Published online Jan 21, 2016. doi: 10.3748/wjg.v22.i3.1139
Peer-review started: June 6, 2015
First decision: August 26, 2015
Revised: September 25, 2015
Accepted: November 24, 2015
Article in press: November 24, 2015
Published online: January 21, 2016
Processing time: 231 Days and 1.4 Hours
Gastric cancer is a common neoplastic disease and, more precisely, is the third leading cause of cancer death in the world, with differences amongst geographic areas. The definition of advanced gastric cancer is still debated. Different stadiating systems lead to slightly different stadiation of the disease, thus leading to variations between the single countries in the treatment and outcomes. In the present review all the possibilities of treatment for advanced gastric cancer have been analyzed. Surgery, the cornerstone of treatment for advanced gastric cancer, is analyzed first, followed by an investigation of the different forms and drugs of chemotherapy and radiotherapy. New frontiers in treatment suggest the growing consideration for intraperitoneal administration of chemotherapeutics and combination of traditional drugs with new ones. Moreover, the necessity to prevent the relapse of the disease leads to the consideration of administering intraperitoneal chemotherapy earlier in the therapeutical algorithm.
Core tip: New frontiers in treatment suggest the growing consideration for intraperitoneal administration of chemotherapeutics and combination of traditional drugs with new ones. Moreover, the necessity to prevent the relapse of the disease leads to the consideration of administering intraperitoneal chemotherapy earlier in the therapeutical algorithm.
- Citation: Coccolini F, Montori G, Ceresoli M, Cima S, Valli MC, Nita GE, Heyer A, Catena F, Ansaloni L. Advanced gastric cancer: What we know and what we still have to learn. World J Gastroenterol 2016; 22(3): 1139-1159
- URL: https://www.wjgnet.com/1007-9327/full/v22/i3/1139.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i3.1139
Gastric cancer (GC) is a common neoplastic disease and, more precisely, is the third leading cause of cancer death in the world, with differences amongst geographic areas. In fact the GC and advanced gastric cancer (AC) incidence and related mortality vary between the latitudes with an higher peak of mortality in the western countries and a lower mortality rates in the eastern ones. In fact the United States account for about 21600 new cases of GC each year, the South Korea accounts for about 33000 new cases per year; China has the highest incidence of GC followed by Mongolia, Japan and South Korea[1,2]. Patients suffering from GC in eastern countries have a better prognosis than in western ones. This is mainly due to successful, decade-old screening programs that detect GC as early as possible; in Japan the survival for resectable GC is almost 70%[3]. The same results have not been achieved in Europe and US where the 5-year survival is almost 25% in advanced cancer (AC)[4]. The major differences in eastern and western countries is summarized in Table 1. They start from the staging systems [Japanese classification of GC (JCGC) and American Joint Committee on Cancer Staging Manual (AJCCSM-TNM)] and pass through all the step of chemotherapeutical and surgical management of GC (neoadjuvant, perioperative and adjuvant treatment, and lymphadenectomy)[5-8].
| ESMO | Germany | United Kingdom | United States | Canada | Japan | |
| Type of standard lymphadenectomy | D2 | D2 | D2 | D1 | D1 | D1 (for T1) |
| D2 (not routinely) | Modified D2 (see in the text) |
The most commonly used classification around the world is the 7th edition TNM[9,10].
The definition of early gastric cancer is well established[11]. As a counterpart the definition of AC is still matter of debate. Some authors define as AC the T3 and T4 cancers. However the vast majority considers as AC the tumors infiltrating beyond the submucosal layer that are not-early and not-metastatic even with N0 staging. According to the AJCC-TNM (7th edition) classification, AC are: T2-T4b/N0-3b/M0-M1
The only curative treatment either for early GC or non-metastatic AC is radical surgery with adequate surgical resection and lymphadenectomy. Lymphadenectomy is considered adequate if at least 16 lymphnodes are removed[12].
The concept of adequacy of surgical resection changed through the years. In the past decades total gastrectomy has been considered superior to the partial one for tumors of the antrum. However in the nineties, some trials demonstrated no survival or recurrence advantages between the two techniques[13,14]. At present definitive agreement has been reached about the resection extension in relation to the position of the tumor and its pattern. For large tumors or for tumors of the lesser curve total gastrectomy would be preferable. A proximal margin of at least 3 cm is recommended for T2 or higher degree tumors with “expansive growth pattern” and a proximal margin of at least 5 cm is recommended for those with “infiltrative growth pattern”.
The penetration of the serosa (T3 disease) and the lymphnode involvement[15] are the principal factors strongly related with prognosis. Literature reports that the clearance of lymph-nodes remains crucial[16-18].
The lymphadenectomy has shown an important role in accurate disease staging and in increasing the long-term survival since the first JCGC in 1998[7,15,19]. However the extension of the lymphadenectomy is still a matter of debate. Differences exist between the JCGC and the TNM classification and are related to the different values of the two classifications. Some studies reported that TNM system has greater prognostic power than the JCGC, however TNM does not provide treatment guidance and should primarily be used as a guide for prognosis[20]. In contrast, the JCGC system has been designed as a comprehensive guide to treatment, and the anatomy-based N-staging system was established on the basis of lymphadenectomy effectiveness[20]. Part of the problem was solved considering that in “standard lymphadenectomy” (D1) almost 15-18 lymph-nodes must be removed to have a proper staging. The TNM classification is more accurate in categorizing the number of metastatic lymph-nodes and gives a better prediction of the overall survival for GC[16].
The importance of lymphadenectomy is an issue of continue interest: recently some authors reported the high propensity of GC to involve lymph-nodes, particularly for Lauren mixed/diffuse adenocarcinomas[17,21]. In Europe the state-of-the-art in curative-intent surgery for GC is gastrectomy with a R0 resection associated with a D2 lymphadenectomy and omentectomy[15,17]. This target has been achieved after numerous randomized controlled trials and cohort studies[7,22-24].
D1 lymphadenectomy: According to the JCGC, D1 dissection consists in the resection of the peri-gastric stations (from 1 to 7 stations)[7,25]. In case of esophageal-gastric junction tumors also the infradiaphragmatic, paraesophageal and supradiafragmatic stations (19, 20, 110 and 111 stations) are included[7]. D1 lymphadenectomy is considered appropriate for T1a tumor not suitable for endoscopic resection and for differentiated and small (≤ 1.5 cm) cT1bN0 tumors[26]. Some authors reported a D1-plus lymphadenectomy consisting in the removal of nodal stations 8a, 9, and 11p in cT1N0 tumors or as alternative to D2 in high-risk patients)[26].
D2 lymphadenectomy: D2 dissection consists in the resection of the peri-gastric stations (D1) and of second echelon lymph-nodes [hepatic artery (station 8), Celiac artery (station 9), splenic artery (station 11) and anterior hepatoduodenal ligament (station 12a-b)][7]. This procedure should yield at least 16 or more lymph-nodes for the pathologic evaluation.
D3 lymphadenectomy: The lymphadenectomy can be considered D3 when posterior (stations No. 12p, No. 13, No. 14v) and para-aortic (No. 16) lymph-nodes are removed. D3 is supposed to provide a better local control of disease in advanced gastric tumors with mixed-diffuse histotype[21]. The inclusion of para-aortic lymph-node stations (16-a para-aortic nodes between the level of the celiac axis and the left renal vein, and 16-b para-aortic nodes between the left renal vein and the inferior mesenteric artery) (considered also as D2 plus) is important in upper third tumors in large tumors or in tumor with involvement of station n. 7 (29% of para-aortic involved lymph-nodes compared to the 7% in middle and lower third GC; P < 0.001)[27,28]. However extended lymphadenectomy and routinely removal of para-aortic lymph-nodes does not correlate with a benefit in terms of survival[29,30].
Super-extender D3 lymphadenectomy: Splenectomy or distal pancreatectomy is strongly discouraged unless deemed necessary based on tumor involvement[18,31,32]. Even in scenarios of higher risk for splenic hilum node involvement, i.e., with proximal and mid greater curvature primaries, spleen-preserving hilum lymphadenectomy can be performed with satisfactory results[33].
Table 2 reports the data about mortality, morbidity and survival in different types of lymphadenectomy in the most important published trials. Results from the first Randomized controlled trials (RCTs)[34-36] reported a superiority of the D1 compared with D2 lymphadenectomy, but these data were not confirmed by other RCTs, meta-analysis and prospective studies[23,24,37,38]. A recent meta-analysis by Jiang et al[18] showed that the results from these two trials seem to be related with the high rate of splenectomy and pancreatic resection included in the D2 resection (65% and 56% respectively), as previously highlighted in other papers[32,39]. The analysis of the data from the Italian Gastric Cancer Study Group showed that D2 dissection without splenectomy and pancreatic resection is feasible and safe with comparable results to those of D1[39]. Splenectomy and pancreatectomy might be considered beneficial only in case where the primary tumor or the lymph-node metastasis involve these organs[18,32]. In 2010 a 15-years follow up of the Dutch trial[30,34] showed an increased survival rate in patients underwent to D2 dissection compared to D1 (29% vs 21%, P = 0.34) with a gastric-cancer-related death and a regional recurrence rates increase in D1 group (48% vs 37% and 19% vs 13% respectively). In 2015 Galizia et al[26] published a RCT to evaluate the difference between D1 plus and D2 lymphadenectomy D2 lymphadenectomyincluded splenectomy. The results reported a similar median recurrence rate (47.2% vs 51.4% in D2, P = NS). D2 lymphadenectomy is also considered the standard in elderly patients with acceptable survival[40].
| Ref. | Year | Country | Type of study | N° pts | Perioperative mortality (%) | Overall morbidity (%) | Overall survival (%) | |||||||||||||||
| D1 | D1+ | D2 | D2+ | D3 | P value | D1 | D1+ | D2 | D2+ | D3 | P value | D1 | D1+ | D2 | D2+ | D3 | P value | |||||
| [34] | 1999 | Holland | RCT | 711 | 4 | - | 10 | - | - | 0.004 | 25 | - | 43 | - | - | < 0.0001 | 45 | - | 47 | - | - | NS |
| [35,36] | 1996 | United Kingdom | RCT | 400 | 6.5 | - | 13 | - | - | 0.04 | 28 | - | 46 | - | - | < 0.001 | 35 | - | 33 | - | - | NS |
| 1999 | ||||||||||||||||||||||
| [23,24] | 1998 | Italy | Prospective | 191 | - | - | 3.1 | - | - | - | - | - | 20.9 | - | - | - | - | - | 55 | - | - | - |
| 2004 | ||||||||||||||||||||||
| [37] | 2004 | Italy | RCT | 162 | 1.3 | - | 0 | - | - | NS | 10.5 | - | 16.3 | - | - | NS | ||||||
| [42,43] | 2004 | China | RCT | 221 | 0 | - | - | - | 0 | NS | 7.3 | - | - | - | 17.1 | 0.012 | 53.6 | - | - | - | 59.5 | 0.041 |
| 2006 | Taiwan | |||||||||||||||||||||
| [28,44] | 2004 | Japan | RCT | 523 | - | - | 0.8 | 0.8 | - | NS | - | - | 20.9 | 28.1 | - | NS | - | - | 69.2 | 70.3 | - | NS |
| 2008 | ||||||||||||||||||||||
| [22] | 2007 | Japan | RCT | 275 | - | - | 4.9 | 2.2 | - | NS | - | - | 27.7 | 21.6 | - | NS | - | - | - | - | - | - |
| [30] | 2010 | Holland | Prospective | 711 | - | - | - | - | - | - | - | - | - | - | - | - | 21 | - | 29 | - | - | NS |
| [39] | 2010 | Italy | RCT | 267 | 3 | - | 2.2 | - | - | NS | 12 | - | 17.9 | - | - | NS | - | - | - | - | - | - |
| [21] | 2015 | Italy | Retrospective | - | - | 4 | - | 2.4 | NS | - | - | - | - | - | - | - | - | - | - | - | - | |
The evaluation of the possible role of an extended lymphadenectomy in reducing the risk of a local recurrence has been reported in several studies[21,28,29,41,42]. A Japanese study[22] showed a better outcome in terms of mortality and morbidity in patients underwent to a D2 with para-aortic lymph-nodes dissection compared to the only D2, due to the frequent rates of involvement of Para Aortic lymph-nodes (17%-40%). However, in a similar study in 2008, Sasako et al[28] reported that D2 plus para-aortic lymph-nodes dissection in T2-subserosa, T3, T4 stages was not associated with an improving survival (70.3% in D2 plus vs 69.2%, P = NS) or recurrence free survival (61.7% in D2 plus vs 62.6%, P = NS), with a similar perioperative mortality and an increase morbidity in extended surgery group.
Wu et al[41] randomized patients to receive D1 vs D3 lymphadenectomy: the rate of morbidity was higher in extended surgery (17.1% vs 7.3%, P = 0.012), but with no reported mortality in the groups. The overall survival was significantly higher in D3 group (59.5% vs 53.6% in D3 resection, P = 0.041) with a decreased regional recurrence rate in D3 (40.3% vs 47.6%, P = 0.063)[29]. An extended lymphadenectomy (as D3) with an acceptable rate of mortality could be useful in the future to be compared with perioperative chemotherapy (as in MAGIC trial)[43] or adjuvant chemoradiotherapy (as in McDonald trial)[44] and to achieve a good long-term survival only with surgery[29]. de Manzoni et al[21] in their analysis emphasized the importance of the interaction between the histology and the extension of lymphadenectomy (P = 0.004), and reported a higher rate of relapse in D3 group in case of intestinal pattern (45.1% vs 35.3%, P = NS), then in mixed/diffuse pattern (48.3% vs 61.5%, P = NS, caused by a pronounced lymphotropism and grater propensity to metastasize to third level lymph-nodes), with a similar mortality in the two groups.
The lymph node ratio is A novel promising prognostic factor(ratio between metastatic and harvested lymph nodes)[45]: in a retrospective study on a large sample of patients an higher N ratio was significantly correlated with a worse prognosis and was a significant prognostic factor, differently from the N stage. N Ratio could be a really interesting prognostic tool, able to standardize data on lymphadenectomy extension. However further prospective studies are needed to assess its real value.
In addition, recent studies reported the importance of the sentinel node to detect the first lymph nodes apt to receive cancer cell drainage, as in breast cancer and melanoma[46]. The aims are to give a sentinel node mapping and intra-operative biopsy to prevent complications of extended and unnecessary lymphadenectomy particularly in early stage patients (2%-18% lymph nodes invasion in T1, and about 20% in T2) where the greater part of lymph-nodes resected is not involved[46]. The techniques to determine sentinel node with a high sensitivity are intraoperative radiation with a gamma probe, or indocyanine green, or with the Maruyama technique[47] with Indian ink. However these techniques are not yet validated and showed a high rate of false negative. Some trials are on going to determine their efficacy and further evidence is needed to standardize the technique.
Cytoreductive surgery: In the treatment of AC with local or diffuse peritoneal carcinosis (PC) the multimodal treatment combines systemic chemotherapy, radical surgery and intra-peritoneal chemotherapy (IPC). This new approach radically changed the outcome in locally advanced and advanced gastric cancer[48-50].
The necessity of complete removal of disseminated intra-peritoneal disease has already been demonstrated in different diseases. In fact, few previous studies showed potential middle/long-term survival benefit of the complete removal of the primary disease and the disseminated macroscopic nodules[51,52]. In ovarian cancer the complete removal of PC has been demonstrated to increase significantly the survival rate[52].
In patients with GC and PC, no survival benefits have been reported for treatment with Cyto Reductive Surgery (CRS) alone. Kodera et al[53] showed that PC cannot be cured using only CRS because of invisible cancer cells remain even after CRS. In fact they found in peri-gastric peritoneum and in macroscopically intact peritoneum even distant from the surgical field is possible to detect CEA/cytokeratin 20 mRNA. This could suggest that without an intraoperative chemotherapy effective in penetrating the peritoneum no gain in term of reduction of free cancer cells is possible even with a complete macroscopic removal of cancer nodules[54]. Recently, also multi-drug intra-peritoneal chemotherapy has been demonstrated to be safe and effective[55].
As a counterpart, CRS in addition to intraperitoneal peri-operative chemotherapy assures a significant benefit in survival rate even in GC with PC[29,56-58]. A previous meta-analysis clearly demonstrated that patients affected by advanced GC, either with either without PC benefit from IPC[51].
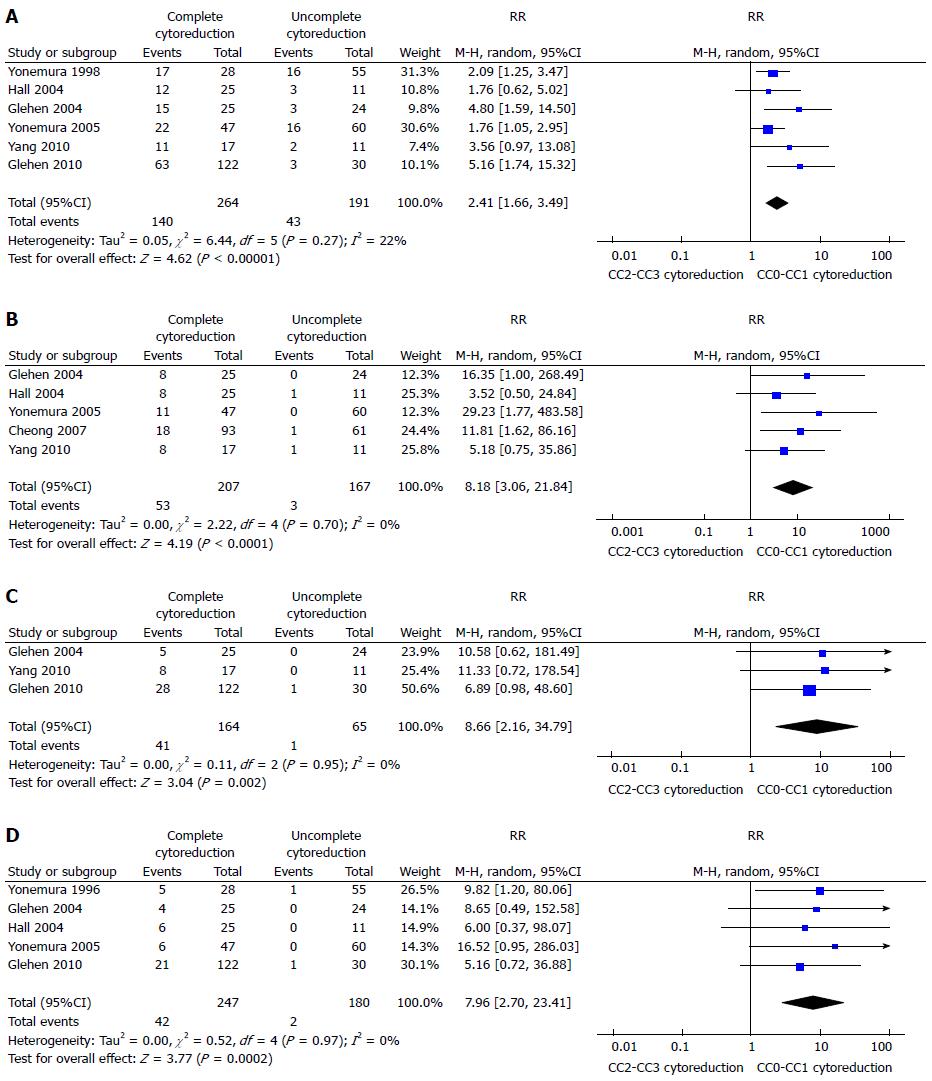
Some studies demonstrated that during CRS, if associated to IPC, the completeness of cytoreduction was an independent favourable prognostic factor[56]. Yonemura et al[59,60] reported that complete cytoreduction was associated with a median survival of 19.2 mo and a 5-year survival rate of 27%. Glehen et al[61] confirmed those results in one of the bigger prospective cohort study published about gastrointestinal diseases.
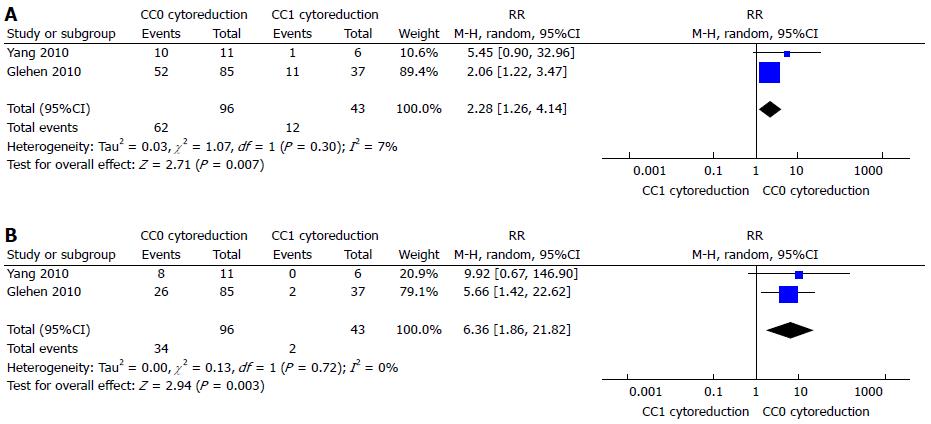
A recent meta-analysis demonstrated as the completeness of cytoreduction (CC) results in a real gain in survival[62]. It reported a 1, 2, 3, and 5-years survival rate increased in CC0-CC1 cytoreduction (1 year: RR = 2.41, 95%CI: 1.66-3.49; 2 year: RR = 8.18, 95%CI: 3.06-21.84; 3 year: RR = 8.66, 95%CI: 2.16-34.79; 5 years: RR = 7.96, 95%CI: 2.70-23.41) (Figure 1) The gain in terms of survival is progressively higher, with the increasing of the years of follow-up. This shows that the gain is a long-term result. Moreover it has been shown a gain also for minimal differences in terms of millimetres of residual disease. CC-0 in fact showed better outcomes than CC-1 cytoreduction. An increased survival has been demonstrated also in the comparison between CC0 and CC1 cytoreduction at 1 and 3 years (1 year: RR = 2.28, 95%CI: 1.26-4.14; 3 years: RR = 6.36, 95%CI: 1.86-21.82) (Figure 2). The reported morbidity rates ranged between 1.1% and 38.5%.
Certainly these data should be considered at the light of the supposed increased complication rate in such aggressive surgical procedure. As a consequence a PCI cut-off evaluating the reasonability of CRS + IPC treatment is needed.
A recent study by Yonemura et al[63] evaluating 95 patients with PC from GC, showed that it was possible to obtain a complete cytoreduction in 91% (42/46) of the patients with a PCI ≤ 6 but in only 42% (12/29) of the patients with a PCI ≥ 7. In addition, the study demonstrated as the survival of patients with a PCI score ≤ 6 was significantly better than those with a PCI score ≥ 7.
The reported 1, 2, 3, 5 years survival change significantly above and below a PCI of 12. In fact the reported overall median 1, 2, 3, 5 years survival for a 0-6 PCI are 56%-36%-33%-30% respectively, for a 7-12 PCI are 65%-25%-18%-0% respectively while for a 13-19 PCI are 35%-22%-0%-0% respectively and are all 0% for a PCI > 19[61]. Other studies reported a median survival for PCI below and above 20 of 3-27.7 and 6.4-10.2 mo respectively[64-66].
No study dedicated to the use of minimally invasive surgery in advanced gastric cancer exists. All the papers considered patients mixed together. However the results could be considered as indicative about the possibility to apply this kind of surgery to gastric cancer. One of the main concerns has historically been the lymph node retrieval.
Laparoscopic surgery: On the one hand, several studies showed that laparoscopic gastrectomy is feasible and effective to treat early gastric cancer with a D1 lymphadenectomy obtaining better results than the open technique in terms of postoperative pain, time to return to normal bowel function and resumption of oral feeding, time to recovery, length of hospital stay, cosmetic results and financial outcome[67-70]. In terms of morbidity and mortality rates laparoscopy reached results comparable to open resections[36,39,71]. On the other hand, however, for D2 or higher lymphadenectomy laparoscopic intervention reduces the possibility to be accurate in dissecting lymphnodes especially from high-risk nodal stations (i.e., stations 10 and 12a).
A recent meta-analysis by Wang et al[72] including 17 studies considered a total of 2313 patients (955 undergone to laparoscopic total gastrectomy and 1358 to open total gastrectomy). Laparoscopy showed longer operative time (WMD = 47.00, 95%CI: 31.67-62.33, P = 0.001), less blood loss (WMD = 2179.60, 95%CI: 2251.80-2107.89, P = 0.001), fewer analgesic uses (WMD = 22.46, 95%CI: 22.71-22.22, P = 0.001), earlier passage of flatus (WMD = 20.80, 95%CI: 21.11-20.50, P = 0.001), quicker resumption of oral intake (WMD = 21.11, 95%CI: 21.57-20.64, P = 0.001), earlier hospital discharge (WMD = 23.37, 95%CI: 24.58-22.16, P = 0.001), and reduced postoperative morbidity. No statistical difference was found between the two groups in the number of harvested lymph nodes (WMD = 2.33, 95%CI: 20.04-4.71, P = 0.054), proximal resection margin, hospital mortality, 5-year OS and DFS were similar.
Another meta-analysis about the comparison between laparoscopic and open gastrectomy analyzed 15 non-randomized comparative studies with 2022 patients (811 undergone laparoscopic total gastrectomy and 1211 to open intervention) partially confirmed the outcome of the other studies[73].
Robotic surgery: The feasibility, safety and eventual advantages of robotic gastrectomy compared to open or laparoscopic gastrectomy in treating gastric cancer are not well defined.
A meta-analysis of four studies considering 5780 patients with 520 (9.00%) that underwent robotic gastrectomy and 5260 (91.00%) that underwent open gastrectomy has been published by Liao et al[74]. Robotic gastrectomy has a significantly longer operation time (WMD = 92.37 min, 95%CI: 55.63-129.12 min, P < 0.00001), lower blood loss [WMD: -126.08, 95%CI: -189.02-(-63.13), P < 0.0001], and shorter hospital stay [WMD = -2.87, 95%CI: -4.17-(-1.56), P < 0.0001]. No statistical difference was noted in overall postoperative complication, wound infection, bleeding, number of harvested lymph nodes, anastomotic leakage and postoperative mortality rate.
Shen et al[75] in another meta-analysis considering eight studies with 1.875 patients, compared robotic and laparoscopic gastrectomy. The study showed as robotic gastrectomy was associated with a longer operative time (WMD = 48.46 min, 95 %CI: 29.49-67.43, P < 0.05), lower estimated blood loss [WMD = -38.43 mL, 95%CI: -67.55-(-9.30), P < 0.05], and a longer distal margin (WMD = 1.04 cm, 95%CI: 0.46-1.62, P < 0.05). In this meta-analysis complications (OR = 0.95, 95%CI: 0.7-1.28, P > 0.05), hospital stay (WMD = -1.00, 95%CI: -2.57-0.56, P > 0.05), proximal margin (WMD = 0.1 cm, 95%CI: -0.25-0.45, P > 0.05), and harvested lymph nodes (WMD = 1.06, 95%CI: - 2.33-4.45, P > 0.05) for robotic and laparoscopic gastrectomy were similar.
Neo-adjuvant chemotherapy is administrated in order to reduce the tumoral extension increasing the potential of a radical surgery and to reduce the biological potential of tumor cells with particular attention to subclinical micrometastases.
As surgery is considered the only curative approach to GC, a suggested potential disadvantage of the pre-operative chemotherapy could be the delay in surgery.
At present no clear evidences exists about the value of neo-adjuvant chemotherapy in gastric cancer treatment: all the proposed phase III randomized studies have been closed prematurely.
The Dutch FAMTX trial[76,77] failed to provide any definitive answer. The study was prematurely closed with only 59 patients enrolled. Patients were randomized to receive methotrexate, 5-fluorouracil, leucovorin and doxorubicin every four weeks for 4 cycles prior to surgery or to undergo surgery alone. Forty percent of patients in the experimental group interrupted chemotherapy because of toxicity. The rate of curative resections (R0) was similar in both groups and lymphadenectomy was limited to D1 in both groups. No significant differences in term of complication were recorded. In available data no survival differences were showed: 5-year survival rate was 21% in the experimental group and 34% in controls (P = 0.17).
Also the EORTC 40954[78] trial was closed prematurely and given the low accrual it was ultimately underpowered at 25%. The study randomized patients with gastric and cardias cancer stage II and III to receive neo-adjuvant chemotherapy with i.v. cisplatin, folinic acid, fluorouracil, 2 cycles of 48 d plus surgery, or surgery alone. D2 gastrectomy was performed in the majority of patients. Available results showed an increased rate of R0 resections in neoadjuvant chemotherapy group (81.9% vs 66.7%, P = 0.036), more frequent postoperative morbidity (P = 0.09) and positive HR favor to NACT in survival but not significant (HR = 0.84, 95%CI: 0.52-1.35; P = 0.466).
Perioperative chemotherapy combines the administration of chemotherapy before surgery as in neoadjuvant setting plus post-operative chemotherapy with interval surgery. The aim of this combined approach is to add the advantages of the neoadjuvant chemotherapy in reducing tumor size and facilitating radical surgery with the advantages of the post-operative chemotherapy. This approach has become quite frequent in Europe since the publication of two large randomized trials.
The main clinical study evaluating this strategy is the MAGIC trial, involving 503 patients with gastric or distal esophagus adenocarcinoma[43]. Patients were randomized to receive three cycles of the ECF regimen (epirubicin, cisplatin and 5-fluorouracil) - before and after surgery or surgery alone. 6.1% of patients in the chemotherapy arm did not proceed to surgery compared to the 2.4% in the surgery alone arm due to the progression of disease during the neoadjuvant chemotherapy phase. Preoperative chemotherapy resulted effective in improving curative resection with R0 observed in 79% vs 69% of patients (P = 0.03); D2 lymphadenectomy was performed in 41% of patients. Forty-nine point five percent of the patients that underwent preoperative treatment in the study received the full courses of the planned postoperative chemotherapy. Perioperative chemotherapy resulted in a reduced risk of relapse [HR = 0.66 (95%CI: 0.53-0.81, P < 0.001)] and in improved median OS (HR = 0.75, 95%CI: 0.60-0.93, P = 0.009) with a 5-year survival rate of 36% vs 23%. The clinical importance of the adjuvant component of the MAGIC regimen was still not certain, this issue was addressed by a retrospective study from the United Kingdom on a series of 66 patients undergoing perioperative chemotherapy according to the MAGIC protocol. The results of this study showed a considerable prognostic benefit in terms of disease free survival (DFS) for patients receiving neoadjuvant as well as adjuvant treatment compared with patients who did not undergo postoperative chemotherapy, while OS was not significantly different between the two groups[79].
Another Randomized trial on perioperative chemotherapy in gastric cancer was the French ACCORD07 trial. In this study 234 patients with gastro esophageal junction cancer were randomized to receive cisplatin (100 mg/m2) and 5FU (800 mg/m2 D1-5) every 28 d for up to 3 cycles prior to surgery and up to 4 cycles after surgery, vs surgery alone with a recommended D2 lymphadenectomy[80]. However the study was originally designed to include patients with cancer of the esophagus and was extended to include cancer of the stomach only later. Consequently, 64% of accrued patients had disease of the gastro esophageal junction while only 25% had gastric carcinoma. Patients who underwent perioperative chemotherapy presented with higher rates of curative resection (87% vs 74%, P = 0.004). Eighty-seven percent of patients received at least 2 preoperative cycles. Three point five percent of patients in chemotherapy arm did not receive surgery due to progression of disease and chemotherapy toxicity vs 1% in surgery alone arm. R0 resection rate was 84% in the perioperative chemotherapy group vs 74% in the surgery group (P = 0.04). Perioperative treatment with the CF regimen was associated with a reduced risk of relapse (HR = 0.65; 95%CI: 0.48-0.89, P = 0.003) and a reduced risk of death (HR = 0.69, 95%CI: 0.50-0.95, P = 0.02) with a 5-year survival rates of 38% vs 24%.
In 2013 was published by Ronellenfitsch et al[81] a Cochrane single patient data meta-analysis on the perioperative chemo(radio)therapy in resectable gastric adenocarcinoma. There were included 14 randomized trials showing an improvement in overall survival (HR = 0.81, 95%CI: 0.79-0.89, P < 0.0001) with a five year survival gain of 9% (from 23% to 32%) for patients undergoing perioperative chemo(radio)therapy; the effect was seen 18 mo. after surgery and lasted at least 10 years. Radical resection was 1.4 times higher in perioperative chemotherapy group with a borderline statistical significance and was confirmed as the strongest prognostic factor. In the subgroups analyzed, the advantage offered by perioperative treatment was more pronounced in tumor of the gastroesophageal junction. The addition of radiotherapy in the perioperative treatment also resulted in a better overall survival. Perioperative treatment was associated with longer disease free survival, higher radical resection rate with no differences in term of mortality and morbidity. Perioperative chemotherapy’s effect also associated with patient age, with a larger effect in younger patients and with no survival benefit for elderly patients. In a multivariate analysis perioperative chemotherapy lost its effect on overall survival while age, tumor site, performance status and radical resection remained significantly associated with better survival.
A major criticism is that in perioperative chemotherapy only a small percent of patients, ranging from 22% to 42%, could receive all the planned post-operative cycles[81]. A British study[82] on patients treated with a protocol similar to the MAGIC study showed a considerable prognostic benefit in terms of DFS for patients receiving neoadjuvant as well as adjuvant treatment compared with patients who did not undergo postoperative chemotherapy, while OS was not significantly different between the two groups. This could demonstrate a relative role of the post-operative chemotherapy; at the moment a polish randomized trial (NCT01787539) in recruiting patients. Patients after preoperative chemotherapy and surgery are randomized to receive post-operative chemotherapy or not. Results, not expected before 2022, could clarify the exact role of post-operative treatment.
Adjuvant chemotherapy after radical surgery is the preferred treatment most parts of the world. Because the surgery is considered the only curative option for gastric cancer many surgeons and oncologist prefer to assail directly the tumor attempting to a radical surgery.
Evidences about adjuvant chemotherapy were collected and summarized in a single patient data meta-analysis by the GASTRIC group in 2010[83]. In the study were included 17 RCT with 3838 patients randomized to receive chemotherapy, in various regimens, after surgery or surgery alone. Adjuvant chemotherapy resulted in a prolonged five years survival (55.3% vs 49.6% respectively, HR = 0.82, 95%CI: 0.76-0.90, P = 0.001) with similar disease free survival. No differences in term of drug regimen, mono-therapy vs poly-chemotherapy were demonstrated; all the chemotherapic regimens was fluoropyrimidine based. Two large trials published after the meta-analysis confirmed the same results showing the role of post-operative adjuvant chemotherapy.
The ACTSGC study involved 1059 patients with disease stages II and III submitted to curative resection associated with D2 lymphadenectomy[84]. Patients were randomized to receive surgery alone or surgery plus adjuvant chemotherapy with systemic S-1 administration for one year. The adjuvant chemotherapy resulted in a prolonged five-year survival from 61.1% in surgery alone arm to 71.7% (HR = 0.66, 95%CI: 0.54-0.82) with a low rate of severe complications.
The CLASSIC trial included patients with a similar study protocol randomizing patients to receive surgery alone vs surgery plus 6 mo of adjuvant XELOX (oral capecitabine 1000 mg/m2 twice daily on days 1 to 14 plus intravenous oxaliplatin 130 mg/m2 on day 1 of each cycle) chemotherapy[85]. Five year follow-up showed an increased estimated survival rate in the adjuvant chemotherapy group, 78% (95%CI: 74-82) in the adjuvant group and 69% (95%CI: 64-73) in the surgery alone one; HR = 0.66 (95%CI: 0.51-0.85, P = 0.0015). A significant reduction in the disease free survival was also demonstrated (HR = 0.58, 95%CI: 0.47-0.72, P < 0.0001)[86].
Right now evidences supporting the adjuvant chemotherapy are lacking focus on which patients, stage and clinical status, could benefit better from the treatment. Three randomized clinical trials are undergoing to evaluate different drug regimens of adjuvant therapy only in patients with stage III disease (clinicaltrials.gov NCT01618474, NCT01935778, NCT00182611).
Increased knowledge of tumor biology and the cellular and molecular mechanisms of malignant proliferation is leading to the development of targeted therapies against these specific mechanisms, in order to reduce the toxicity of traditional chemotherapic agents and improve survival. Several biological pathways have been individuated in gastric cancer, adopting knowledge from other tumors.
Her-2/neu (ERBB2) right now is the main molecular target where monoclonal antibodies have been demonstrated to be effective. HER2 is a cell membrane receptor involved in cell growth and differentiation; it’s over-expressed in 10%-40% of gastric cancer. Several meta-analysis assessed the prognostic role of HER2 over-expression in gastric cancer with contrasting results[87-91] depending on the diagnostic technique adopted on the expression assessment. Although these criticisms of the over expression of HER2 seems to be related with an instestinal tumor type, according to the Lauren classification, venous and lymphovascular invasion, lymphnode metastasis and, above all, overall survival. Against HER2 a monoclonal antibody, Trastuzumab (Herceptin®, Genentech) has been developed and was demostrated to be effective. The ToGA Trial was a randomized phase III study including patients with metastatic or unresectable gastric cancer with HER2 over-expression; patients received cisplatin plus fluoropyrimidines based chemotherapy plus Trastuzumab or chemotherapy alone[92]. The addition of the monoclonal antibody resulted in a reduced relative risk of death by 26% (HR = 0.74, 95%CI: 0.60-0.91), and the risk reduction was more pronounced in the HER2-enriched population, with 3+ or 2+ immunohistochemistry and FISH-positive status (HR = 0.65, 95%CI: 0.51-0.83). Trastuzumab has been approved in several countries and has become standard treatment in advanced gastric cancer. Right now there are no trial evaluating the role of Trastuzumab in neadjuvant or adjuvant settings: the positive results in advanced setting lead to test its efficacy after courative resection and randomized trials are now recruiting patients: a phase II trials is evaluating the combination of capecitabine, oxaliplatin and trastuzumab in the adjuvant setting (NCT 01748773); a phase III trial is evaluating the same drug regimens in perioperative setting (NCT 01130337).
Lapatinib is tyrosine kinase inhibitor agaist EGFR and HER2, developed and approved in breast cancer. It has been tested in advanced gastric cancer in two phase III studies but did not produce an improvement in OS[93,94]: at the moment there are no evidences for the application of this new drug in resectable gastric cancer.
Epithelial Grow Factor Receptor (EGFR) is one of the implicated molecular pathway with a reported over-expression in 30%-50% of gastric cancer[95,96]: the activation of the cell membrane receptor leads to a signaling cascade involved in the regulation of intracellular/intercellular processes such as cell cycle progression, apoptosis and cell survival, proliferation, angiogenesis and metastasis. Several targeted therapies against this agent have been developed but all have been tested on metastatic or inoperable cancers. Cetuximab (Erbitux®), and Panitumumab (Vectibix®, Amgen), monoclonal antibodies, seem to slightly improve the progression-free survival in advanced gastric cancer with contrasting results[96,97] and their roles are still unclear. Several trials are needed to estimate the real benefit and the eventual translation in operable gastric cancer in perioperative settings. Gefitinib (Iressa®, AstraZeneca Pharmaceuticals) and Erlotinib (Tarceva®, Roche-Genetech), tyosin-kinase inhibitors, have been demonstrated as ineffective in gastric cancer[96].
Angiogenesis is another target for novel drug agents due to its role in tumoral growth, survival and metastatic diffusion. VEGF and its receptors (VEGFR-1 and VEGFR-2) are the molecular targets involved in the angiogenic pathways to which were developed novel agents.
Bevacizumab is monoclonal antibody against VEGF, which initially developed for colorectal, lung, ovarian, and renal cell cancers. In AGC it was tested in two randomized phase III trial, the AVAGAST and the AVATAR trials[98,99]: the addition of Bevacizumab to the chemiotherapic scheme did not show any difference in the overall survival; however both median Progression Free Survival and overall response rate were significantly improved in the bevacizumab group. The new agents are now under evaluation in perioperative setting in the MAGIC-B trial (NCT00450203) in operable gastric cancer.
Ramucirumab is a monoclonal antibody against VEGFR-2: in two different randomized phase III trials was tested on patients with advanced gastric and gastro-esophageal cancer after disease progression after first line chemotherapy[100,101]. In both studies overall survival was significantly higher in ramucirumab group [(HR = 0.774, 95%CI: 0.605-0.991); P = 0.042 and 0.807 (95%CI: 0.678-0.962), P = 0.017 respectively]. However the real median gain in overall survival was only 1.4 and 2.6 mo respectively.
The most important spread of malignant cells in GC are into the lymphatic torrent and the serosa invasion: for this reason the 53%-60% of that patients had a disseminated peritoneal disease (stage III-IV), while only a 40% of patients have hepatic metastases through the hematic torrent[49,51]. Moreover these patients die more frequently for peritoneal spread of the disease then distant metastases (77% of patient M0 die for peritoneal progression of disease), despite a radical surgery, and radiotherapy or systemic chemotherapy regimens[49,102-104]. Positive cytology was found in 11% to 27% of patients with GC[105]. the administration of chemotherapics directly into the peritoneal cavity has been suggested as a potential therapy of GC at this stages.
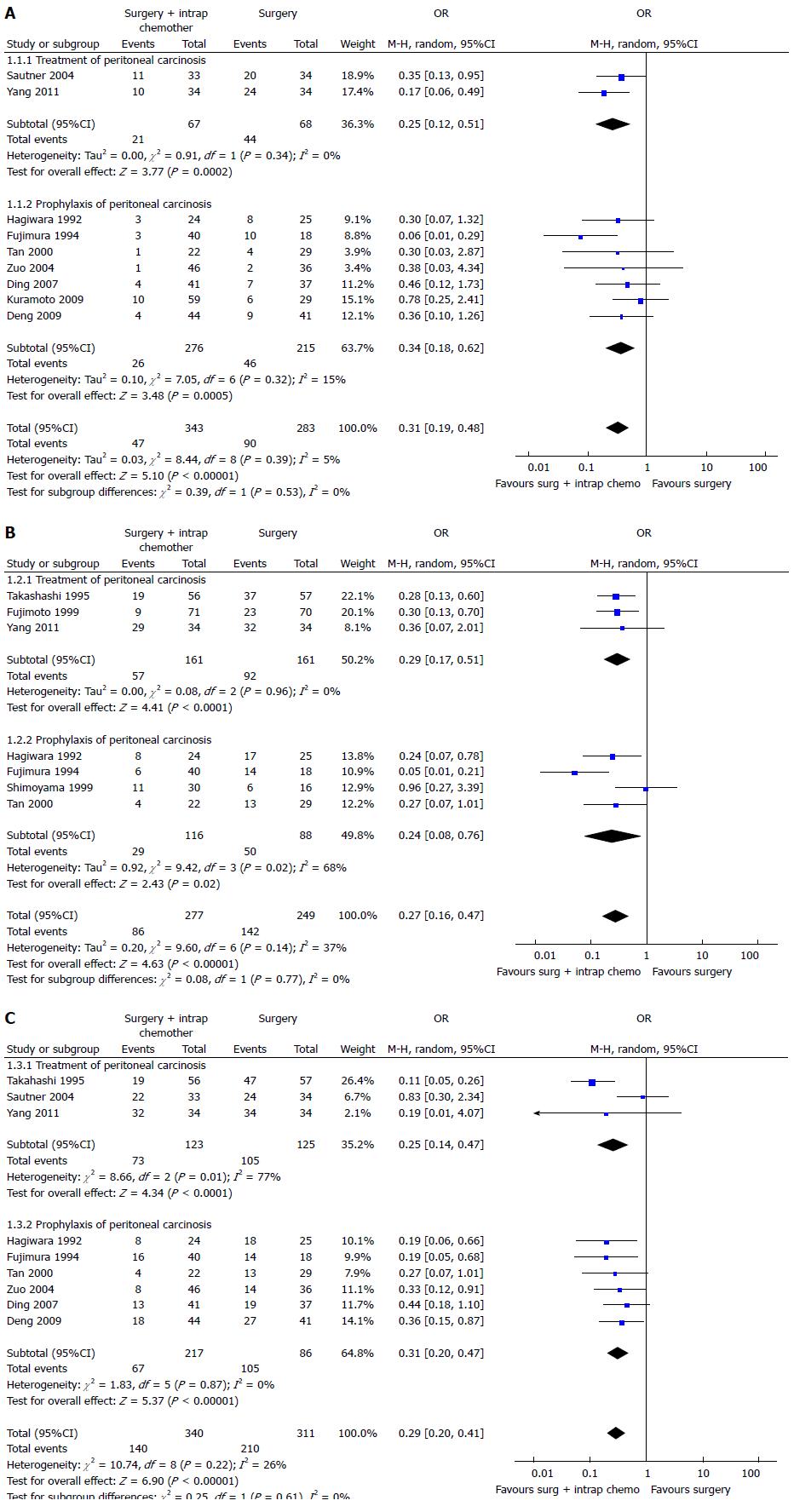
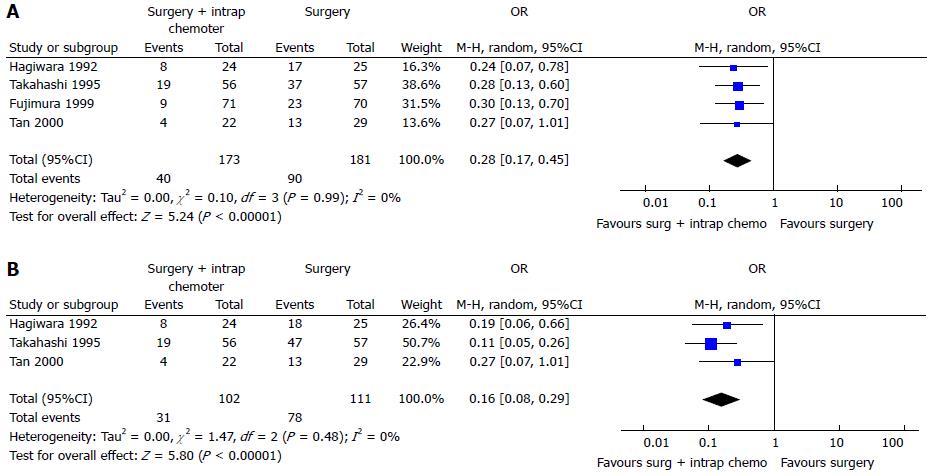
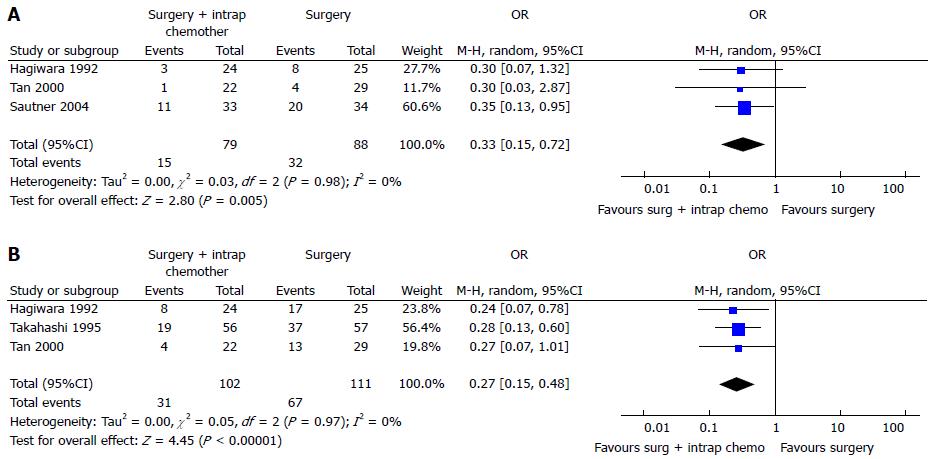
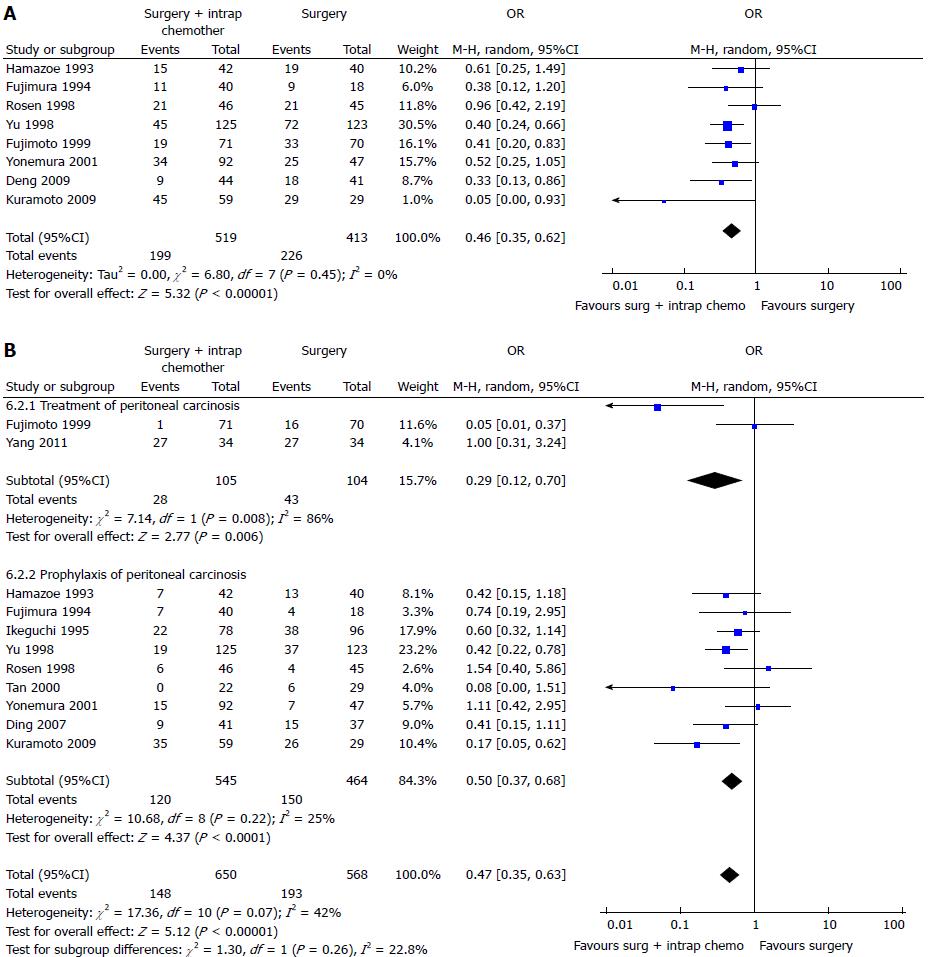
Two recent meta-analysis[102,103] have investigated its role in GC with or without peritoneal, nodal and distant metastasis after radical surgery. The first[102] evaluated the effect of IPC plus CRS in patients with GC with peritoneal, nodal and distant metastasis. They analyzed 20 RCTs (2145 patients) and reported an increase of overall survival in patients who underwent CRS plus IPC. IPC reduced 1, 2, 3-years mortality (OR = 0.31, 0.27, 0.29 respectively) (Figure 3), 2 and 3-years mortality in patients with loco regional nodal metastasis (OR = 0.28, 0.16 respectively) (Figure 4), 1 and 2-year mortality rate in patients with serosal infiltration (OR = 0.33, 0.27 respectively) (Figure 5). However morbidity rate was increased by surgery plus IPC (OR = 1.82). The overall recurrence and the peritoneal recurrence rates were improved by surgery plus IPC (OR = 0.46 and 0.47 respectively) (Figure 6). There was no statistically significant difference in lymph nodal recurrence rate. The rate of hematogenous metastasis was improved by surgery plus IPC (OR = 0.63).
In the second meta-analysis, Mi et al[103] evaluate the effect of hypertermic intraperitoneal chemotherapy (HIPEC) on patients without peritoneal metastasis who have undergone radical surgery. They analyzed 16 RCTs (1906 pts.) in which was compared surgery alone vs CRS plus HIPEC. Data reported an improvement in survival rate at 1, 2, 3, 5, 9 years (HR = 2.99, 2.43, 2.63, 2.49, 2.14 respectively, P < 0.05). A significant reduction in recurrence rate in HIPEC plus surgery group was found after 2, 3, 5-years (RR = 0.42, 0.35, 0.47, P < 0.00001). Despite the previous meta-analysis, HIPEC group was not associated with higher risks of anastomotic leakage, ileus, bowel perforation, myelosuppression, gastrointestinal reaction and hypohepatia, but it increased the incidence of abdominal pain (RR = 21.46, P < 0.00001).
Another recent meta-analysis by Sun et al[106] reported data about HIPEC plus surgery vs surgery alone in patients with macroscopic serosal invasion without distant metastasis or peritoneal carcinomatosis (10 trials, 1062 patients). The overall survival was improved in HIPEC group particularly in Mitomycin subgroup [RR = 0.75 (P < 0.00001) and in 5-Fluorouriacil (5FU) group RR = 0.69 (P < 0.00001)].
Neoadjuvant regimen could downstage tumor and improve the efficacy of radical surgery, and IPC plus CRS could effect on the tumor and on peritoneal disease and free malignant cells. In 2012, Yonemura et al[107] proposed a new therapeutic approach called “bidirectional chemotherapy” aims to induce a reduction of the peritoneal disease and reduce the free malignant cells. He proposed a neoadjuvant intraperitoneal and systemic chemotherapy (NIPS) that can act on peritoneal carcinomatosis from inside of peritoneum and from the subperitoneal blood vessels. He proposed a drug regimen with oral S-1, i.v. taxotere and cisplatinum and intraperitoneal cisplatinum and docetaxel. Yonemura reported a reduction of positive cytology from 70.8% to 22.9% after NIPS, and 69% of the first 70.8% positive cytology became negative. After NIPS a complete cytoreduction (CC0) was achieved in 70.7% of patients and in a 36.8% of cases was achieved a complete pathological response of PC. The overall morbidity was 24.4% and overall mortality was 3.7%. The median survival time was 14.4 mo, with a survival rate at 1, 3, 5-years of 61%, 16% and 16% respectively However due to the high morbidity and mortality rates the procedure should be chosen only after a strict patients selection (patients with good pathological response and a PCI ≤ 6). In 2014 was published a similar study[108] that confirmed these data about mortality (3.9%) and morbidity (23.6%) and showed better survival rates (66%, 32% and 10.7% at 1, 2, 5-years). Moreover the authors reported that in the literature 25% of patients with negative cytology before induction chemotherapy became positive, while in their study no patient with negative cytology switched in a positive cytology after bidirectional chemotherapy. These data suggested that bidirectional chemotherapy could give a better control than usual therapy in case of peritoneal disseminated disease. Further studies are needed to confirm it.
In the last years the literature has shown the fundamental role of free peritoneal tumor cells and positive cytology for the survival, particularly in Advanced GC[109-111].
When gastric serosa is involved, Peritoneal Carcinosis could be considered practically unavoidable[102]. In the case of free peritoneal tumor cells in the abdominal cavity the natural evolution in peritoneal carcinomatosis occur in 80% of cases, with a distant survival near to 0%[112]. Positive cytology was considered the most important prognostic factor (more than T or N) for advanced disease, early recurrence, and it decreased disease-specific survival following curative resection in patients with GC[109]. In the AJCC-NCCN 7th edition, the positive cytology at the staging laparoscopy is considered as M1 disease[113,114]. The risk factors of positive cytology are increasing T stage and serosal invasion (increase the relative risk of positive cytology of 11 over that of patients without serosal involvement; P = 0.03), and positive node disease (increase of 5 fold the risk of positive cytology)[109].
Recent studies have shown that more than 30% of patients with a disease beyond T1 on EUS and absent metastasis on CT-scan have evident peritoneal disease on laparoscopy[115]. Despite the importance of a staging laparoscopy (in locally advanced GC T ≥ 3 and N ≥ 1) this procedure is not always performed before surgery[109,110]. Some authors stratified with Endoscopic UltraSounds into high and low risk patients (T1/T2, N0 vs T3/T4, N+) before proceed to a staging laparoscopy and found peritoneal dissemination (M1 disease) in 25% of high risk patients 25% vs 4% in low risk[109].
Some authors proposed an algorithm for patients with locally advanced GC in which after a complete pre-treatment staging (including staging laparoscopy and peritoneal washing), if cytology was positive they underwent to palliative chemotherapy and/or surgery, and if is negative they underwent to neoadjuvant chemotherapy with a successive second look laparoscopy with peritoneal cytology evaluation[109]. However, despite neoadjuvant therapy, patients with cytology conversion from positive to negative have not a significant increase survival[109].
The main criticism of peritoneal washing cytology remains its low sensitivity (14%-70% but it should be noticed that these rates are extrapolated from heterogeneous cohorts of patients in different disease’s stage)[110]. Ang et al[110] found in his series that 10.2% of patients with negative radiological and staging laparoscopy for metastatic disease have a positive cytology, and that 8/20 patients have a peritoneal biopsy positive for peritoneal disease with a negative cytology. To improve the sensitivity Homma et al[111] suggested to perform the washing in multiple cavities (right and left subphrenic space, inside the omental bursa, and Douglas pouch), and not only in the Douglas pouch[105].
External beam Radiotherapy (RT) has a role in the treatment of locally advanced gastric cancer in preoperative, postoperative or palliative setting.
Neoadjuvant or adjuvant radiochemotherapy (RCTh) improves overall survival, compared to surgery alone. However a clear consensus on the better treatments integration hasn’t already been defined.
Neoadjuvant therapy increases the probability of curative resection due to down-staging. The pre-operative RT target volume is generally smaller and the presence of the macroscopic tumor displaces surrounding normal structures (organ at risk). On the contrary, organs at risk receive higher dose if they fill the original tumor site in the postoperative setting. Moreover postoperative strategies generally require higher doses to achieve the same local control effect, because of the increased tissue hypoxia. The irradiated volumes are the tumor with a safety margin surrounding it, in the preoperative setting, or the tumor bed in the postoperative one. Regional lymph nodes (perigastric, celiac axis, pancreaticoduodenal, porta hepatis, paraaortic and splenic hilar lymph nodes) are part of the target volume according to the stage or the extension of disease.
The prescribed total dose is usually 45 Gy in 25 daily fractions.
The modern RT techniques (i.e., 3D-conformal, intensity-modulated RT, Arc techniques, Tomotherapy) seem to allow a better target coverage and a lower radiation related toxicity, even if clinical trials are still ongoing.
The role of neoadjuvant RCT have been explored considering the excellent results in terms of local control and overall survival (OS) in the treatment of esophageal and rectal cancer[116,117]. Trials of neoadjuvant chemotherapy (CT) or RCTh compared to surgery alone have shown an OS improvement in patients with esophageal and esophago-gastric junction Siewert I-II adenocarcinomas.
Small prospective studies have examined the use of induction CT prior to preoperative RCTh for potentially resectable gastric cancer showing good rates of pathological response and R0 resection, with acceptable acute and late toxicity[118-120].
Single institution retrospective data have also been published[121-124], but we lack significant evidence on the effectiveness of neoadjuvant approach in gastric cancer.
The randomized trial “Preoperative Therapy for Gastric and Esophagogastric Junction Adenocarcinoma” compares neoadjuvant CT vs RCTh. It started with randomization in 2009 and the end of enrollment is planned for 2020[125].
In 2001 the pivotal INT0116 study has established the role of adjuvant RCTh in the management of locally advanced gastric cancer demonstrating that adjuvant RT (45 Gy in 25 fractions over 5 wk) plus 5-fluorouracil based CT increases relapse-free and OS, compared with surgery alone, despite high toxicity[44]. This result is confirmed significant with a hazard ratio for OS of 1.32 in favour of adjuvant RCT[126] after ten years follow-up.
The main limitation of the study is the suboptimal surgical treatment because 54% of patients have been treated with a D1 lymphadenectomy and only 10% with D2 lymphadenectomy, suggesting that postoperative RCTh may compensate for sub-optimal surgery. This hypothesis is supported by the Dutch D1D2 trial, which results confirm reduction of local recurrence rate if adjuvant RCTh is given in case of D1 lymphadenectomy, but provides no benefit in case of D2 nodal dissection[127].
The ARTIST trial, designed for comparing adjuvant chemotherapy with capecitabine plus cisplatin to RCTh, failed to demonstrate differences in DFS and OS between the two groups. Noteworthy the subgroup analysis evidenced a benefit in terms of disease free survival if Radiotherapy was added in patients with lymph node metastasis[128].
The subsequent phase III trial (ARTIST-II) is still ongoing for reconfirming these results.
The CRITICS trial investigates if perioperative chemotherapy followed by postoperative RCTh (45 Gy in 25 fractions plus cisplatin and capecitabine) improves clinical outcome. Patients accrual has been started in 2006 for recruiting 788 patients.
One observational and one randomized study suggested potential benefits from postoperative RCTh even after optimal D2 dissection[129,130]. However RCTh as adjuvant treatment after D2 dissection still remains controversial.
Radiotherapy has been shown to be effective for palliation in case of gastric bleeding, pain and obstruction.
Fields et al[122] have analyzed treatment outcomes of RCT vs chemotherapy alone in 79 patients with locoregional recurrence of gastric cancer, showing better overall symptom-control rate in the former group without significant differences in toxicity rate.
Tey et al[131] have conducted a retrospective review of 115 patients, treated with palliative intent, showing symptoms control at one month in 46%-81% of cases with low toxicity profile. Dose fractionation regimen ranged from 8Gy single fraction to 40 Gy in 16 fractions. The gastric bleeding control could safely be obtained with low radiotherapy doses without significant side effects[132]. The role of a higher dose (> 41 Gy), remains debatable in the obstruction treatment[133].
Gastric cancer is an aggressive disease with a high risk of peritoneal dissemination either at early stages. Surgical therapy of GC should be based on radical surgery aiming to eradicate all the macroscopic disease. As peritoneal dissemination of GC is the main cause of long term failure of the treatment a peritoneal fluid cytology should always be done. Uncertainty about its results however suggests the importance of preventing peritoneal dissemination and subsequent carcinosis with an anticipated use of the intraperitoneal chemotherapy. Moreover the use of perioperative and bidirectional chemotherapy should be considered. Advanced gastric cancer with positive cytology at stadiative laparoscopy should be treated in experienced centre in order to introduce the use of “preventive intraperitoneal chemotherapy” either in the absence of macroscopic peritoneal dissemination associated to perioperative chemotherapy regimen.
P- Reviewer: Aoyagi K S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | American Cancer Society. Cancer Facts and Figures 2013. Atlanta: American Cancer Society 2013; Available from: http://www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2013. |
| 2. | Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Prediction of cancer incidence and mortality in Korea, 2013. Cancer Res Treat. 2013;45:15-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | World Health Organization. GLOBOCAN 2012: Estimated Cancer Incidence. Mortality and Prevalence: Worldwide 2012; . |
| 4. | Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 289] [Reference Citation Analysis (0)] |
| 5. | Bauer K, Schroeder M, Porzsolt F, Henne-Bruns D. Comparison of international guidelines on the accompanying therapy for advanced gastric cancer: reasons for the differences. J Gastric Cancer. 2015;15:10-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Wang J, Sun Y, Bertagnolli MM. Comparison of gastric cancer survival between Caucasian and Asian patients treated in the United States: results from the Surveillance Epidemiology and End Results (SEER) database. Ann Surg Oncol. 2015;22:2965-2971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 7. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2872] [Article Influence: 205.1] [Reference Citation Analysis (0)] |
| 8. | Verlato G, Giacopuzzi S, Bencivenga M, Morgagni P, De Manzoni G. Problems faced by evidence-based medicine in evaluating lymphadenectomy for gastric cancer. World J Gastroenterol. 2014;20:12883-12891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077-3079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 814] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 10. | Sobin LH, Gospodarowicz MK, Wittekind C. International Union Against Cancer (UICC) TNM Classification of Malignant Tumors. 7th ed. New York: Wiley-Liss 2010; . |
| 11. | Murakami T. Early cancer of the stomach. World J Surg. 1979;3:685-692. [PubMed] |
| 12. | Seevaratnam R, Bocicariu A, Cardoso R, Yohanathan L, Dixon M, Law C, Helyer L, Coburn NG. How many lymph nodes should be assessed in patients with gastric cancer? A systematic review. Gastric Cancer. 2012;15 Suppl 1:S70-S88. [PubMed] |
| 13. | Gouzi JL, Huguier M, Fagniez PL, Launois B, Flamant Y, Lacaine F, Paquet JC, Hay JM. Total versus subtotal gastrectomy for adenocarcinoma of the gastric antrum. A French prospective controlled study. Ann Surg. 1989;209:162-166. [PubMed] |
| 14. | Bozzetti F, Marubini E, Bonfanti G, Miceli R, Piano C, Gennari L. Subtotal versus total gastrectomy for gastric cancer: five-year survival rates in a multicenter randomized Italian trial. Italian Gastrointestinal Tumor Study Group. Ann Surg. 1999;230:170-178. [PubMed] |
| 15. | McCulloch P, Niita ME, Kazi H, Gama-Rodrigues JJ. Gastrectomy with extended lymphadenectomy for primary treatment of gastric cancer. Br J Surg. 2005;92:5-13. [PubMed] |
| 16. | Deng J, Zhang R, Pan Y, Wang B, Wu L, Jiao X, Bao T, Hao X, Liang H. Comparison of the staging of regional lymph nodes using the sixth and seventh editions of the tumor-node-metastasis (TNM) classification system for the evaluation of overall survival in gastric cancer patients: findings of a case-control analysis involving a single institution in China. Surgery. 2014;156:64-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Schwarz RE. Current status of management of malignant disease: current management of gastric cancer. J Gastrointest Surg. 2015;19:782-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Jiang L, Yang KH, Chen Y, Guan QL, Zhao P, Tian JH, Wang Q. Systematic review and meta-analysis of the effectiveness and safety of extended lymphadenectomy in patients with resectable gastric cancer. Br J Surg. 2014;101:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition -. Gastric Cancer. 1998;1:10-24. [PubMed] |
| 20. | Wu W, Dong P, Wu X, Li M, Ding Q, Zhang L, Yang J, Weng H, Ding Q, Tan Z. Three-step method for systematic lymphadenectomy in gastric cancer surgery using the ‘curettage and aspiration dissection technique’ with Peng’s multifunctional operative dissector. World J Surg Oncol. 2014;12:322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | de Manzoni G, Verlato G, Bencivenga M, Marrelli D, Di Leo A, Giacopuzzi S, Cipollari C, Roviello F. Impact of super-extended lymphadenectomy on relapse in advanced gastric cancer. Eur J Surg Oncol. 2015;41:534-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Kulig J, Popiela T, Kolodziejczyk P, Sierzega M, Szczepanik A; Polish Gastric Cancer Study Group. Standard D2 versus extended D2 (D2+) lymphadenectomy for gastric cancer: an interim safety analysis of a multicenter, randomized, clinical trial. Am J Surg. 2007;193:10-15. [PubMed] |
| 23. | Degiuli M, Sasako M, Ponti A, Soldati T, Danese F, Calvo F. Morbidity and mortality after D2 gastrectomy for gastric cancer: results of the Italian Gastric Cancer Study Group prospective multicenter surgical study. Clin Oncol. 1998;16:1490-1493. |
| 24. | Degiuli M, Sasako M, Ponti A, Soldati T, Danese F, Calvo F. Morbidity and mortality after D2 gastrectomy for gastric cancer: results of the Italian Gastric Cancer Study Group prospective multicenter surgical study. J Clin Oncol. 1998;16:1490-1493. [PubMed] |
| 25. | Song W, He Y, Wang S, He W, Xu J. Significance of the lymph nodes in the 7th station in rational dissection for metastasis of distal gastric cancer with different T categories. Chin J Cancer Res. 2014;26:423-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 26. | Galizia G, Lieto E, De Vita F, Castellano P, Ferraraccio F, Zamboli A, Mabilia A, Auricchio A, De Sena G, De Stefano L. Modified versus standard D2 lymphadenectomy in total gastrectomy for nonjunctional gastric carcinoma with lymph node metastasis. Surgery. 2015;157:285-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Roviello F, Pedrazzani C, Marrelli D, Di Leo A, Caruso S, Giacopuzzi S, Corso G, de Manzoni G. Super-extended (D3) lymphadenectomy in advanced gastric cancer. Eur J Surg Oncol. 2010;36:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 753] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 29. | Wu CW, Hsiung CA, Lo SS, Hsieh MC, Chen JH, Li AF, Lui WY, Whang-Peng J. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol. 2006;7:309-315. [PubMed] |
| 30. | Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1140] [Cited by in RCA: 1306] [Article Influence: 87.1] [Reference Citation Analysis (1)] |
| 31. | Kodera Y, Schwarz RE, Nakao A. Extended lymph node dissection in gastric carcinoma: where do we stand after the Dutch and British randomized trials? J Am Coll Surg. 2002;195:855-864. [PubMed] |
| 32. | Kuo CY, Chao Y, Li CP. Update on treatment of gastric cancer. J Chin Med Assoc. 2014;77:345-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Schwarz RE. Spleen-preserving splenic hilar lymphadenectomy at the time of gastrectomy for cancer: technical feasibility and early results. J Surg Oncol. 2002;79:73-76. [PubMed] |
| 34. | Bonenkamp JJ, Hermans J, Sasako M, van de Velde CJ, Welvaart K, Songun I, Meyer S, Plukker JT, Van Elk P, Obertop H. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999;340:908-914. [PubMed] |
| 35. | Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, Sydes M, Fayers P. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999;79:1522-1530. [PubMed] |
| 36. | Cuschieri A, Fayers P, Fielding J, Craven J, Bancewicz J, Joypaul V, Cook P. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. The Surgical Cooperative Group. Lancet. 1996;347:995-999. [PubMed] |
| 37. | Degiuli M, Sasako M, Calgaro M, Garino M, Rebecchi F, Mineccia M, Scaglione D, Andreone D, Ponti A, Calvo F; Italian Gastric Cancer Study Group. Morbidity and mortality after D1 and D2 gastrectomy for cancer: interim analysis of the Italian Gastric Cancer Study Group (IGCSG) randomised surgical trial. Eur J Surg Oncol. 2004;30:303-308. [PubMed] |
| 38. | McCulloch P, Nita ME, Kazi H, Gama-Rodrigues J. Extended versus limited lymph nodes dissection technique for adenocarcinoma of the stomach. Cochrane Database Syst Rev. 2004;CD001964. [PubMed] |
| 39. | Degiuli M, Sasako M, Ponti A; Italian Gastric Cancer Study Group. Morbidity and mortality in the Italian Gastric Cancer Study Group randomized clinical trial of D1 versus D2 resection for gastric cancer. Br J Surg. 2010;97:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 40. | Kiyokawa T, Hiki N, Nunobe S, Honda M, Ohashi M, Sano T, Yamaguchi T. Feasibility of Gastrectomy with Standard Lymphadenectomy for Patients Over 85 Years Old with Gastric Cancer. Ann Surg Oncol. 2015;22:3962-3969. [PubMed] |
| 41. | Wu CW, Hsiung CA, Lo SS, Hsieh MC, Shia LT, Whang-Peng J. Randomized clinical trial of morbidity after D1 and D3 surgery for gastric cancer. Br J Surg. 2004;91:283-287. [PubMed] |
| 42. | Sano T, Sasako M, Yamamoto S, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, Yamamura Y. Gastric cancer surgery: morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para-aortic lymphadenectomy--Japan Clinical Oncology Group study 9501. J Clin Oncol. 2004;22:2767-2773. [PubMed] |
| 43. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [PubMed] |
| 44. | Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725-730. [PubMed] |
| 45. | Marchet A, Mocellin S, Ambrosi A, Morgagni P, Garcea D, Marrelli D, Roviello F, de Manzoni G, Minicozzi A, Natalini G. The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: results from an Italian multicentric study in 1853 patients. Ann Surg. 2007;245:543-552. [PubMed] |
| 46. | Yashiro M, Matsuoka T. Sentinel node navigation surgery for gastric cancer: Overview and perspective. World J Gastrointest Surg. 2015;7:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 47. | Maruyama K, Sasako M, Kinoshita T, Sano T, Katai H. Can sentinel node biopsy indicate rational extent of lymphadenectomy in gastric cancer surgery? Fundamental and new information on lymph-node dissection. Langenbecks Arch Surg. 1999;384:149-157. [PubMed] |
| 48. | Cohen DJ, Leichman L. Controversies in the treatment of local and locally advanced gastric and esophageal cancers. J Clin Oncol. 2015;33:1754-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 49. | Montori G, Coccolini F, Ceresoli M, Catena F, Colaianni N, Poletti E, Ansaloni L. The treatment of peritoneal carcinomatosis in advanced gastric cancer: state of the art. Int J Surg Oncol. 2014;2014:912418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 50. | Rudloff U, Langan RC, Mullinax JE, Beane JD, Steinberg SM, Beresnev T, Webb CC, Walker M, Toomey MA, Schrump D. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: results of the GYMSSA trial. J Surg Oncol. 2014;110:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 169] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 51. | Coccolini F, Gheza F, Lotti M, Virzì S, Iusco D, Ghermandi C, Melotti R, Baiocchi G, Giulini SM, Ansaloni L. Peritoneal carcinomatosis. World J Gastroenterol. 2013;19:6979-6994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 253] [Cited by in RCA: 232] [Article Influence: 19.3] [Reference Citation Analysis (1)] |
| 52. | Bristow RE, Chi DS. Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: a meta-analysis. Gynecol Oncol. 2006;103:1070-1076. [PubMed] |
| 53. | Kodera Y, Nakanishi H, Ito S, Yamamura Y, Fujiwara M, Koike M, Hibi K, Ito K, Tatematsu M, Nakao A. Prognostic significance of intraperitoneal cancer cells in gastric carcinoma: detection of cytokeratin 20 mRNA in peritoneal washes, in addition to detection of carcinoembryonic antigen. Gastric Cancer. 2005;8:142-148. [PubMed] |
| 54. | Ansaloni L, Coccolini F, Morosi L, Ballerini A, Ceresoli M, Grosso G, Bertoli P, Busci LM, Lotti M, Cambria F. Pharmacokinetics of concomitant cisplatin and paclitaxel administered by hyperthermic intraperitoneal chemotherapy to patients with peritoneal carcinomatosis from epithelial ovarian cancer. Br J Cancer. 2015;112:306-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 55. | Coccolini F, Campanati L, Catena F, Ceni V, Ceresoli M, Jimenez Cruz J, Lotti M, Magnone S, Napoli J, Rossetti D. Hyperthermic intraperitoneal chemotherapy with cisplatin and paclitaxel in advanced ovarian cancer: a multicenter prospective observational study. J Gynecol Oncol. 2015;26:54-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 56. | Yonemura Y, Shinbo M, Hagiwara A. Treatment for potentially curable gastric cancer patients with intraperitoneal free cancer cells. Gastroenterological Surg. 2008;31:802-812. |
| 57. | Yonemura Y, Bandou E, Sawa T, Yoshimitsu Y, Endou Y, Sasaki T, Sugarbaker PH. Neoadjuvant treatment of gastric cancer with peritoneal dissemination. Eur J Surg Oncol. 2006;32:661-665. [PubMed] |
| 58. | Glehen O, Gilly FN, Arvieux C, Cotte E, Boutitie F, Mansvelt B, Bereder JM, Lorimier G, Quenet F, Elias D; Association Française de Chirurgie. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2010;17:2370-2377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 341] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 59. | Yonemura Y, Endou Y, Shinbo M, Sasaki T, Hirano M, Mizumoto A, Matsuda T, Takao N, Ichinose M, Mizuno M. Safety and efficacy of bidirectional chemotherapy for treatment of patients with peritoneal dissemination from gastric cancer: Selection for cytoreductive surgery. J Surg Oncol. 2009;100:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 60. | Yonemura Y, Kawamura T, Bandou E, Takahashi S, Sawa T, Matsuki N. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br J Surg. 2005;92:370-375. [PubMed] |
| 61. | Glehen O, Gilly FN, Boutitie F, Bereder JM, Quenet F, Sideris L, Mansvelt B, Lorimier G, Msika S, Elias D; French Surgical Association. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer. 2010;116:5608-5618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 400] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 62. | Coccolini F, Catena F, Glehen O, Yonemura Y, Sugarbaker PH, Piso P, Montori G, Ansaloni L. Complete versus incomplete cytoreduction in peritoneal carcinosis from gastric cancer, with consideration to PCI cut-off. Systematic review and meta-analysis. Eur J Surg Oncol. 2015;41:911-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 63. | Yonemura Y, Elnemr A, Endou Y, Hirano M, Mizumoto A, Takao N, Ichinose M, Miura M, Li Y. Multidisciplinary therapy for treatment of patients with peritoneal carcinomatosis from gastric cancer. World J Gastrointest Oncol. 2010;2:85-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 92] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 64. | Scaringi S, Kianmanesh R, Sabate JM, Facchiano E, Jouet P, Coffin B, Parmentier G, Hay JM, Flamant Y, Msika S. Advanced gastric cancer with or without peritoneal carcinomatosis treated with hyperthermic intraperitoneal chemotherapy: a single western center experience. Eur J Surg Oncol. 2008;34:1246-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 65. | Yang XJ, Li Y, Yonemura Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy to treat gastric cancer with ascites and/or peritoneal carcinomatosis: Results from a Chinese center. J Surg Oncol. 2010;101:457-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 66. | Yang XJ, Huang CQ, Suo T, Mei LJ, Yang GL, Cheng FL, Zhou YF, Xiong B, Yonemura Y, Li Y. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18:1575-1581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 398] [Cited by in RCA: 492] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 67. | Kitano S, Shiraishi N, Fujii K, Yasuda K, Inomata M, Adachi Y. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery. 2002;131:S306-S311. [PubMed] |
| 68. | Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc. 2005;19:168-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 327] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 69. | Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, Ryu SW, Lee HJ, Song KY. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg. 2010;251:417-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 562] [Cited by in RCA: 622] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 70. | Yasunaga H, Horiguchi H, Kuwabara K, Matsuda S, Fushimi K, Hashimoto H, Ayanian JZ. Outcomes after laparoscopic or open distal gastrectomy for early-stage gastric cancer: a propensity-matched analysis. Ann Surg. 2013;257:640-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 71. | Bonenkamp JJ, Songun I, Hermans J, Sasako M, Welvaart K, Plukker JT, van Elk P, Obertop H, Gouma DJ, Taat CW. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet. 1995;345:745-748. [PubMed] |
| 72. | Wang W, Zhang X, Shen C, Zhi X, Wang B, Xu Z. Laparoscopic versus open total gastrectomy for gastric cancer: an updated meta-analysis. PLoS One. 2014;9:e88753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 73. | Xiong JJ, Nunes QM, Huang W, Tan CL, Ke NW, Xie SM, Ran X, Zhang H, Chen YH, Liu XB. Laparoscopic vs open total gastrectomy for gastric cancer: a meta-analysis. World J Gastroenterol. 2013;19:8114-8132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 74. | Liao G, Chen J, Ren C, Li R, Du S, Xie G, Deng H, Yang K, Yuan Y. Robotic versus open gastrectomy for gastric cancer: a meta-analysis. PLoS One. 2013;8:e81946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 75. | Shen WS, Xi HQ, Chen L, Wei B. A meta-analysis of robotic versus laparoscopic gastrectomy for gastric cancer. Surg Endosc. 2014;28:2795-2802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 76. | Songun I, Keizer HJ, Hermans J, Klementschitsch P, de Vries JE, Wils JA, van der Bijl J, van Krieken JH, van de Velde CJ. Chemotherapy for operable gastric cancer: results of the Dutch randomised FAMTX trial. The Dutch Gastric Cancer Group (DGCG). Eur J Cancer. 1999;35:558-562. [PubMed] |
| 77. | Hartgrink HH, van de Velde CJ, Putter H, Songun I, Tesselaar ME, Kranenbarg EK, de Vries JE, Wils JA, van der Bijl J, van Krieken JH; Cooperating Investigators of The Dutch Gastric Cancer Group. Neo-adjuvant chemotherapy for operable gastric cancer: long term results of the Dutch randomised FAMTX trial. Eur J Surg Oncol. 2004;30:643-649. [PubMed] |
| 78. | Schuhmacher C, Gretschel S, Lordick F, Reichardt P, Hohenberger W, Eisenberger CF, Haag C, Mauer ME, Hasan B, Welch J. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28:5210-5218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 532] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 79. | Mirza A, Pritchard S, Welch I. The postoperative component of MAGIC chemotherapy is associated with improved prognosis following surgical resection in gastric and gastrooesophageal junction adenocarcinomas. Int J Surg Oncol. 2013;2013:781742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 80. | Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1501] [Article Influence: 107.2] [Reference Citation Analysis (0)] |
| 81. | Ronellenfitsch U, Schwarzbach M, Hofheinz R, Kienle P, Kieser M, Slanger TE, Jensen K; GE Adenocarcinoma Meta-analysis Group. Perioperative chemo(radio)therapy versus primary surgery for resectable adenocarcinoma of the stomach, gastroesophageal junction, and lower esophagus. Cochrane Database Syst Rev. 2013;5:CD008107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 82. | Reim D, Gertler R, Novotny A, Becker K, zum Büschenfelde CM, Ebert M, Dobritz M, Langer R, Hoefler H, Friess H. Adenocarcinomas of the esophagogastric junction are more likely to respond to preoperative chemotherapy than distal gastric cancer. Ann Surg Oncol. 2012;19:2108-2118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 83. | Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303:1729-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 604] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 84. | Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387-4393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1089] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 85. | Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1290] [Article Influence: 99.2] [Reference Citation Analysis (0)] |
| 86. | Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, Kim HH, Choi JH, Kim HK, Yu W. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 775] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 87. | Liang JW, Zhang JJ, Zhang T, Zheng ZC. Clinicopathological and prognostic significance of HER2 overexpression in gastric cancer: a meta-analysis of the literature. Tumour Biol. 2014;35:4849-4858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 88. | Gu J, Zheng L, Wang Y, Zhu M, Wang Q, Li X. Prognostic significance of HER2 expression based on trastuzumab for gastric cancer (ToGA) criteria in gastric cancer: an updated meta-analysis. Tumour Biol. 2014;35:5315-5321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 89. | Chen C, Yang JM, Hu TT, Xu TJ, Yan G, Hu SL, Wei W, Xu WP. Prognostic role of human epidermal growth factor receptor in gastric cancer: a systematic review and meta-analysis. Arch Med Res. 2013;44:380-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 90. | Chua TC, Merrett ND. Clinicopathologic factors associated with HER2-positive gastric cancer and its impact on survival outcomes--a systematic review. Int J Cancer. 2012;130:2845-2856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (1)] |
| 91. | Kurokawa Y, Matsuura N, Kimura Y, Adachi S, Fujita J, Imamura H, Kobayashi K, Yokoyama Y, Shaker MN, Takiguchi S. Multicenter large-scale study of prognostic impact of HER2 expression in patients with resectable gastric cancer. Gastric Cancer. 2015;18:691-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 92. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5308] [Article Influence: 353.9] [Reference Citation Analysis (3)] |
| 93. | Satoh T, Xu RH, Chung HC, Sun GP, Doi T, Xu JM, Tsuji A, Omuro Y, Li J, Wang JW. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol. 2014;32:2039-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 488] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 94. | Kothari N, Almhanna K. Current status of novel agents in advanced gastroesophageal adenocarcinoma. J Gastrointest Oncol. 2015;6:60-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 95. | Jørgensen JT. Role of human epidermal growth factor receptor 2 in gastric cancer: biological and pharmacological aspects. World J Gastroenterol. 2014;20:4526-4535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 96. | Cappetta A, Lonardi S, Pastorelli D, Bergamo F, Lombardi G, Zagonel V. Advanced gastric cancer (GC) and cancer of the gastro-oesophageal junction (GEJ): focus on targeted therapies. Crit Rev Oncol Hematol. 2012;81:38-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 97. | Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 680] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 98. | Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968-3976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 887] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 99. | Shen L, Li J, Xu J, Pan H, Dai G, Qin S, Wang L, Wang J, Yang Z, Shu Y. Bevacizumab plus capecitabine and cisplatin in Chinese patients with inoperable locally advanced or metastatic gastric or gastroesophageal junction cancer: randomized, double-blind, phase III study (AVATAR study). Gastric Cancer. 2015;18:168-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 202] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 100. | Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, dos Santos LV, Aprile G, Ferry DR. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1541] [Cited by in RCA: 1572] [Article Influence: 142.9] [Reference Citation Analysis (0)] |
| 101. | Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1613] [Cited by in RCA: 1765] [Article Influence: 160.5] [Reference Citation Analysis (0)] |
| 102. | Coccolini F, Cotte E, Glehen O, Lotti M, Poiasina E, Catena F, Yonemura Y, Ansaloni L. Intraperitoneal chemotherapy in advanced gastric cancer. Meta-analysis of randomized trials. Eur J Surg Oncol. 2014;40:12-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 103. | Mi DH, Li Z, Yang KH, Cao N, Lethaby A, Tian JH, Santesso N, Ma B, Chen YL, Liu YL. Surgery combined with intraoperative hyperthermic intraperitoneal chemotherapy (IHIC) for gastric cancer: a systematic review and meta-analysis of randomised controlled trials. Int J Hyperthermia. 2013;29:156-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 104. | Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, Fontaumard E, Brachet A, Caillot JL, Faure JL. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88:358-363. [PubMed] |
| 105. | Kano Y, Kosugi S, Ishikawa T, Otani T, Muneoka Y, Sato Y, Hanyu T, Hirashima K, Bamba T, Wakai T. Prognostic significance of peritoneal lavage cytology at three cavities in patients with gastric cancer. Surgery. 2015;158:1581-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 106. | Sun J, Song Y, Wang Z, Gao P, Chen X, Xu Y, Liang J, Xu H. Benefits of hyperthermic intraperitoneal chemotherapy for patients with serosal invasion in gastric cancer: a meta-analysis of the randomized controlled trials. BMC Cancer. 2012;12:526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 107. | Yonemura Y, Elnemr A, Endou Y, Ishibashi H, Mizumoto A, Miura M, Li Y. Effects of neoadjuvant intraperitoneal/systemic chemotherapy (bidirectional chemotherapy) for the treatment of patients with peritoneal metastasis from gastric cancer. Int J Surg Oncol. 2012;2012:148420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 108. | Canbay E, Mizumoto A, Ichinose M, Ishibashi H, Sako S, Hirano M, Takao N, Yonemura Y. Outcome data of patients with peritoneal carcinomatosis from gastric origin treated by a strategy of bidirectional chemotherapy prior to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in a single specialized center in Japan. Ann Surg Oncol. 2014;21:1147-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 109. | De Andrade JP, Mezhir JJ. The critical role of peritoneal cytology in the staging of gastric cancer: an evidence-based review. J Surg Oncol. 2014;110:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 110. | Ang CW, Tan LC. Peritoneal cytology in the staging process of gastric cancer: do or don’t? J Gastroint Dig Syst. 2013;3:156. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 111. | Homma Y, Ushida S, Yamada M, Kobayashi H, Suzuki K. Positive peritoneal washing cytology in multiple cavities can predict poor prognosis of advanced gastric cancer patients. Ann Surg Oncol. 2010;17:455-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 112. | Kodera Y, Yamamura Y, Shimizu Y, Torii A, Hirai T, Yasui K, Morimoto T, Kato T. Peritoneal washing cytology: prognostic value of positive findings in patients with gastric carcinoma undergoing a potentially curative resection. J Surg Oncol. 1999;72:60-64; discussion 64-65. [PubMed] |
| 113. | Ajani JA, Bentrem DJ, Besh S, D’Amico TA, Das P, Denlinger C, Fakih MG, Fuchs CS, Gerdes H, Glasgow RE. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11:531-546. [PubMed] |
| 114. | Edge S. Cancer AJCo: AJCC cancer staging manual. New York: Springer 2010; . |
| 115. | Sarela AI, Lefkowitz R, Brennan MF, Karpeh MS. Selection of patients with gastric adenocarcinoma for laparoscopic staging. Am J Surg. 2006;191:134-138. [PubMed] |
| 116. | van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3288] [Cited by in RCA: 4075] [Article Influence: 313.5] [Reference Citation Analysis (0)] |
| 117. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [PubMed] |
| 118. | Ajani JA, Winter K, Okawara GS, Donohue JH, Pisters PW, Crane CH, Greskovich JF, Anne PR, Bradley JD, Willett C. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol. 2006;24:3953-3958. [PubMed] |
| 119. | Ajani JA, Mansfield PF, Crane CH, Wu TT, Lunagomez S, Lynch PM, Janjan N, Feig B, Faust J, Yao JC. Paclitaxel-based chemoradiotherapy in localized gastric carcinoma: degree of pathologic response and not clinical parameters dictated patient outcome. J Clin Oncol. 2005;23:1237-1244. [PubMed] |
| 120. | Ajani JA, Mansfield PF, Janjan N, Morris J, Pisters PW, Lynch PM, Feig B, Myerson R, Nivers R, Cohen DS. Multi-institutional trial of preoperative chemoradiotherapy in patients with potentially resectable gastric carcinoma. J Clin Oncol. 2004;22:2774-2780. [PubMed] |
| 121. | Reed VK, Krishnan S, Mansfield PF, Bhosale PR, Kim M, Das P, Janjan NA, Delclos ME, Lowy AM, Feig BW. Incidence, natural history, and patterns of locoregional recurrence in gastric cancer patients treated with preoperative chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2008;71:741-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 122. | Fields RC, Strong VE, Gönen M, Goodman KA, Rizk NP, Kelsen DP, Ilson DH, Tang LH, Brennan MF, Coit DG. Recurrence and survival after pathologic complete response to preoperative therapy followed by surgery for gastric or gastrooesophageal adenocarcinoma. Br J Cancer. 2011;104:1840-1847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 123. | Díaz-González JA, Rodríguez J, Hernández-Lizoain JL, Ciérvide R, Gaztañaga M, San Miguel I, Arbea L, Aristu JJ, Chopitea A, Martínez-Regueira F. Patterns of response after preoperative treatment in gastric cancer. Int J Radiat Oncol Biol Phys. 2011;80:698-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 124. | Kim MS, Lim JS, Hyung WJ, Lee YC, Rha SY, Keum KC, Koom WS. Neoadjuvant chemoradiotherapy followed by D2 gastrectomy in locally advanced gastric cancer. World J Gastroenterol. 2015;21:2711-2718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 125. | Leong T, Smithers BM, Michael M, Gebski V, Boussioutas A, Miller D, Simes J, Zalcberg J, Haustermans K, Lordick F. TOPGEAR: a randomised phase III trial of perioperative ECF chemotherapy versus preoperative chemoradiation plus perioperative ECF chemotherapy for resectable gastric cancer (an international, intergroup trial of the AGITG/TROG/EORTC/NCIC CTG). BMC Cancer. 2015;15:532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 126. | Smalley SR, Benedetti JK, Haller DG, Hundahl SA, Estes NC, Ajani JA, Gunderson LL, Goldman B, Martenson JA, Jessup JM. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30:2327-2333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 627] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 127. | Dikken JL, Jansen EP, Cats A, Bakker B, Hartgrink HH, Kranenbarg EM, Boot H, Putter H, Peeters KC, van de Velde CJ. Impact of the extent of surgery and postoperative chemoradiotherapy on recurrence patterns in gastric cancer. J Clin Oncol. 2010;28:2430-2436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 128. | Lee J, Lim do H, Kim S, Park SH, Park JO, Park YS, Lim HY, Choi MG, Sohn TS, Noh JH. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol. 2012;30:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 580] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 129. | Kim S, Lim DH, Lee J, Kang WK, MacDonald JS, Park CH, Park SH, Lee SH, Kim K, Park JO. An observational study suggesting clinical benefit for adjuvant postoperative chemoradiation in a population of over 500 cases after gastric resection with D2 nodal dissection for adenocarcinoma of the stomach. Int J Radiat Oncol Biol Phys. 2005;63:1279-1285. [PubMed] |
| 130. | Zhu WG, Xua DF, Pu J, Zong CD, Li T, Tao GZ, Ji FZ, Zhou XL, Han JH, Wang CS. A randomized, controlled, multicenter study comparing intensity-modulated radiotherapy plus concurrent chemotherapy with chemotherapy alone in gastric cancer patients with D2 resection. Radiother Oncol. 2012;104:361-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 131. | Tey J, Back MF, Shakespeare TP, Mukherjee RK, Lu JJ, Lee KM, Wong LC, Leong CN, Zhu M. The role of palliative radiation therapy in symptomatic locally advanced gastric cancer. Int J Radiat Oncol Biol Phys. 2007;67:385-388. [PubMed] |
| 132. | Asakura H, Hashimoto T, Harada H, Mizumoto M, Furutani K, Hasuike N, Matsuoka M, Ono H, Boku N, Nishimura T. Palliative radiotherapy for bleeding from advanced gastric cancer: is a schedule of 30 Gy in 10 fractions adequate? J Cancer Res Clin Oncol. 2011;137:125-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 133. | Wong RK, Jang R, Darling G. Postoperative chemoradiotherapy vs. preoperative chemoradiotherapy for locally advanced (operable) gastric cancer: clarifying the role and technique of radiotherapy. J Gastrointest Oncol. 2015;6:89-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |