, Ficus carica L. and Perilla frutescens. Chemotherapy. 2014;60:379-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 90. | Leung PC, Ko EC, Siu WS, Pang ES, Lau CB. Selected Topical Agents Used in Traditional Chinese Medicine in the Treatment of Minor Injuries- A Review. Front Pharmacol. 2016;7:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 91. | Xu Y, Zhang M, Wu T, Dai S, Xu J, Zhou Z. The anti-obesity effect of green tea polysaccharides, polyphenols and caffeine in rats fed with a high-fat diet. Food Funct. 2015;6:297-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 92. | Zhang D, Liu L, Jia Z, Yao X, Yang M. Flavonoids of Herba Epimedii stimulate osteogenic differentiation and suppress adipogenic differentiation of primary mesenchymal stem cells via estrogen receptor pathway. Pharm Biol. 2016;54:954-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 93. | Zhang TT, Yang L, Jiang JG. Bioactive comparison of main components from unripe fruits of Rubus chingii Hu and identification of the effective component. Food Funct. 2015;6:2205-2214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 94. | Wang Y, Li JY, Han M, Wang WL, Li YZ. Prevention and treatment effect of total flavonoids in Stellera chamaejasme L. on nonalcoholic fatty liver in rats. Lipids Health Dis. 2015;14:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 95. | Qin N, Chen Y, Jin MN, Zhang C, Qiao W, Yue XL, Duan HQ, Niu WY. Anti-obesity and anti-diabetic effects of flavonoid derivative (Fla-CN) via microRNA in high fat diet induced obesity mice. Eur J Pharm Sci. 2016;82:52-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 96. | Kim CS, Kwon Y, Choe SY, Hong SM, Yoo H, Goto T, Kawada T, Choi HS, Joe Y, Chung HT. Quercetin reduces obesity-induced hepatosteatosis by enhancing mitochondrial oxidative metabolism via heme oxygenase-1. Nutr Metab (Lond). 2015;12:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 97. | Mohan SK, Veeraraghavan VP, Jainu M. Effect of pioglitazone, quercetin and hydroxy citric acid on extracellular matrix components in experimentally induced non-alcoholic steatohepatitis. Iran J Basic Med Sci. 2015;18:832-836. [PubMed] |
| 98. | Pisonero-Vaquero S, Martínez-Ferreras Á, García-Mediavilla MV, Martínez-Flórez S, Fernández A, Benet M, Olcoz JL, Jover R, González-Gallego J, Sánchez-Campos S. Quercetin ameliorates dysregulation of lipid metabolism genes via the PI3K/AKT pathway in a diet-induced mouse model of nonalcoholic fatty liver disease. Mol Nutr Food Res. 2015;59:879-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 99. | Panchal SK, Poudyal H, Arumugam TV, Brown L. Rutin attenuates metabolic changes, nonalcoholic steatohepatitis, and cardiovascular remodeling in high-carbohydrate, high-fat diet-fed rats. J Nutr. 2011;141:1062-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 100. | Ok HM, Gebreamanuel MR, Oh SA, Jeon H, Lee WJ, Kwon O. A Root-Based Combination Supplement Containing Pueraria lobata and Rehmannia glutinosa and Exercise Preserve Bone Mass in Ovariectomized Rats Fed a High-Fat Diet. Calcif Tissue Int. 2015;97:624-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 101. | Fu Y, Luo J, Jia Z, Zhen W, Zhou K, Gilbert E, Liu D. Baicalein Protects against Type 2 Diabetes via Promoting Islet β-Cell Function in Obese Diabetic Mice. Int J Endocrinol. 2014;2014:846742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 102. | Gao M, Ma Y, Liu D. Rutin suppresses palmitic acids-triggered inflammation in macrophages and blocks high fat diet-induced obesity and fatty liver in mice. Pharm Res. 2013;30:2940-2950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 103. | Zhang ZF, Fan SH, Zheng YL, Lu J, Wu DM, Shan Q, Hu B. Troxerutin improves hepatic lipid homeostasis by restoring NAD(+)-depletion-mediated dysfunction of lipin 1 signaling in high-fat diet-treated mice. Biochem Pharmacol. 2014;91:74-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 104. | Liu Y, Fu X, Lan N, Li S, Zhang J, Wang S, Li C, Shang Y, Huang T, Zhang L. Luteolin protects against high fat diet-induced cognitive deficits in obesity mice. Behav Brain Res. 2014;267:178-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 105. | Liu Q, Wang CY, Liu Z, Ma XS, He YH, Chen SS, Bai XY. Hydroxysafflor yellow A suppresses liver fibrosis induced by carbon tetrachloride with high-fat diet by regulating PPAR-γ/p38 MAPK signaling. Pharm Biol. 2014;52:1085-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 106. | Susutlertpanya W, Werawatganon D, Siriviriyakul P, Klaikeaw N. Genistein Attenuates Nonalcoholic Steatohepatitis and Increases Hepatic PPARγ in a Rat Model. Evid Based Complement Alternat Med. 2015;2015:509057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 107. | Zhang YB, Yan JD, Yang SQ, Guo JP, Zhang X, Sun XX, Na XL, Dai SC. Maternal Genistein Intake Can Reduce Body Weight in Male Offspring. Biomed Environ Sci. 2015;28:769-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 108. | Loguercio C, Andreone P, Brisc C, Brisc MC, Bugianesi E, Chiaramonte M, Cursaro C, Danila M, de Sio I, Floreani A. Silybin combined with phosphatidylcholine and vitamin E in patients with nonalcoholic fatty liver disease: a randomized controlled trial. Free Radic Biol Med. 2012;52:1658-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 109. | Zhang Y, Gu M, Cai W, Yu L, Feng L, Zhang L, Zang Q, Wang Y, Wang D, Chen H. Dietary component isorhamnetin is a PPARγ antagonist and ameliorates metabolic disorders induced by diet or leptin deficiency. Sci Rep. 2016;6:19288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 110. | Zhang HJ, Zhang XF, Ma ZM, Pan LL, Chen Z, Han HW, Han CK, Zhuang XJ, Lu Y, Li XJ. Irisin is inversely associated with intrahepatic triglyceride contents in obese adults. J Hepatol. 2013;59:557-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 111. | Wang D, Yan J, Chen J, Wu W, Zhu X, Wang Y. Naringin Improves Neuronal Insulin Signaling, Brain Mitochondrial Function, and Cognitive Function in High-Fat Diet-Induced Obese Mice. Cell Mol Neurobiol. 2015;35:1061-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 112. | Lee H, Bae S, Kim K, Kim W, Chung SI, Yang Y, Yoon Y. Shikonin inhibits adipogenesis by modulation of the WNT/β-catenin pathway. Life Sci. 2011;88:294-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 113. | Hoek-van den Hil EF, van Schothorst EM, van der Stelt I, Swarts HJ, van Vliet M, Amolo T, Vervoort JJ, Venema D, Hollman PC, Rietjens IM. Direct comparison of metabolic health effects of the flavonoids quercetin, hesperetin, epicatechin, apigenin and anthocyanins in high-fat-diet-fed mice. Genes Nutr. 2015;10:469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 114. | Alkhalidy H, Moore W, Zhang Y, McMillan R, Wang A, Ali M, Suh KS, Zhen W, Cheng Z, Jia Z. Small Molecule Kaempferol Promotes Insulin Sensitivity and Preserved Pancreatic β -Cell Mass in Middle-Aged Obese Diabetic Mice. J Diabetes Res. 2015;2015:532984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 115. | Choi HN, Kang MJ, Lee SJ, Kim JI. Ameliorative effect of myricetin on insulin resistance in mice fed a high-fat, high-sucrose diet. Nutr Res Pract. 2014;8:544-549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 116. | Sang H, Yuan N, Yao S, Li F, Wang J, Fang Y, Qin S. Inhibitory effect of the combination therapy of simvastatin and pinocembrin on atherosclerosis in ApoE-deficient mice. Lipids Health Dis. 2012;11:166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 117. | Faghihzadeh F, Adibi P, Rafiei R, Hekmatdoost A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr Res. 2014;34:837-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 251] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 118. | Peluso I, Manafikhi H, Reggi R, Palmery M. Effects of red wine on postprandial stress: potential implication in non-alcoholic fatty liver disease development. Eur J Nutr. 2015;54:497-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 120. | Bertelli AA, Das DK. Grapes, wines, resveratrol, and heart health. J Cardiovasc Pharmacol. 2009;54:468-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 195] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 121. | Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109-1122. [PubMed] |
| 122. | Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337-342. [PubMed] |
| 123. | Shang J, Chen LL, Xiao FX, Sun H, Ding HC, Xiao H. Resveratrol improves non-alcoholic fatty liver disease by activating AMP-activated protein kinase. Acta Pharmacol Sin. 2008;29:698-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 232] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 124. | Jeon BT, Jeong EA, Shin HJ, Lee Y, Lee DH, Kim HJ, Kang SS, Cho GJ, Choi WS, Roh GS. Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes. 2012;61:1444-1454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 271] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 125. | Cho IJ, Ahn JY, Kim S, Choi MS, Ha TY. Resveratrol attenuates the expression of HMG-CoA reductase mRNA in hamsters. Biochem Biophys Res Commun. 2008;367:190-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 126. | Gómez-Zorita S, Fernández-Quintela A, Macarulla MT, Aguirre L, Hijona E, Bujanda L, Milagro F, Martínez JA, Portillo MP. Resveratrol attenuates steatosis in obese Zucker rats by decreasing fatty acid availability and reducing oxidative stress. Br J Nutr. 2012;107:202-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 127. | Alberdi G, Rodríguez VM, Macarulla MT, Miranda J, Churruca I, Portillo MP. Hepatic lipid metabolic pathways modified by resveratrol in rats fed an obesogenic diet. Nutrition. 2013;29:562-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 128. | Bagul PK, Middela H, Matapally S, Padiya R, Bastia T, Madhusudana K, Reddy BR, Chakravarty S, Banerjee SK. Attenuation of insulin resistance, metabolic syndrome and hepatic oxidative stress by resveratrol in fructose-fed rats. Pharmacol Res. 2012;66:260-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 158] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 129. | Qiao Y, Sun J, Xia S, Tang X, Shi Y, Le G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014;5:1241-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 270] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 130. | Chachay VS, Macdonald GA, Martin JH, Whitehead JP, O’Moore-Sullivan TM, Lee P, Franklin M, Klein K, Taylor PJ, Ferguson M. Resveratrol does not benefit patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2014;12:2092-2103.e1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 228] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 131. | Noorafshan A, Ashkani-Esfahani S. A review of therapeutic effects of curcumin. Curr Pharm Des. 2013;19:2032-2046. [PubMed] |
| 132. | Pan MH, Lai CS, Tsai ML, Ho CT. Chemoprevention of nonalcoholic fatty liver disease by dietary natural compounds. Mol Nutr Food Res. 2014;58:147-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 133. | Leclercq IA, Farrell GC, Sempoux C, dela Peña A, Horsmans Y. Curcumin inhibits NF-kappaB activation and reduces the severity of experimental steatohepatitis in mice. J Hepatol. 2004;41:926-934. [PubMed] |
| 134. | Vizzutti F, Provenzano A, Galastri S, Milani S, Delogu W, Novo E, Caligiuri A, Zamara E, Arena U, Laffi G. Curcumin limits the fibrogenic evolution of experimental steatohepatitis. Lab Invest. 2010;90:104-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 135. | Kuzu N, Bahcecioglu IH, Dagli AF, Ozercan IH, Ustündag B, Sahin K. Epigallocatechin gallate attenuates experimental non-alcoholic steatohepatitis induced by high fat diet. J Gastroenterol Hepatol. 2008;23:e465-e470. [PubMed] |
| 136. | Wang YC, Kong WZ, Jin QM, Chen J, Dong L. Effects of salvianolic acid B on liver mitochondria of rats with nonalcoholic steatohepatitis. World J Gastroenterol. 2015;21:10104-10112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 137. | Panchal SK, Ward L, Brown L. Ellagic acid attenuates high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. Eur J Nutr. 2013;52:559-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 138. | Guo H, Xia M, Zou T, Ling W, Zhong R, Zhang W. Cyanidin 3-glucoside attenuates obesity-associated insulin resistance and hepatic steatosis in high-fat diet-fed and db/db mice via the transcription factor FoxO1. J Nutr Biochem. 2012;23:349-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 175] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 139. | Tori M. Terpenoid Composition and Base Sequences of Ligularia virgaurea (Asteraceae) Grown in the Hengduan Mountain Area in China and a Comment on Drawing Structures. Chem Pharm Bull (Tokyo). 2016;64:193-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 140. | Arendt P, Pollier J, Callewaert N, Goossens A. Synthetic biology for production of natural and new-to-nature terpenoids in photosynthetic organisms. Plant J. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 141. | Choi YJ, Park SY, Kim JY, Won KC, Kim BR, Son JK, Lee SH, Kim YW. Combined treatment of betulinic acid, a PTP1B inhibitor, with Orthosiphon stamineus extract decreases body weight in high-fat-fed mice. J Med Food. 2013;16:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 142. | de Melo CL, Queiroz MG, Arruda Filho AC, Rodrigues AM, de Sousa DF, Almeida JG, Pessoa OD, Silveira ER, Menezes DB, Melo TS. Betulinic acid, a natural pentacyclic triterpenoid, prevents abdominal fat accumulation in mice fed a high-fat diet. J Agric Food Chem. 2009;57:8776-8781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 143. | Quan HY, Kim do Y, Kim SJ, Jo HK, Kim GW, Chung SH. Betulinic acid alleviates non-alcoholic fatty liver by inhibiting SREBP1 activity via the AMPK-mTOR-SREBP signaling pathway. Biochem Pharmacol. 2013;85:1330-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 144. | Jia Y, Kim S, Kim J, Kim B, Wu C, Lee JH, Jun HJ, Kim N, Lee D, Lee SJ. Ursolic acid improves lipid and glucose metabolism in high-fat-fed C57BL/6J mice by activating peroxisome proliferator-activated receptor alpha and hepatic autophagy. Mol Nutr Food Res. 2015;59:344-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 145. | Wang CH, Wang ZT, Bligh SW, White KN, White CJ. Pharmacokinetics and tissue distribution of gentiopicroside following oral and intravenous administration in mice. Eur J Drug Metab Pharmacokinet. 2004;29:199-203. [PubMed] |
| 146. | Kim KE, Ko KH, Heo RW, Yi CO, Shin HJ, Kim JY, Park JH, Nam S, Kim H, Roh GS. Artemisia annua Leaf Extract Attenuates Hepatic Steatosis and Inflammation in High-Fat Diet-Fed Mice. J Med Food. 2016;19:290-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 147. | Li J, Wang RF, Yang L, Wang ZT. [Structure and biological action on cardiovascular systems of saponins from Panax notoginseng]. Zhongguo Zhong Yao Zazhi. 2015;40:3480-3487. [PubMed] |
| 148. | Liu X, Yu JL, Liu M, Shu JC, Huang HL. Research progress of bioactivity of steroidal saponins in recent ten years. Zhongguo Zhong Yao Zazhi. 2015;40:2518-2523. [PubMed] |
| 149. | Zhang ZY, Wu JP, Gao BB, Ren HT, Liu YL, Li XR, Li KP, Xu QM, Yang SL. Two new 28-nor-oleanane-type triterpene saponins from roots of Camellia oleifera and their cytotoxic activity. J Asian Nat Prod Res. 2016;18:669-676. [PubMed] |
| 150. | Yin LH, Xu LN, Wang XN, Lu BN, Liu YT, Peng JY. An Economical method for isolation of dioscin from Dioscorea nipponica Makino by HSCCC coupled with ELSD, and a computer-aided UNIFAC mathematical model. Chromatographia. 2009;71:15-23. [RCA] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 151. | Hsieh MJ, Tsai TL, Hsieh YS, Wang CJ, Chiou HL. Dioscin-induced autophagy mitigates cell apoptosis through modulation of PI3K/Akt and ERK and JNK signaling pathways in human lung cancer cell lines. Arch Toxicol. 2013;87:1927-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 152. | Li H, Huang W, Wen Y, Gong G, Zhao Q, Yu G. Anti-thrombotic activity and chemical characterization of steroidal saponins from Dioscorea zingiberensis C.H. Wright. Fitoterapia. 2010;81:1147-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 153. | Li M, Han X, Yu B. Synthesis of monomethylated dioscin derivatives and their antitumor activities. Carbohydr Res. 2003;338:117-121. [PubMed] |
| 154. | Liu M, Xu L, Yin L, Qi Y, Xu Y, Han X, Zhao Y, Sun H, Yao J, Lin Y. Potent effects of dioscin against obesity in mice. Sci Rep. 2015;5:7973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 155. | Shen L, Xiong Y, Wang DQ, Howles P, Basford JE, Wang J, Xiong YQ, Hui DY, Woods SC, Liu M. Ginsenoside Rb1 reduces fatty liver by activating AMP-activated protein kinase in obese rats. J Lipid Res. 2013;54:1430-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 156. | Yu H, Zhen J, Yang Y, Gu J, Wu S, Liu Q. Ginsenoside Rg1 ameliorates diabetic cardiomyopathy by inhibiting endoplasmic reticulum stress-induced apoptosis in a streptozotocin-induced diabetes rat model. J Cell Mol Med. 2016;20:623-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 157. | Wang T, Choi RC, Li J, Bi CW, Ran W, Chen X, Dong TT, Bi K, Tsim KW. Trillin, a steroidal saponin isolated from the rhizomes of Dioscorea nipponica, exerts protective effects against hyperlipidemia and oxidative stress. J Ethnopharmacol. 2012;139:214-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 158. | Stegelmeier BL, Brown AW, Welch KD. Safety concerns of herbal products and traditional Chinese herbal medicines: dehydropyrrolizidine alkaloids and aristolochic acid. J Appl Toxicol. 2015;35:1433-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 159. | Kukula-Koch W, Koch W, Angelis A, Halabalaki M, Aligiannis N. Application of pH-zone refining hydrostatic countercurrent chromatography (hCCC) for the recovery of antioxidant phenolics and the isolation of alkaloids from Siberian barberry herb. Food Chem. 2016;203:394-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 160. | Zhang J, Cao H, Zhang B, Cao H, Xu X, Ruan H, Yi T, Tan L, Qu R, Song G. Berberine potently attenuates intestinal polyps growth in ApcMin mice and familial adenomatous polyposis patients through inhibition of Wnt signalling. J Cell Mol Med. 2013;17:1484-1493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 161. | Zhang H, Wei J, Xue R, Wu JD, Zhao W, Wang ZZ, Wang SK, Zhou ZX, Song DQ, Wang YM. Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism. 2010;59:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 315] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 162. | Zhou L, Wang X, Yang Y, Wu L, Li F, Zhang R, Yuan G, Wang N, Chen M, Ning G. Berberine attenuates cAMP-induced lipolysis via reducing the inhibition of phosphodiesterase in 3T3-L1 adipocytes. Biochim Biophys Acta. 2011;1812:527-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 163. | Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, Wang Y, Wang Z, Si S, Pan H. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344-1351. [PubMed] |
| 164. | Yuan X, Wang J, Tang X, Li Y, Xia P, Gao X. Berberine ameliorates nonalcoholic fatty liver disease by a global modulation of hepatic mRNA and lncRNA expression profiles. J Transl Med. 2015;13:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 165. | Zhang Y, Wang S, Li Y, Xiao Z, Hu Z, Zhang J. Sophocarpine and matrine inhibit the production of TNF-alpha and IL-6 in murine macrophages and prevent cachexia-related symptoms induced by colon26 adenocarcinoma in mice. Int Immunopharmacol. 2008;8:1767-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 166. | Kim SJ, Lee SJ, Lee S, Chae S, Han MD, Mar W, Nam KW. Rutecarpine ameliorates bodyweight gain through the inhibition of orexigenic neuropeptides NPY and AgRP in mice. Biochem Biophys Res Commun. 2009;389:437-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 167. | Shi L, Shi L, Zhang H, Hu Z, Wang C, Zhang D, Song G. Oxymatrine ameliorates non-alcoholic fatty liver disease in rats through peroxisome proliferator-activated receptor-α activation. Mol Med Rep. 2013;8:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 168. | Kwan HY, Niu X, Dai W, Tong T, Chao X, Su T, Chan CL, Lee KC, Fu X, Yi H. Lipidomic-based investigation into the regulatory effect of Schisandrin B on palmitic acid level in non-alcoholic steatotic livers. Sci Rep. 2015;5:9114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 169. | Lee JH, Jung JY, Jang EJ, Jegal KH, Moon SY, Ku SK, Kang SH, Cho IJ, Park SJ, Lee JR. Combination of honokiol and magnolol inhibits hepatic steatosis through AMPK-SREBP-1c pathway. Exp Biol Med (Maywood). 2015;240:508-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 170. | Zhang Y, Fan S, Hu N, Gu M, Chu C, Li Y, Lu X, Huang C. Rhein Reduces Fat Weight in db/db Mouse and Prevents Diet-Induced Obesity in C57Bl/6 Mouse through the Inhibition of PPARγ Signaling. PPAR Res. 2012;2012:374936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 171. | Li J, Ding L, Song B, Xiao X, Qi M, Yang Q, Yang Q, Tang X, Wang Z, Yang L. Emodin improves lipid and glucose metabolism in high fat diet-induced obese mice through regulating SREBP pathway. Eur J Pharmacol. 2016;770:99-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |