INTRODUCTION
Before the 1980s the epithelial-mesenchymal transition (EMT) was mainly known as a process involved in embryogenesis and tissue repair. Then, it was proved in the experimental studies that the conversions of epithelial cells to mesenchymal cells could play a role in carcinogenesis and metastasis[1-5]. During EMT, the epithelial cells become more elongated and spindle-shaped, with fibroblastoid aspect, experienced loss of polarity, pseudopodia formation, and disintegration of the E-cadherin-related cell-cell adhesion[1-5]. In cancer cells, these changes increase the mobility and favor the metastasizing process[1-5]. Although several markers were described between 1989 and 2016 as being involved in the EMT, this process is still poorly understood. Moreover, conflicting and insufficient data was published about EMT particularities in colorectal cancer (CRC).
In this review, the old and new concepts about the role of EMT in prognosis and therapy of CRC are presented. Molecular mechanisms, the interaction EMT-angiogenesis, and the possible role of EMT in targeted therapy of CRC were also analyzed.
METHODOLOGY
For this review, a systematic search of the literature was done in the PubMed database to identify papers reporting data about EMT in CRC. Keywords “epithelial mesenchymal transition and colorectal cancer” were used. Studies published until the first of March 2016 were considered in this paper. The review focused on the possible role of EMT in CRC, from tumor cells invasion, metastatic capacity, possible re-expression in metastatic tissue, and therapeutic inhibition pathways. Both experimental studies and publications referring to human tissues were taken into account. Additional studies were identified by searching bibliographies and papers previously published by our team in field of CRC. The following articles were excluded: reviews, meta-analysis, duplicate records, letters, and papers that did not add significant information (Figure 1). Data assessment was conducted independently by two authors (Gurzu S, and Jung I) using predefined terms.
Figure 1 Preferred reported items for systematic reviews and meta-analyses (PRISMA) flow diagram adapted for data about epithelial mesenchymal transition in colorectal cancer in the PubMed database between 1995 and 2016 (First of March).
Based on these criteria, from about 750 publications identified in PubMed database using these terms, 426 articles were selected for further analysis, first identified study being published in 1995 (only 5 articles were published before 2004). From the 426 papers, a total of 74 representative studies have been further selected to be eligible for the present review (Figure 1).
EMT AND TUMOR BUDDING
The EMT is known as the process of conversion of an epithelial cell into an elongated cell with mesenchymal phenotype, which can occur in physiologic and pathologic processes[1-3]. In CRC, this process was described to favor increasing motility of the tumor cells in the invasion front and increasing cell resistance to apoptosis. Moreover, it is considered, nowadays, as the critical mechanism that underlies the initiation of cancer metastasis[3,4].
The EMT can be histologically quantified in the tumor budding areas. The tumor buds, which are considered as an important prognostic factor in CRC, are represented by the isolated cells or poorly differentiated clusters from the invasion front[6,7]. In these areas, changing morphology of the tumor cells can be seen (more elongated and discohesive cells) in parallel with gaining EMT phenotype[3-6]. Based on the number of isolated cells, the cases were histologically classified in G1 (single cells or clusters with fewer than 5 cells), G2 (clusters of 5-9 cells), and G3 (at least 10 isolated cells in the invasion front)[6,7]. Recently, a suggestion was made for this histological classification, to be completed with the aspect of the stroma. The following three groups of CRCs were defined: Category A - tumors with G1 buds and mature-type stroma (fibrotic stroma without keloid-like collagen, without myxoid component), Category C - tumors with G3 buds and immature-type (myxoid) stroma, and Category B - tumors with G2 buds and intermediate-type (usually keloid-type) stroma[6]. These categories were suggested for a better correlation with the EMT status[6].
Immunohistochemically (IHC), the EMT refers to down-regulation of the epithelial cell-cell adhesion molecules E-cadherin, claudins (types 3, 4, and 7), α-catenin, γ-catenin, occludin, desmoplakin and plakoglobin, and positivity of the tumor cells for mesenchymal markers such vimentin, Twist1, Twist2, β-catenin, VSIG (V-set and immunoglobulin domain-containing protein 1), N-cadherin (and the subtype cadherin 12), ZEB1 (zinc finger E-box-binding homeobox 1), fibronectin, smooth muscle actin (SMA), desmin, Sox, SNAIL1/SNAIL, SNAIL2/SLUG, Axl, Notch-1, ETS1 (v-ets erythroblastosis virus E26 oncogene homologue 1), FLT1 (fms-related tyrosine kinase 1), stromelysin-3, FOXC2, HOXB7, ACTA2, PDGF (platelet derived growth factor), integrin αVβ6, etc.[1-6].
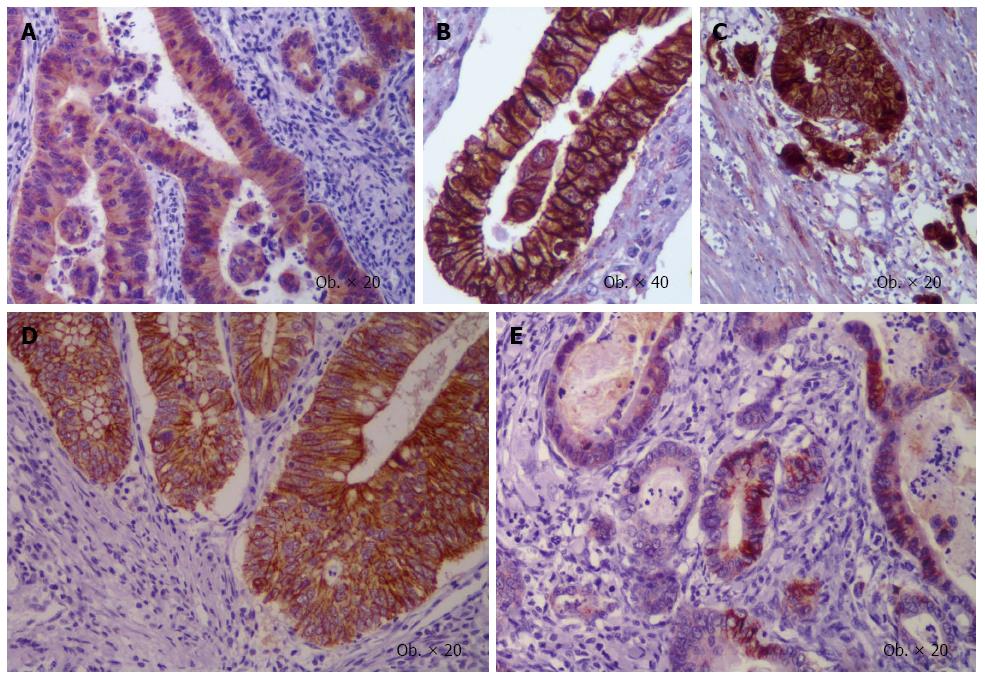
The paucity of the IHC markers used to examine the EMT particularities[1,5,6,8] increases the difficulty of its quantification and comparing the results between studies is practically impossible. Decreasing E-cadherin membrane expression in the tumor buddings (Figure 2) is a well-proven aspect that is mentioned in almost all of the studies in this field[1-5]. Regarding the mesenchymal markers, the results are totally confusing, several markers and clones are being used in the hundreds of studies published in the last years. Tumor heterogeneity and the systems used for quantification increase the difficulty of IHC evaluation. Although a high number of markers were described to be involved in EMT, β-catenin remains the main oncoprotein of the EMT of CRC cells[5,9]. Its role in EMT of the CRC cells was first proved by Brabletz in 2001[10].
Figure 2 Epithelial mesenchymal transition-related immunoprofile of buds of colorectal cancer.
β-catenin displays cytoplasmic (A) or membrane positivity in the tumor core (B) with nuclear switch in buds (C), whereas membrane E-cadherin expression (D) is lost in the invasion front (E).
Another aspect is the aberrant stain of the markers: in some studies they were quantified in cytoplasm, whereas other authors accounted it for membrane or nuclei staining aspects. E-cadherin and catenins should be quantified in the cell membrane (Figure 2), the N-cadherin, vimentin, and PDGF present a predominant cytoplasmic expression, the Notch-1 and ZEB1 mark the nuclei, whereas SNAIL, SLUG (Figure 3), and Twist present positivity for both cytoplasm and nuclei of the tumor cells[1]. Moreover, dual epithelial-mesenchymal expression (e.g., E-cadherin/N-cadherin) can occur in some tumor cells[1,11,12]. Regarding β-catenin, the membrane positivity can be seen in the normal mucosa and tumor cells, cytoplasm increased positivity can be noted in the tumor cells, compared to the normal mucosa, whereas nuclear switch can be seen in the invasion front (Figure 2) in about 80% of the cases[5]. It means that β-catenin nuclear positivity should be considered as an indicator of EMT[9], no changes being added till 2001[10].
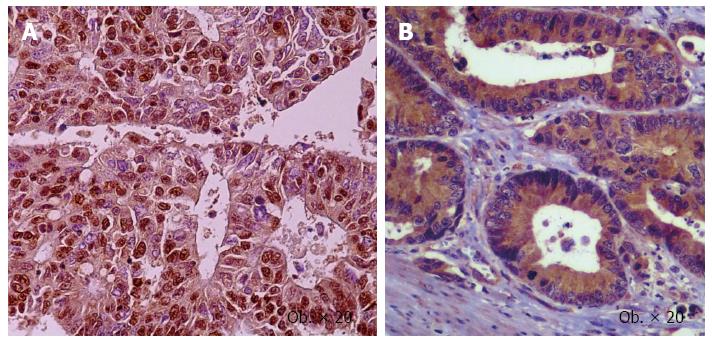
Figure 3 Nuclear positivity for Slug (A), and cytoplasmic expression of vascular endothelial growth factor-A (B) in the colorectal cancer cells.
However, aberrant expressions can be seen. For example, inhibition of the renin-angiotensin system down-regulates the EMT in the CRC cells, down regulating the E-cadherin, but no change is noted in ZEB1 or vimentin expression[11].
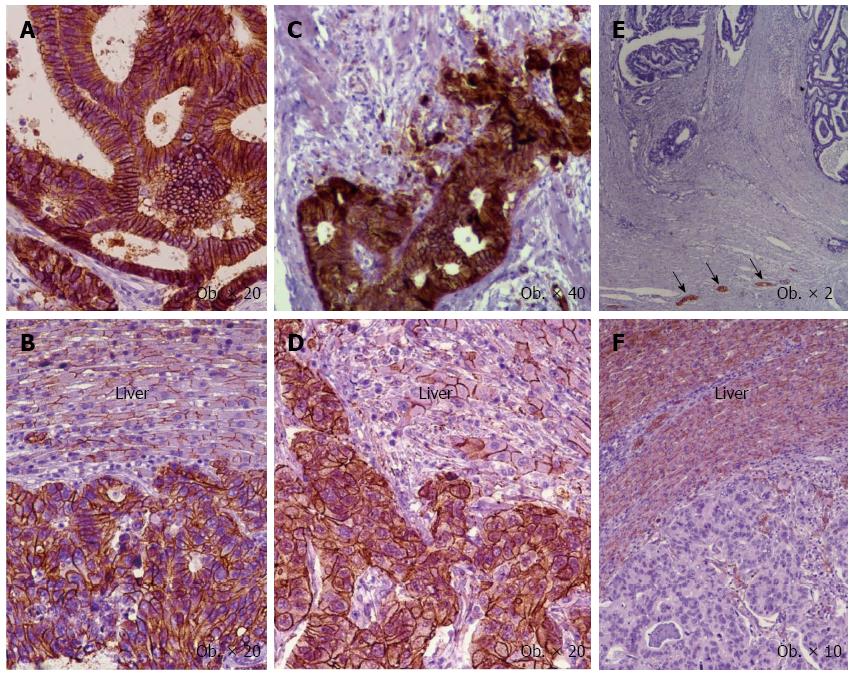
In daily practice in CRC samples, loss of membrane E-cadherin positivity and focally β-catenin membrane to nuclear switch have been confirmed in the tumor buds (Figure 2). In the case of N-cadherin, although its cytoplasmic and/or membrane-associated immunoreactivity was previously reported to be expressed in 44% of the cases[12], its positivity is not confirmed; only a few cases (less than 5%) were marked in the material that include over 150 consecutive cases. Valuable internal positive control cells for N-cadherin are the neural structures (Figure 4).
Figure 4 Immunoexpression of the epithelial mesenchymal transition-related markers in primary tumor (A, C, E) vs hepatic metastases (B, D, F).
Membrane positivity for E-cadherin (A, B) and β-catenin (C, D), and negativity for N-cadherin (E, F) with positive neural structures (arrows). The normal hepatocytes express E-cadherin (B), β-catenin (D), and N-cadherin (F).
Due to the fact that intraepithelial vimentin is difficult to be counted, its quantification must be performed by at least two experienced pathologist. A weak point of the literature in the field of EMT markers is the absence of an established cut point.
EMT MARKERS IN PRIMARY TUMOR VS METASTATIC TISSUE
Although it is relatively well proven that EMT in the buds increases the cell motility and risk for metastasis, few aspects are known about the heterogeneous immunoprofile of the metastatic tissue. E-cadherin expression decreases in the invasion front but seems to be re-expressed in metastatic nodal tissue and is negatively correlated with the nuclear and/or cytoplasmic β-catenin and nuclear Slug positivity[5,8,9,11,13]. The Ki67 labeling index seems to decrease in the invasive front and increase in metastatic nodal lesions[13]. The adenomatous polyposis coli (APC) expression is diffused in normal mucosa, decreased in the tumor cells and re-expressed in liver metastases[5]. No other data has been found to reveal the immunoprofile of EMT-related markers in the primary tumor vs metastatic tissue.
Similar immunoprofile was noted in the liver metastatic tissue vs primary tumor for E-cadherin, N-cadherin, and β-catenin (Figure 4).
EMT AND ANGIOGENESIS
The aspects of angiogenesis were extensively studied in patients with CRC, being also one of the main fields of our team[14-16]. Intense angiogenesis is considered as an indicator of aggressivity of CRC cells and poor survival rate[15]. The antiangiogenic therapy is used successfully in metastatic CRCs. It was proven in 1970s that the tumor cells, in hypoxic conditions, have the ability to secrete pro-angiogenic factors such vascular endothelial growth factor (VEGF-A) and hypoxia inducible factor (HIF-1α) that stimulate the sprouting of pre-existent vessels and subsequently tumor growing and spread in the newly formed vessels. The density and type of neoformed vessel are prognostic factors for patients with CRC and can also indicate success or failure of the antiangiogenic treatment[14-16]. The role of transforming growth factor-beta (TGF-β) in EMT-induced carcinoma cells invasiveness was described in 1998, and has proven its simultaneous role in tumor angiogenesis[17]. In this material, simultaneous IHC expression of VEGF-A and EMT markers such SLUG in the CRC samples have been observed (Figure 3).
The most recent studies had proven that the EMT is also a hypoxia-dependent mechanism. In hypoxic conditions, up-regulation of Snail, Slug, Twist, and integrin αVβ6 was observed in CRC cells[3,18]. The angiogenesis also seems to be modulated through Akt/HIF-1α signalling[19]. Notch-1/MMP9 axis is involved in promoting EMT[18] but MMP9 and Leucine-rich-alpha-2-glycoprotein 1 (LRG1) are also important pro-angiogenic agents[19-22] stimulated by the renin angiotensin system[11]. LRG1 is involved in the immune response, cell proliferation, cell migration, cell apoptosis, and VEGF-A-mediated promotion of angiogenesis[22].
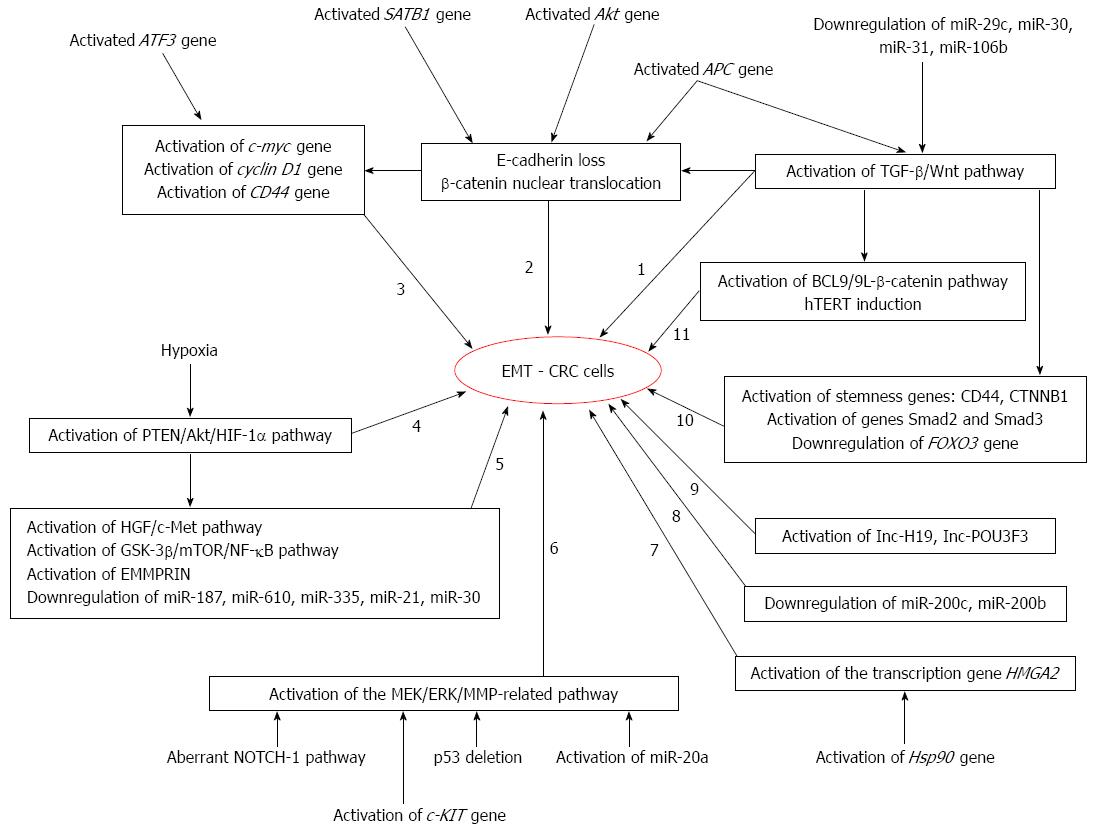
MOLECULAR PATHWAYS OF EMT
The exact molecular mechanisms of EMT in CRC are not known. At least 11 EMT-related molecular pathways have been described in literature in the CRC cells (Figure 5).
Figure 5 Molecular pathways of epithelial mesenchymal transition in colorectal cancer cells.
EMT: Epithelial mesenchymal transition; CRC: Colorectal cancer; hTERT: Human telomerase reverse transcriptase; TGF-β: Transforming growth factor-beta.
The oncogenic activation of the Akt gene was longtime considered as the key molecular step for EMT by activating β-catenin and Snail in solid tumors including CRC[19]. In experimental studies that consider the hypoxia-induced EMT, it was proved that it was partially mediated via PTEN/Akt/HIF-1α pathway[3]. The phosphoinositide 3-kinase/Akt signaling pathway also interacts with the hepatocyte growth factor (HGF)/c-Met signaling pathway, GSK-3β/mTOR/NF-κB signaling axis, microRNAs (mRNAs), and proteins such EMMPRIN, influencing the EMT of CRC cells[3,19,21]. The HIF-1α-dependent EMT can also be initiated in a concentration- and time-dependent manner by LRG1[22].
The β-catenin-related EMT is a central mechanism of CRC behavior that is mediated by the APC tumor suppressor gene[9]. During EMT, the membrane protein β-catenin is accumulated in cytoplasm and a nuclear switch can be seen in the de-differentiated mesenchymal-like tumor cells from the invasion front[8]. The nuclear β-catenin triggers the other genes like c-myc, cyclin D1, matrilysin, fibronectin, CD44, and uPAR[9]. The aberrant expression of β-catenin is promoted by various factors such as the global gene regulator SATB1[23].
Another molecular pathway seems to be related to the transcriptional activation of stemness-related genes like CTNNB1, which are up-regulated during EMT and has exerted an anti-apoptotic and pro-migration effect upon the tumor cells. The CTNNB1 gene activity is reversely correlated with the expression of FOXO3[24].
The gene transcription-dependent growth and EMT is mediated by the nonhistone chromatin-binding protein high mobility group AT-hook 2 (HMGA2). This protein is regulated by the molecular chaperone Heat Shock Protein 90 (Hsp90) and mRNAs[25,26].
The TGF-β and Wnt pathway-dependent EMT of CRC cells is partly regulated by the SERPINI1 protein and LRG1[17,23,27]. The high Wnt signaling is necessary for stemness maintenance[28] and β-catenin translocation to the nucleus[29]. The Wnt signaling is mediated by the BCL9/9L-β-catenin signaling[28] and telomerase activation through human telomerase reverse transcriptase (hTERT) induction[29]. However, some authors say that Wnt signaling is not activated or has a low signal in CRC cells[29,30]. The EMT and stemness maintenance seems to be simultaneously controlled by the specific molecular pathways such as IGF/NANOG/Slug signaling axis via STAT3 phosphorylation[31]. The main Wnt-effector proteins are β-catenin, APC, and Wnt-1[5], the Wnt/β-catenin signaling pathway being mutated in about 90% of the sporadic and hereditary CRCs[30]. On the other hand, TGF-β receptor activates Smad2 and Smad3 pathways through growth differentiation factor 15 (GDF15)[32].
Aberrant NOTCH-1 signaling induces EMT through metalloproteinase-9 (MMP9) mediated pro-inflammatory effect[20], whereas the MEK/ERK/MMP-related EMT is facilitated by the adaptor protein Grb2-associated binder 2 (Gab2)[33]. Gab2 has the capacity of increasing the expression of MMP7 and MMP9 in the CRC cells[33]. The MMP7-related EMT via ERK can also be promoted by the c-KIT gene activation[34]. On the other hand, Notch activation has a synergistic effect with p53 deletion, inducing EMT and promoting metastasis, in CRC culture cells[35].
A challenging molecular aspect is the dual role of some markers like the stress-inducible gene also known as activating transcription factor 3. It down-regulates the cell apoptosis via Bcl-2 gene inhibition and increases CD44 expression and cell migration, but also decrease expression of the EMT-associated transcription factors β-catenin, Snail and Slug[36]. These aspects probably involve the tumor heterogeneity.
Other proteins described to be involved in the EMT of CRC are the SOX-2[37], hTERT/ZEB1 complex (that binds to the E-cadherin and inhibits its expression)[29], etc.
Several mRNAs and long intergenic non-coding RNAs (lncRNAs) were also described to influence the EMT in CRC cells[22,38-41]. mRNAs, transcribed by RNA polymerase II (RNA pol II), are small, noncoding, single-stranded RNAs that are usually composed of 20-25 nucleotides[40,41]. lncRNAs, also transcribed by RNA pol II, are composed by over 200 nucleotides to approximately 100 kilobases and are characterized by the absence of an open reading frame[39]. They were recently identified as regulators of tumorigenesis and EMT-related tumour progression and they do not code for proteins, but interact with them[39-44].
In CRC, the IncRNAs described to be involved in the activation of the EMT-related genes are the Taurine up-regulated gene 1 (TUG1)[39] Inc-POU3F3[42], and Inc-H19[42]. They play a role in carcinogenesis, tumor cell colony formation, migration and CRC progression[39,42,43]. The newest described Inc-H19 proved to antagonize the activity of miR-138 and miR-200a, and their EMT endogenous targets vimentin, ZEB1, and ZEB2[43]. At the same time, the BRAF-activated lncRNA, which is located on chromosome 9 and is known for its role in melanoma cell migration, can induce EMT in CRC cells through an MEK/extracellular signal-regulated kinase-dependent mechanism[44]. It is closely associated with the V600EBRAF gene and is poorly characterized in CRC cells[44].
The newest mRNAs explored in CRC are the miR-187 and miR-610 (first studies were published in CRC in 2016 and 2015, respectively)[38,45]. The miR-610 suppresses the EMT, cell proliferation, migration and invasion, up-regulates the E-cadherin, and down-regulates vimentin in the cells[38]. It seems to negatively influence the expression of hepatoma-derived growth factor (HDGF) by binding to its 3’UTR[38]. The miR-187 is down-regulated in aggressive CRC cells compared with normal mucosa, being suppressed by the TGF-β. Its normal level prevents EMT via inactivation of Smad pathway and subsequent decreasing levels of SOX4, NT5E, and PTK6[45].
The LRG1 mediated HIF-1α activation is a target of miR-335[21]. The miR-21 is overexpressed in CRC cells targeting the angiogenesis activator miR-30 via TGF-β pathway[1,40,46], whereas miR-200c is a crucial EMT inhibitor, being more expressed in hepatic metastases, compared with primary tumor. miR-200c also induces, together with miR-200b, increased E-cadherin and decreased vimentin expression in the invasion front, and negative regulation of its gene targets ZEB1, ETS1, and FLT1[1,41,47]. Other mRNAs that influence the EMT of CRC cells are the miR-29c, which stimulates EMT via PTP4A and GNA13 regulation of Wnt/β-catenin signaling and inhibits it via PI3K/AKT and GSK-3β/β-catenin pathway[1,48], miR-31 (via TGF-β pathway)[46], miR-212 (via dysregulation of manganese superoxide dismutase, it exerts antiproliferative/anti-EMT effect)[49], miR-20a (enhances EMT via MMP-2 and MMP-9)[50], miR-106b (induces cytoskeletal reorganization and increases TGF-β1 expression)[51], miR-138[43], miR-141[52], miR-200a[43], etc. Gene expression was described to be strongly influenced by the circadian rhythm, which is regulated by mRNAs[53].
It is also worth mentioning that the EMT activation was described not only in CRC cells but also in the premalignant status like ulcerative colitis. However, only patients with active colitis and with risk for malignization showed EMT pathway activation[54]. This supposition is not yet well proven and it is believed that in inflammatory diseases EMT is rather related on regeneration than tumorigenesis.
EMT MARKERS AND PROGNOSIS
In clinical studies it was proved that diffuse positivity of the tumor cells for the EMT markers usually shows unfavorable prognosis. For example, the IncRNAs TUG1 indicates a short survival as an independent prognostic marker[39].
Slug positivity, observed in 37% of the cases, was directly correlated with Dukes stage and distant metastasis and was also proved to be an independent negative prognostic factor, indicating a low overall survival[55]. The unfavorable prognosis is also predicted by Slug positivity in the stromal fibroblasts[1,56].
Twist overexpression induces both EMT and cancer stem cell phenotype in CRC cells and plays a role in increasing cell migration and invasion, proving the possible common molecular pathway of the EMT and stemness maintenance[28,31,57]. Twist overexpression in CRC cells, but also in the stroma cells, especially in high budding cases, is an indicator of more advanced pT, lymph node metastasis, and a worse overall survival time[58].
The β-catenin gene regulator SATB1 is expressed in 66.5% of the CRCs. It induces dedifferentiation, aberrant expression of β-catenin, decreasing level of the Keratin 20, and vimentin positivity of the tumor cells[23].
Other EMT-related markers, which indicate poor prognosis and increased risk for CRC metastasis, include positivity for the cancer stem cell membrane proteins CD44, CD133, CD166, and ALDH1[59]. Negativity for DAB2IP indicates promotion of EMT and stimulation of the cancer stem cell like activity[60]. High telomerase expression and Inc-POU3F3 also induces tumor aggressiveness and metastatic potential[29,42]. Snail expression was not correlated with the overall survival rate but the 5-year survival rate seems to be negatively influenced by loss of E-cadherin in the invasion front[61].
High serum values of GDF15 also indicate reduced overall survival[32].
As microsatellite instability (MSI-H) is considered an indicator of a favorable prognosis[62-64], the EMT markers are not very well expressed in these cases[58]. However, scarce information can be found about the relationship between MSI status and EMT.
EMT AND CONVENTIONAL CHEMOTHERAPY
Despite the several drugs used in clinical trials, most of the patients with CRC are primarily treated with 5-fluorouracil (5-Fu) based chemotherapy, radiotherapy, and surgery[19,53,65]. One of the most common disadvantages of conventional therapy is the development of drug resistance to conventional chemotherapeutic agents[19,65]. The 5-Fu resistance seems to be partially induced by EMT via Akt gene[19] or mediated by Twist, miR-200c, miR-141, or the CD44 isoform containing variant exon v6 (CD44v6)[52,59]. EMT-induced 5-Fu chemoresistance seems to also be related on gain-of-function mutant p53/ephrin-B2 signaling axis[66]. The 5-FU response seems to be chronomodulated and its administration should be adjusted for circadian rhythm[53]. It was suggested that it is better processed with a reduced toxicity when administered at night[53].
Chemoresistance to oxaliplatin was experimentally proved to be induced by the CD44v6 and/or Twist overexpression in the CRC cells[57,59]. Moreover, Twist over-expression can induce tumor cell positivity for the transmembrane glycoprotein P-gp, which is known as an inductor of multidrug-resistance[57]. Acquired oxaliplatin resistance can also be induced by down-regulation of the EMT-related miR-200c and miR-141[54]. Chemoresistance to doxorubicin can be induced by EMT promoted by the oncogene Eukaryotic translation initiation factor 5A2[67]. This oncogene can be inhibited by Twist siRNA, and it has been suggested to be a potential inductor of the reversal of drug resistance in CRC therapy[67]. However, Twist is an EMT promoter[1-5].
Irinotecan resistance can also be induced by down-regulation of the EMT-related miR-200c and miR-141[52].
Another unusual aspect is the drugs-induced EMT. Oxaliplatin-induced EMT can be mediated by several substances such reactive oxygen species[68]. At the same time, 5-Fu can stimulate ephrin-B2 reverse signaling and, as a result, EMT and tumor cells proliferation are promoted via Src-FAK, and Src-ERK respectively[66]. Moreover, radiotherapy can also be responsible for inducing EMT in the irradiated CRC cells[69].
A relatively new aspect that can explain the drug resistance, drug-induced EMT, and the risk for relapse, is the so called “cellular senescence”[70,71]. It refers to the permanent cell-cycle arrest[70] that can be induced by the telomerase activation. The proliferated cells do not die but remain in this status for long time and can be re-activated. In CRC cells, the senescence can be induced by the ionizing radiations or DNA damaging agents such 5-FU and doxorubicin[71]. The senescent cells are positive for the senescence-associated β-galactosidase, which is capable of paracrine induction of EMT, tumor cells proliferation and invasion[71]. In patients with rectal cancer treated with neoadjuvant chemotherapy, the senescent cells can display positivity for Slug, SNAIL, and vimentin[71]. No specific data about telomerase expression and the senescence susceptibility in CRC cells were reported, although telomerase-induced EMT was experimentally proven[29].
EMT AND TARGETED THERAPY
In patients with CRC, the development of therapeutic strategies targeting the EMT is ongoing[26] but no conclusive data had been reported until now. There are some substances in clinical evaluation but the results of the clinical trials are still under examination and the discovery of new biomarkers and/or therapeutic targets is necessary[39]. EMT-induced chemoresistance to targeted drugs such as the anti-epidermal growth factor receptor (EGFR) agents (cetuximab, erlotinib, gefitinib) has already been described, and it is supposed to be induced via Akt/STAT3 genes, independently from EGFR staus[72].
A lot of experimental studies have been published in the field of targeting EMT and a lot of hypothesis regarding inhibition of EMT in CRC cells were emitted. Because of the paucity of data in this field, it is very difficult to identify the most suitable component that could be used to synthesize a new drug for the targeted therapy of CRC.
Based on the key role of Akt gene in activating EMT, an ingredient of the traditional Chinese herbal medicine Rumex japonicus Houtt called Physicon 8-O-β-glucopyranoside has been proven, in cell cultures-based studies, to exert anti-hypoxic/anti-EMT effects via PTEN/Akt/HIF-1α pathway. It also inhibits the hypoxia-induced up-regulation of Snail, Slug, and Twist. Based on its low toxicity for healthy tissues, it was suggested that Physicon 8-O-β-glucopyranoside can be a safe anti-metastatic agent in patients with CRC[3]. The oncogenic activation of the Akt gene can also be inhibited by other substances such as withaferin A and dexamethasone, which can be orally administrated. The natural compound withaferin A from the winter cherry (Withania somnifera), that was historically used in oriental medicine for the treatment of inflammatory and neurological disorders, seems to also induce down-regulation of the EMT markers Snail, Slug, β-catenin, and vimentin, and decreasing intensity of microvessels density[19]. Dexamethasone inhibits TGF-β1 and MMP-induced EMT by regulating the ERK and AKT pathways through cysteine-rich angiogenic inducer R61, which is also a VEGF inhibitor via STAT3 down-regulation[73]. The Akt/HIF-1α hypoxia-dependent signaling is also inhibited by dexamethasone[18]. This drug can also down-regulate the mRNA levels of hypoxia-induced Snail, Slug, Twist, and integrin αVβ6[18]. The MMP-9 down regulation is also induced through blockage of the renin-angiotensin system[11] or by Eicosapentaenoic Free Fatty Acid via Notch-1 signaling[20]. Moreover, the hypoxia and/or EGF-induced EMT in CRC cells were proved to be inhibited by the selective cyclooxygenase-2 inhibitors such celecoxib[74].
Another promising substance is the proteasome inhibitor called PS341 (Bortezomib), which is currently approved for patients with multiple myeloma, was tested in both in vitro and in vivo studies. Besides the inhibition of cell proliferation, EMT, cell migration and invasiveness, the expression of stemness-related genes such CTNNB1 was down-regulated. Bortezomib also suppresses the VEGF activity, having indirect dose-dependent antiangiogenic effect and exerting pro-apoptotic activity through inhibition of NF-κB, increasing activity of p53 and Bax proteins, or accumulation of cyclin dependent kinase inhibitors p27 and p21[24].
Although the Hsp90 inhibition was supposed to suppress the EMT, the Hsp90 inhibitor benzoquinone ansamycin 17-allylamino-17-demethoxygeldanamycin (17-AAG), that is currently (2016) in clinical evaluation, proved to have acquired resistance. The resistant cells showed down-regulated E-cadherin and diffuse positivity for the EMT markers N-cadherin and β-catenin, and also for Mucin 1 (MUC1). Based on these facts, it was supposed that the Hsp90 resistance is mediated by MUC1[26]. The second-generation Hsp90 inhibitor, NVP-AUY922, proved to inhibit the Hsp90-HMGA2 axis that is partially responsible for maintaining CRC cell survival and migration[25]. It is necessary to prove its superiority to the previously described Hsp90 inhibitor 17-AAG[26].
Other supposed proteins/pathways whose drug-mediated modulation seems to prevent or inhibit the EMT are the HGF/c-Met signaling pathway[21], SERPINI1-mediated TGF-β signaling and BCL9/9L-β-catenin mediated Wnt pathway[27,28], ephrin-B2[66], telomerase inhibition[38], etc.
Identification of the mRNAs and/or IncRNAs antagonists was mainly focused on the miR-610/HDGF axis[38], miR-21[40,46], miR-30[40], miR-200c[47], miR-187[45], miR-20a[50], miR-106b[51], and knockdown of Inc-H19)[43] or Inc-POU3F3, which also inhibits cell proliferation and induces G1 cell cycle arrest and autophagy, which are mediated by cyclin D1, CDK4, p18, Rb, and phosphorylated Rb)[42], etc.
SUMMARY AND FUTURE PERSPECTIVES
The old concept of EMT in CRC cells take into account the Akt gene as the key modulator of this process and the discovery of the EMT-specific markers like Twist, Slug, Snail, VSIG, β-catenin, vimentin, N-cadherin, etc.
The newest studies in the field of EMT in CRC are mainly focused on the molecular pathways of this process, angiogenesis, and its potential therapeutically uses. The IHC quantification of EMT on histological slides remains a real challenge because of the paucity of markers and clones that are currently in use, the lack of an established cut-point, and the aberrant expression of some of these markers. Moreover, in the rare types of CRC such as serrated carcinomas, basaloid carcinomas, clear cell carcinomas, or neuroendocrine tumors the role of EMT is practically unknown.
Because Akt/HIF-1α hypoxia-dependent signaling, LRG-induced HIF-1α/VEGF-A up-regulation, Notch-1/MMP-9 interaction, and other similar mechanisms were proved to be involved in both EMT and angiogenesis, this interaction should be more explored in the case of CRC cells. This idea is also sustained by the involvement of mRNAs like miR-30 in both EMT and angiogenesis.
Based on the clinical use of antiangiogenic therapy in metastatic CRC, the EMT-angiogenesis and EMT-stemness links in the CRC cells, newly synthesized drugs with antiangiogenic/anti-EMT properties could be one of the medications used in the future in the targeted therapy of patients with CRC. Moreover, these drugs should be administrated not only in metastatic cases and through preventing metastasis they could have real clinical benefits. On the other hand, because the renin-angiotensin system seems to stimulate EMT of CRC cell, association of angiotensin converting enzyme inhibitors with chemotherapy, adjusted according to the blood pressure values of the patient, could have clinical benefits.