INTRODUCTION
Inflammatory bowel disease (IBD) with its sub-forms Crohn’s disease (CD) and ulcerative colitis (UC) are chronic inflammatory disorders of the gastrointestinal tract of multi-factorial aetiology. The current hypothesis suggests that an intestinal epithelial barrier defect coupled with a dysfunctional immune response of the innate as well as the acquired immune system to the commensal microbiota results in dysregulation of inflammatory events, and subsequent development of chronic intestinal inflammation[1]. This indicates that the regulation of intestinal epithelial barrier function, as well as of factors that regulate innate as well as adaptive immune responses, are crucial for maintaining intestinal homeostasis. The predisposition to develop IBD is partially genetically determined and genome wide association studies (GWAS) identified variations in more than 160 gene loci being associated with IBD[2] that contribute about 30% of disease aetiology. Many of the identified risk genes for IBD are critically involved in bacterial recognition, induction of antimicrobial factors, activation and modulation of innate as well as adaptive immune responses and in the maintenance of intestinal epithelial barrier function.
Among IBD risk genes, the gene locus encoding protein tyrosine phosphatase non-receptor type 2 (PTPN2) is of distinct interest, as several studies demonstrated a pivotal role for PTPN2 in the regulation of epithelial barrier properties and inflammatory responses[3-8]. Protein tyrosine phosphatases remove phosphate groups from tyrosine residues of their target proteins. As (tyrosine) phosphorylation is a fundamental mechanism of activation or deactivation of cell signalling molecules, tyrosine phosphatases regulate the functional activity of their targets. PTPN2 in particular, is capable of dephosphorylating many protein tyrosine kinase-targets such as the insulin receptor, epidermal growth factor receptor (EGFR), Src family kinases as well as several Janus kinases and signal transducer and activator of transcription (STAT) family members[9-15]. Subsequently, PTPN2 not only influences proliferation, differentiation and cell survival[16], but also partially determines how cells respond to inflammatory conditions[6,7].
In this review we will summarize the most recent knowledge about the role of PTPN2 in the pathogenesis of chronic intestinal inflammation, in particular IBD.
GENETIC VARIANTS WITHIN THE PTPN2 GENE ARE ASSOCIATED WITH IBD
The gene locus encoding PTPN2 has emerged as a site of important clinical significance due to the association of a number of SNPs in the PTPN2 locus (18p11) with chronic inflammatory conditions such as CD, UC, type 1 diabetes and celiac disease[17-19]. The rs2542151 SNP is the most widely identified and best analysed PTPN2 SNP associated with IBD. The Welcome Trust Case Control Consortium (WTCCC) study published the initial findings of a genetic association between the rs2542151 SNP in PTPN2 and CD (P = 4.6 × 106; OR = 1.3)[20]. Follow-up studies confirmed this association and also identified links between the rs2542151 SNP with CD and UC[18,21-25]. Additional SNPs in the PTPN2 gene locus have also been associated with IBD and disease outcomes. This includes the rs7234029 SNP that has a potential association with a stricturing disease phenotype in CD subjects (P = 6.62 × 10-3), and may be linked to early onset CD [P = 1.30 × 10-3; OR = 1.35 (1.13-1.62)] and UC [P = 7.53 × 10-2; OR = 1.26 (0.98-1.62)][26]. The rs1893217 SNP was originally reported to be associated with type 1 diabetes, however it has emerged as a candidate SNP in both adult [P = 1.29 × 10-14; OR = 1.25 (1.18-1.32)] and early-onset pediatric CD (P = 0.005) as well as UC [4.78 × 10-5; OR = 1.12 (1.06-1.18)], although the effect of this SNP on PTPN2 gene and protein function remains to be determined[17,18,27]. Nevertheless, a first study points towards the presence of a loss-of-function PTPN2 protein in variant carrying cells[28].
PROTEIN STRUCTURE AND SPLICING VARIANTS OF PTPN2
PTPN2 - also known as T-cell protein tyrosine phosphatase (TCPTP) as it was originally cloned from a T-cell cDNA library - is almost ubiquitously expressed in embryonic and adult tissues[29,30]. PTPN2 is a cytosolic tyrosine phosphatase that, in addition to an N-terminal phosphatase domain, harbours a nuclear localization sequence (NLS)[31]. As a consequence, PTPN2 is able to dephosphorylate and thereby inactivate its targets not only in the cytosol, but also after translocation to the nucleus. This is of interest, as among the substrates of PTPN2 are the STAT family of transcription factors[13], which are found in the nucleus after activation.
In humans two functional variants of PTPN2 exist, which originate from alternative splicing. The larger 48 kD form is restricted to the endoplasmic reticulum (ER) by a hydrophobic C-terminus that masks the NLS. The enzymatically more active 45 kD variant lacks the hydrophobic C-terminus and can transit to the nucleus via the NLS and is thus regarded as the mobile form of PTPN2[32,33]. In response to an appropriate stimulus the 45 kD form can exit the nucleus and dephosphorylate target substrates in the cytoplasm and at the plasma membrane[15].
PTPN2 controls innate host defence mechanisms
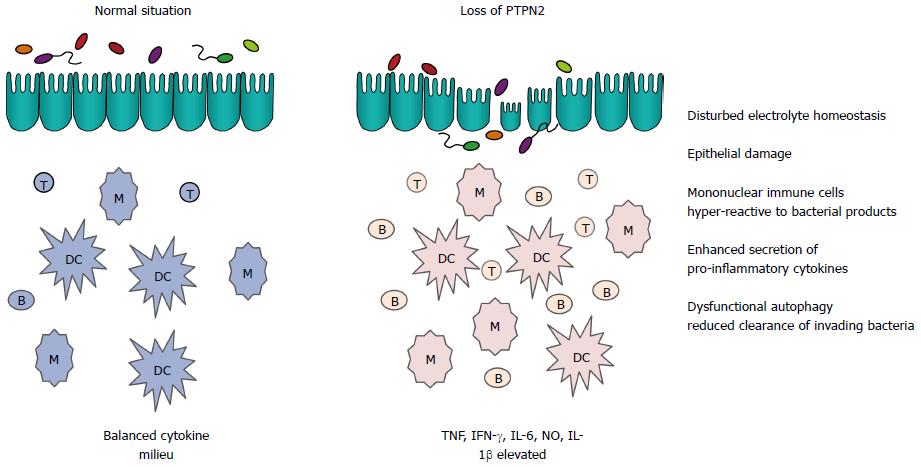
Studies with mice demonstrated that PTPN2 is a key negative regulator of cytokine signalling. Ptpn2-/- animals develop progressive systemic inflammatory disease as indicated by chronic myocarditis, gastritis, nephritis, and sialadenitis as well as elevated serum levels of interferon-gamma (IFN-γ) IL-12, tumour necrosis factor (TNF) and nitric oxide. These inflammatory mediators are mainly produced by mononuclear cells. Interestingly, Ptpn2-/- mice also exhibit increased sensitivity to the bacterial cell wall component lipopolysaccharide (LPS) in vivo, and in vitro cultured macrophages from Ptpn2-/- mice are hypersensitive to (LPS)[4]. Further, loss of PTPN2 results in pronounced IFN-γ mediated barrier disruption in epithelial cell cultures[8]. Taken together, this demonstrates a crucial role of PTPN2 in innate immune functions (Figure 1).
Figure 1 Effect of loss of protein tyrosine phosphatase non-receptor type 2 on innate immune functions in the intestine.
When protein tyrosine phosphatase non-receptor type 2 (PTPN2) is lost either by genetic deletion in the mouse or due to genetic variants in inflammatory bowel disease (IBD) patients, several aspects of innate immunity are affected, ultimately resulting in inflammation. Depicted are mechanisms that play pivotal roles in intestinal homeostasis. B: B cell; DC: Dendritic cell; IFN: Interferon; M: Macrophage; NO: Nitric oxide; T: T cell; TNF: Tumour necrosis factor; IL: Interleukin.
PTPN2 regulates inflammatory responses
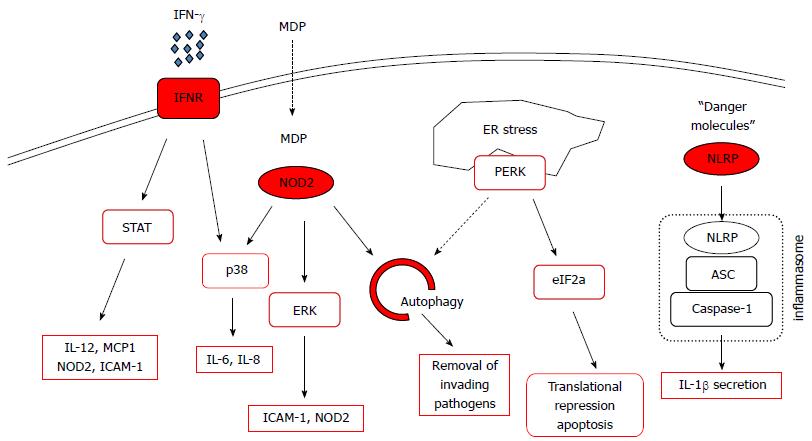
An important function of PTPN2 is to dephosphorylate STAT1 leading to its inactivation[13]. Upon ligand binding, cytokine-receptor associated kinases phosphorylate STAT molecules, which subsequently dimerize and translocate to the nucleus, where they act as transcription factors. PTPN2 counteracts the activity of receptor-associated kinases by de-phosphorylation of STAT molecules, ultimately repressing cytokine signalling[14]. One inflammatory molecule that crucially depends on STAT activation is IFN-γ. Not surprisingly, recent data have demonstrated that PTPN2 regulates IFN-γ-induced signalling and effects in cell models of inflammation. Treatment of human intestinal epithelial cells with IFN-γ increases PTPN2 mRNA and protein levels, elevates enzymatic PTPN2 activity, and causes cytoplasmic accumulation of PTPN2. These effects are mediated via the cellular energy sensor, adenosine-monophosphate activated protein kinase. In these cells, knockdown of PTPN2 resulted in increased STAT1 and STAT3 phosphorylation upon IFN-γ treatment[8]. Further, in PTPN2 deficient human THP-1 monocytic cells, IFN-γ-induced activity of the mitogen-activated protein kinase (MAPK) family member, p38, and secretion of monocyte chemo-attractant protein and interleukin (IL)-6 were enhanced[6]. Additionally, PTPN2 regulates signalling responses to the bacterial cell wall component muramyl-dipeptide (MDP), which is a NOD2 ligand in human monocytes, both in intestinal epithelial cells and monocytic cells. Loss of PTPN2 results in enhanced IFN-γ, but reduced IL-8 and TNF secretion in MDP-treated THP-1 cells. This might be due to the fact that dysfunction of PTPN2 in human monocytes causes enhanced MAPK signalling in response to MDP. Of note, PTPN2 dysfunction also resulted in enhanced cleavage of caspase-1 and increased IL-1β secretion, indicative of increased inflammasome activation in response to MDP[28]. TNF induces PTPN2 protein and mRNA levels in human intestinal epithelial cells via an NF-κB-dependent mechanism. PTPN2 in turn regulates TNF-induced ERK- and p38-MAPK activity as well as IL-6 and IL-8 secretion[7]. Further studies also demonstrated that PTPN2 controls TNF-induced IL-6 secretion in mouse embryonic fibroblasts[12], and in synovial fibroblasts from rheumatoid arthritis patients[34]. Additionally, PTPN2 has also been demonstrated to play a critical role in regulating ER stress responses in intestinal epithelial cells and monocytes, while it also controls cytokine secretion in response to ER stress triggers[35,36]. These findings strongly suggest that PTPN2 is crucial for controlling cytokine secretion from intestinal epithelial cells, fibroblasts and mononuclear cells. Figure 2 gives an overview on cellular pathways that are regulated by PTPN2.
Figure 2 Signaling pathways affected by protein tyrosine phosphatase non-receptor type 2.
Depicted are pathways that play important roles in intestinal homeostasis, factors with red margins are directly influenced by protein tyrosine phosphatase non-receptor type 2 (PTPN2). ASC: Apoptosis-associated speck containing protein; eIF2a: Eukaryotic translation initiation factor 2A; ER: Endoplasmatic reticulum; ERK: Extracellular-stress activated kinase; ICAM-1: Intercellular adhesion molecule-1; IFN: Interferon; IL: Interleukin; MCP1: Monocyte-chemoattracting protein 1; MDP: Muramyl-dipeptide; NLRP: Nod-line receptor protein; NOD2: Nucleotide oligomerization containing 2; PERK: Protein Kinase RNA-like endoplasmic reticulum kinase; STAT: Signal transducer and activator of transcription.
PTPN2 regulates autophagosome formation
Autophagy is a fundamental process for bulk degradation of cytoplasmic compartments, damaged organelles, and/or misfolded proteins. In autophagosomes, target proteins/organelles are sequestered into double-membrane-enclosed vesicles and delivered to lysosomes for final degradation[37-39]. Stress conditions, such as starvation or hypoxia, enhance autophagy, and numerous pathologies, including cancer or neurodegeneration have been linked with aberrant autophagy function[40]. Of note for intestinal homeostasis, autophagy is also critically involved in host defence against intracellular pathogens such as Listeria monocytogenes (LM) or Salmonella typhimurium[41-43]. Previous data have clearly demonstrated that the presence of genetic variations within autophagy genes results in defective bacterial handling, prolonged intracellular survival of pathogenic bacteria and an elevated inflammatory response[41-43].
Recent data demonstrated that PTPN2 not only regulates cytokine-induced activation and expression of autophagy-related molecules, but is also involved in the regulation of autophagosome formation in intestinal epithelial cells[44]. siRNA-induced knock-down of PTPN2 in intestinal epithelial cells inhibits the expression of several autophagy-associated molecules, including beclin-1, ATG5, ATG7, ATG12, ATG16L1 and IRGM in response to IFN-γ and TNF treatment. Of note, reduced protein levels of all of these autophagy markers have also been observed in intestinal tissue samples derived from patients with active CD when compared to tissue samples from non-IBD control patients[44].
On a functional level, loss of PTPN2 in human intestinal epithelial cells reduced autophagosome formation in response to TNF and IFN-γ co-treatment. PTPN2-deficient cells featured only a small number of LC3B+ vesicles and TNF+IFN-γ co-treatment caused the formation of fewer, but larger LC3B+ vacuoles that were localized close to cell borders[44]. The appearance of such abnormal, large autophagic vacuoles has been regarded as a marker of an ineffective formation of dysfunctional autophagosomes due to a defective autophagy process in these cells[45]. Similar findings were observed in MDP-treated human monocytes[28]. Interestingly, the effects of PTPN2 on autophagosome formation seem to be mediated by controlling the phosphorylation status of the EGF receptor and subsequently of PI3K, Akt and mTOR activity[44]. More recent data found a role for STAT3 in inhibition of autophagy (summarized in[46]), hence increased STAT3 activation upon loss of PTPN2 might provide an additional mechanism how loss of PTPN2 affects autophagy.
In primary colonic lamina propria fibroblasts (CLPF) isolated from CD patients, presence of a disease-associated PTPN2 variant exerts similar effects to siRNA-induced loss of PTPN2 expression[44]. In particular, CLPFs featuring the CD-associated PTPN2 variant revealed reduced basal levels of PTPN2 protein when compared to PTPN2-WT fibroblasts and the TNF+IFN-γ-induced increase in PTPN2 protein was absent. As in intestinal epithelial cells, PTPN2 dysfunction also prevented the cytokine-induced increase in the expression of autophagy markers, such as IRGM, and also resulted in diminished formation of autophagosomes in PTPN2-variant carrying CLPF[44].
Impaired autophagy has been described to result in defective handling of invading bacteria[42,43] and defective handling of luminal and/or invading bacteria might critically contribute to the onset of IBD. Studies using GFP-labelled Listeria monocytogenes have demonstrated that loss, or genetically-caused dysfunction of PTPN2, results in impaired autophagosome formation and defective clearance of invading bacteria. Collectively, these data suggest that the presence of the CD-associated PTPN2 variant within intestinal cells could critically contribute to the onset of IBD by causing a defective innate immune response to invading bacteria[44].
PTPN2 maintains intestinal barrier function
PTPN2 is expressed in both, hematopoietic as well as non-hematopoietic cells. In the healthy intestine, where highest PTPN2 expression is found in immune cells, PTPN2 is also detectable in intestinal epithelial cells[8,44]. In active lesions of CD patients, colonic PTPN2 mRNA and protein expression is increased, with expression being most prominent in the epithelium[7,8]. Consistent with this, we have shown that in intestinal epithelial cell lines the IBD-associated inflammatory cytokines IFN-γ and TNF are capable of increasing expression of PTPN2[7,8]. This suggests that these inflammatory cytokines induce expression of a negative regulator of their own signalling in an apparent negative feedback loop. While expression of PTPN2 is increased in CD, the impact of non-coding SNPs appears to manifest in a loss of enzymatic activity or efficacy[28]. Loss of PTPN2 expression has been shown to have dramatic consequences for intestinal epithelial cells and their ability to form an effective barrier. Knockdown of PTPN2 in intestinal epithelial cells resulted in a pronounced decrease in trans-epithelial resistance in response to IFN-γ coupled with a higher increase in expression of the cation-selective pore-forming molecule, claudin-2[8]. Claudin-2 expression is elevated in colonic tissues in IBD patients, especially in UC, and functionally this could contribute to symptoms of disease by permitting increased paracellular passage of sodium ions into the intestinal lumen, thus leading to intestinal fluid loss associated with IBD[47-49]. In addition, PTPN2-deficient cells also displayed increased macromolecule permeability following IFN-γ treatment as determined by increased apical-to-basolateral passage of FITC-dextran across polarized intestinal epithelial cell monolayers[8]. Due to the width of the pore size generated by claudin-2 being insufficient to permit passage of FITC-dextran (10 kD), this strongly suggests that additional mechanisms capable of modifying tight junction components responsible for regulation of macromolecule permeability are recruited by IFN-γ in cells lacking PTPN2[5]. Conclusive evidence of this has not yet been achieved: PTPN2 knockdown did not cause further decreases in expression of the tight junction proteins occludin or ZO-1 by IFN-γ. On the other hand, a possible influence of PTPN2 on re-localization of tight junction proteins has not been investigated[8]. These data suggest that PTPN2 plays an important role in protecting intestinal epithelial barrier function. A protective role for PTPN2 in intestinal barrier function has also been indicated in vivo. Ptpn2 knockout mice suffer from systemic inflammation, hematopoietic defects, increased levels of pro-inflammatory cytokines, splenomegaly and diarrhea, and die within 3-5 wk after birth[50,51]. Murine bone marrow chimeric studies indicated that the inflammation and mortality were governed by loss of PTPN2 in the non-hematopoietic compartment[4]. Studies using heterozygous (Ptpn2+/-) mice demonstrated no overt inflammatory phenotype and normal survival rates. However, Ptpn2+/- mice are more susceptible to dextran sulfate sodium (DSS)-induced colitis, suggesting that PTPN2 deficiency increases the susceptibility to agents that disrupt the epithelial barrier[52].
PTPN2 regulates electrolyte transport
Another important epithelial function that plays a critical role in intestinal homeostasis is appropriate regulation of electrolyte transport. This is essential for absorption and secretion of electrolytes and fluids as well as for the absorption of nutrients, maintenance of luminal pH and preserving the sterility of intestinal crypts[53]. In IBD, epithelial electrolyte transport is suppressed, contributing to overall fluid loss due to decreased absorptive capacity. This creates an environment conducive to increased bacterial interactions with the intestinal epithelium[54-56]. PTPN2 has been shown to play a role in regulating fluid secretion. Specifically, PTPN2 knockdown in T84 colonic epithelial cells accentuated EGF inhibition of Ca2+-stimulated chloride secretion thus promoting EGFR suppression of electrolyte secretion[57]. Thus, it is possible that PTPN2 mutations resulting in a loss of enzymatic activity could mediate elevated or prolonged EGFR phosphorylation and exacerbate overall dysregulation of intestinal fluid homeostasis.
PTPN2 CONTROLS ADAPTIVE IMMUNE FUNCTIONS
Many of the signalling cascades involved in innate immunity, are also of fundamental importance for adaptive immune cells. For example STAT molecules, which are an important target of PTPN2, are crucially involved in T and B cell differentiation and maturation[58,59]. PTPN2 protein had initially been termed T cell protein tyrosine phosphatase (TCPTP), which reflects the original cellular compartment of detection and characterization[60,61]. Subsequently a number of functions for PTPN2 in the adaptive immune system have been described. Similar to the innate immune system PTPN2 also exerts an anti-inflammatory role, both in the T and B cell compartments. Aside from anomalies in bone marrow development and changes in innate immunity[50], Ptpn2-/- mice show severe alterations in the adaptive immune system including splenomegaly, lymphadenopathy and altered T and B cell functions[50]. These data indicate an important role of PTPN2 in the adaptive immune system and in the maturation/function of B and T cells and subsequently in autoimmunity.
PTPN2 in B cell development
As alluded to above, B cell development is crucially affected by PTPN2. Ptpn2 knock-out mice develop an early bone marrow B cell deficiency which is caused by a block of the transition from pre-B cells to immature B cells[3]. The impairment of Pre-B to immature B cell transition is associated with the secretion of abnormally high amounts of IFN-γ by bone marrow stromal cells. High levels of IFN-γ in turn result in phosphorylation of STAT1 in the pre-B cell compartment[62]. As STAT1 activity impairs pre-B cell differentiation to immature B cells, and blocks IL-7 induced pre-B cell proliferation[63], loss of PTPN2 crucially influences this developmental stage. Reduced numbers of immature B cells have been associated with the survival of auto-reactive B cells, which are normally deleted at this stage due to competition for B cell survival factors. Therefore, loss of PTPN2 might result in increased levels of potentially auto-reactive B cells, however experimental evidence is still missing. In contrast to pre-B cells, IFN-γ signalling and STAT activation plays an important mitogenic and differentiation-inducing role in mature B cells. In the germinal centre, where B cells proliferate and differentiate with the help of CD4+ T cells into antibody-secreting plasma cells, high IFN-γ levels (and subsequent STAT1 phosphorylation) promote IgM and IgG secretion, while it represses the switch to IgA production[64]. Therefore it is likely, that loss of PTPN2 might also influence the terminal stage of B cell differentiation and IgA production. Of note, IgA is secreted in large amounts at mucosal surfaces and seems to play a role in bacterial handling[65].
PTPN2 in T cells
T cells express especially high levels of PTPN2[3], suggesting a pivotal role of this phosphatase in T cell development/function. Besides the above-mentioned role of PTPN2 in regulating STAT molecules, PTPN2 is also a key negative regulator of T-cell receptor (TCR) signalling: PTPN2 dephosphorylates and thereby inactivates Src family kinases. Src family kinases mediate signalling downstream of the TCR, hence PTPN2 directly influences how T cells respond to antigens[66]. By interfering with TCR signalling, PTPN2 attenuates T cell activation and proliferation and in general limits antigen-induced responses. Of note, TCR signalling strength determines the fate of activated T cells: on one hand, strong TCR signalling is involved in priming cytotoxic CD8+ T cells to pathogens and pathologic antigens, while on the other hand, low levels of TCR signalling induces peripheral tolerance to self-antigens and commensal microbes in the gut. Due to enhanced TCR signalling strength, PTPN2-deficient CD8+ T cells loose tolerance to low-affinity TCR ligands - ligands often found in the body’s own tissues. Therefore, enhanced TCR signalling strength enables T cells to react against tissues such as pancreatic β cells in an auto-reactive manner, finally resulting in the development of diabetes even in the absence of CD4+ T cells[67]. Thus, PTPN2 variants can re-direct a normally tolerogenic CD8+ T cell response into an auto-reactive and destructive response.
Correspondingly, a deficiency of PTPN2 was reported to enhance naive T cell responses to low-affinity ligands[66]. This may partially be associated with the fact that STAT3 and STAT5 are further substrates for de-phosphorylation by PTPN2. Wiede et al[66] reported that in the periphery, PTPN2 deficiency resulted in a memory phenotype of CD4+ T cells. In their mouse model the number of T cells with an effector/memory phenotype increased progressively from 4 to 12 wk of age, which was paralleled by a decrease in naive T cell numbers. This may lead to a selection of high-affinity, potentially self-reactive T cells, which represents another pathway of autoimmune disease induction by a lack of PTPN2 function. In contrast, loss of PTPN2 function did not influence the number and function of regulatory T cells (Treg) under physiological conditions in this study[67].
The signalling pathways that are regulated by PTPN2 in innate immune cells, also affect T cell fate: Up-regulation of IFN-γ, IL-12, and other inflammatory cytokines, plays an important role for T cell activation[3]. Further, IL-6-mediated STAT3 activity is important for the development of IL-17 producing T cells[68]. As STAT3 is also a known target of PTPN2, it might well be, that loss of PTPN2 might influence the development of these potentially pathogenic T helper cells.
Normal PTPN2 function seems to have an important role in T cells to prevent auto-reactivity. Of interest, naive CD8+ T cells that leave the thymus express high levels of PTPN2[69]. When PTPN2 is missing in these cells, they undergo rapid proliferation and acquire an antigen-experienced effector phenotype, especially when transferred into T- and B cell deficient hosts. This increase in lymphopenia-induced proliferation is mediated by elevated TCR-dependent responses, which are associated with the development of autoimmunity[69]. Tolerance to self- and commensal-/food-derived antigens is important for intestinal homeostasis. The function of PTPN2 in preventing excessive activation/proliferation of naïve T cells might therefore be very crucial for preventing aberrant intestinal inflammation.
PTPN2 in adaptive immunity during intestinal inflammation
As described above, loss of PTPN2 crucially affects B and T cell function. Few studies, however, have addressed the functional consequences of the resulting changes in the setting of intestinal inflammation. In a recent study using mice featuring loss of PTPN2 in CD4+ cells, we addressed how loss of PTPN2 function in T cells affects intestinal inflammation. We found that loss of PTPN2 specifically in T cells resulted in enhanced induction of Th1 and Th17 cells, but impaired induction of Tregs when colitis was induced by DSS administration. Ultimately, mice lacking PTPN2 in T cells suffered from more severe colitis[70]. Further, transfer of PTPN2 deficient naïve T cells into mice lacking B and T cells resulted in pronounced weight loss and colitis severity when compared to transfer of PTPN2 competent naïve T cells[70]. Of note, this was again attributed to enhanced emergence of Th1 and Th17 cells but reduced induction of Treg cells.
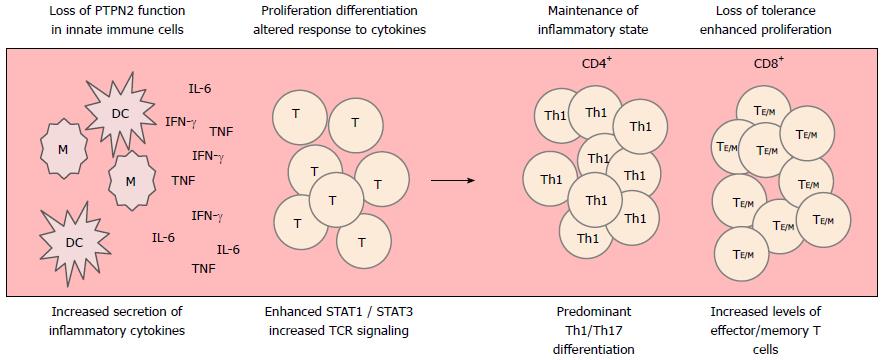
In line with the findings in mice lacking PTPN2 in T cells, CD patients featuring a loss-of-function PTPN2 variant exhibit enhanced Th1 and Th17 cell markers in serum and intestinal tissue samples, when compared with PTPN2 wild-type patients, while reduced Treg markers are found in these patients[70]. Our findings in mice lacking PTPN2 in CD4+ cells and the observation in CD patients with loss-of-function PTPN2 variant contrasts the situation under physiological conditions described by Wiede et al[66], where Treg function/numbers are not changed upon loss of PTPN2. This indicates that under inflamed conditions, PTPN2 has slightly different functions for Th cell differentiation, and PTPN2 activity seems to be especially important for induction of regulatory mechanisms in the inflamed intestine. The observed alterations in T cell differentiation finally resulted in increased susceptibility to intestinal inflammation supporting the role for PTPN2 in IBD pathogenesis. Additionally, mice with loss of PTPN2 in CD4+ cells displayed intestinal dysbiosis. Detailed analysis of the microbiome revealed that the resulting dysbiosis is comparable with that observed in CD patients. We also detected inflammatory infiltrates in liver, kidney, and skin and elevated autoantibody levels in mice with dysfunctional PTPN2. These observations (summarized in Figure 3) strongly indicate a systemic loss of tolerance in PTPN2-deficient animals and suggest how PTPN2 might contribute to the onset of auto-inflammatory diseases, as well as intestinal inflammation[70].
Figure 3 Loss of protein tyrosine phosphatase non-receptor type 2 affects several aspects of T cell development.
Loss of protein tyrosine phosphatase non-receptor type 2 (PTPN2) in innate immune cells results in enhanced secretion of pro-inflammatory cytokines, including IFN-γ, IL-6 and IL-1β. In CD4+ T cells, these cytokines are involved in driving Th1 and Th17 development. Loss of PTPN2 in CD4+ T cells further potentiates the IFN-γ/IL-6-induced activation of STAT1/STAT3, what further strengthens the development of Th1/Th17 cells. Further, loss of PTPN2 results in enhanced TCR signaling strength what drives aberrant activation and proliferation of naïve T cells and escape of auto-reactive T cells from negative selection. Ultimately, this leads to the generation of increased levels of effector and memory T cells. DC: Dendritic cell; IFN: Interferon; M: Macrophage; TNF: Tumour necrosis factor; IL: Interleukin; STAT: Signal transducer and activator of transcription; TCR: T-cell receptor.
On the side of B cells, no functional studies have been addressed to investigate the effect of loss of PTPN2 on intestinal inflammation. However, as loss of PTPN2 in B cells might affect terminal B cell differentiation, it would be of great interest to determine whether loss of PTPN2 specifically in B cells might affect colitis severity. As STAT1 is involved in suppressing the switch from IgG to IgA production from plasma cells, and PTPN2 controls STAT1 activation, it is very likely that B cell specific loss of PTPN2 affects IgA production. IgA is crucially involved in controlling invasive microbes in the intestine, hence PTPN2-mediated changes in IgA production might strongly affect colitis severity, but this has not yet been addressed.
Despite the important role of PTPN2 and the finding of relevant variants in autoimmune diseases and CD and UC, a detailed analysis of modifications of T and B cell functions by those variants on intestinal inflammation has not been performed yet. Animal models may not be optimally suited as there are complex interactions between the immune cells and also between adaptive and innate immune mechanisms.
CONCLUSION
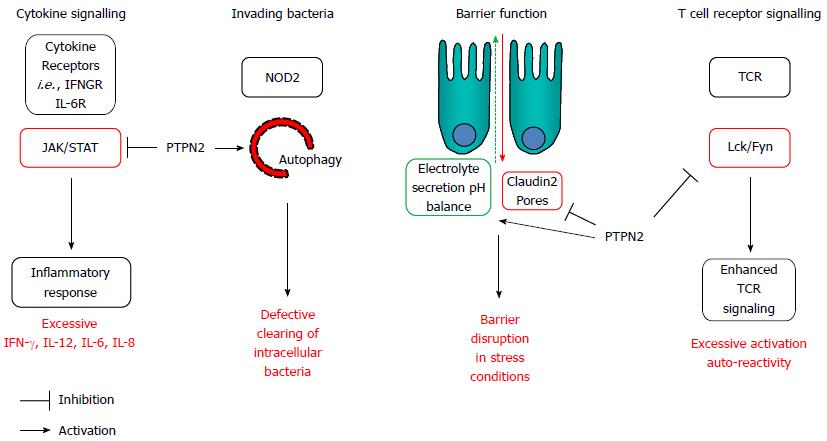
Loss of PTPN2 expression/function has consequences for several aspects of innate and adaptive immunity including epithelial barrier function, autophagy, and T cell development/activation (summarized in Figure 4).
Figure 4 Protein tyrosine phosphatase non-receptor type 2 influences pathways involved in inflammatory bowel disease pathogenesis.
A: Protein tyrosine phosphatase non-receptor type 2 (PTPN2) dephosphorylates JAK1/2 and STAT1/3/5 to control inflammatory cytokine signaling; B: PTPN2 promotes autophagy induction in response to invading bacteria; C: In epithelial cells PTPN2 protects against inflammation-induced barrier defects; D: In T cells, PTPN2 counteracts TCR associated kinases and thereby reduces the reaction towards cognate antigens. IL: Interleukin; JAK: Janus activated tyrosine kinase; Lck: Lymphocyte tyrosine kinase; NOD2: Nucleotide oligomerization domain containing protein 2; STAT: Signal transducer and activator of transcription; TCR: T cell antigen receptor.
Given that PTPN2 represents a point of convergence for multiple aspects of intestinal homeostasis it may therefore play a key role in multiple IBD-associated physiological events and thus, like other IBD candidate genes, make a greater cumulative contribution to IBD pathogenesis than suggested by the genetic prevalence of PTPN2 SNPs in sampled populations. On one hand, clinically relevant loss-of-function mutations in the PTPN2 gene may contribute, at least in part, to the development of IBD and other chronic inflammatory intestinal diseases via a compromised epithelial barrier. Indeed, given that increased intestinal permeability is a feature of IBD, type 1 diabetes and celiac disease, this may be one avenue to explore commonality in the aetiology of these conditions arising from PTPN2 mutations. On the other hand, PTPN2 also crucially affects immune functions. This involves autophagy, a pathway that appears to play an important role in IBD pathogenesis. As autophagy affects bacterial handling in both, intestinal epithelial cells and innate immune cells such as monocytes, macrophages and dendritic cells, this might additionally contribute to the association of PTPN2 with IBD. Loss of functional autophagy results in increased bacterial burden and thus prolonged activation of the immune system. Loss of autophagosome formation due to PTPN2 dysfunction might therefore crucially affect the ability of the innate immune system to clear penetrating bacteria. As loss of PTPN2 additionally increases the cellular response to inflammatory cytokines, this reduced ability to react towards invading bacteria, leads to the generation of a highly pro-inflammatory cytokine milieu. Coupled with enhanced TCR signalling strength, and the role of PTPN2 in preventing proliferation of auto-reactive T cells, this might crucially contributes to loss of tolerance against bacterial antigens in the intestine. Taken together, the involvement of PTPN2 in such diverse aspects of (intestinal) immune homeostasis can explain the association of PTPN2 variants with IBD as well as several other auto-inflammatory disorders.
In this review, we summarized the many aspects how PTPN2 affects intestinal homeostasis. By controlling important pro-inflammatory signalling cascades, such as the IFN-γ-STAT1 and the IL-6-STAT3 pathways, as well as MAPK induction, PTPN2 prevents exacerbated inflammatory reactions. Inflammatory cytokines, such as TNF and IFN-γ, induce PTPN2 expression, what results in their own negative regulation; PTPN2 is therefore regarded as a classical negative feedback mediator of inflammatory signalling. PTPN2 further controls the inflammatory response towards bacterial products including LPS and the NOD2-ligand MDP. Another important aspect of PTPN2 function is the promotion of autophagy, which especially in intestinal epithelial cells and macrophages is importantly involved in removal of invading bacteria. In epithelial cells, PTPN2 has the additional important function to prevent inflammation-induced epithelial barrier defects, and it is crucially involved in electrolyte balance.
Besides this role in innate host defence mechanisms, PTPN2 also exerts crucial roles in adaptive immunity, where it is involved in maturation of naïve B cells and in T cell proliferation and differentiation. PTPN2 controls TCR signalling strength and thus prevents aberrant proliferation/activation. In CD8+ T cells, loss of PTPN2 results in increased proliferation and emergence of auto-reactive cells. In CD4+ cells on the other hand, PTPN2 is involved in controlling CD4+ T cell differentiation into the Th1/Th17 subsets.
These different aspects how PTPN2 is involved in regulating innate immune functions and host defence mechanisms, as well as adaptive immune reactions, explain the association of variants in PTPN2 with IBD, as well as its role in several other inflammatory disorders.