Published online Jan 21, 2016. doi: 10.3748/wjg.v22.i3.1008
Peer-review started: July 4, 2015
First decision: July 20, 2015
Revised: August 27, 2015
Accepted: October 17, 2015
Article in press: October 20, 2015
Published online: January 21, 2016
Processing time: 198 Days and 13 Hours
Crohn’s disease (CD) is a chronic remittent idiopathic disease. Although the early phase of the disease is commonly characterized by inflammation-driven symptoms, such as diarrhea, the frequency of fibrostenotic complications in patients with CD increases over the long-term course of the disease. This review presents the current diagnostic options for assessing CD-associated strictures. In addition to the endoscopic evaluation of CD strictures, this review summarizes the currently available imaging modalities, including ultrasound and cross-sectional imaging techniques. In addition to stricture detection, differentiating between the primarily inflammatory strictures and the predominantly fibrotic ones is essential for selecting the appropriate treatment strategy (anti-inflammatory medical treatment vs endoscopical or surgical approaches). Therefore, recent imaging advances, such as contrast-enhanced ultrasound and ultrasound elastography, contribute to the development of non-invasive non-radiating imaging of CD-associated strictures. Finally, novel magnetic resonance imaging techniques, such as diffusion-weighted, motility and magnetization transfer imaging, as well as 18F-FDG PET/CT, molecular imaging approaches and biomarkers, are critically reviewed with regard to their potential role in assessing stricturing CD.
Core tip: Stricturing Crohn’s disease (CD) significantly decreases patients’ quality of life and often represents a challenging treatment situation that may lead to hospitalization and surgery. Differentiating between the predominantly inflammatory strictures and the primarily fibrotic strictures is essential for selecting the appropriate treatment approach (anti-inflammatory medical treatment vs endoscopic or surgery-based interventions). This review summarizes the available diagnostic procedures and emphasizes the use of endoscopy and imaging modalities, including ultrasound and cross-sectional imaging. Finally, promising recent imaging advances that might enable a more specific characterization of CD-associated strictures in the future are presented.
- Citation: Bettenworth D, Nowacki TM, Cordes F, Buerke B, Lenze F. Assessment of stricturing Crohn's disease: Current clinical practice and future avenues. World J Gastroenterol 2016; 22(3): 1008-1016
- URL: https://www.wjgnet.com/1007-9327/full/v22/i3/1008.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i3.1008
Intestinal strictures represent a frequent complication of Crohn’s disease (CD)[1]. More specifically, approximately 40% of CD patients with ileal disease develop clinically apparent strictures[2]. In patients with CD, intestinal fibrosis can be present at the time of the diagnosis; however, more frequently, a stricturing phenotype and intestinal stenosis develop during the long-term course of the disease[3,4]. Two decades ago, there was hope that the advent of novel immunosuppressive agents, such as anti-TNF antibodies, might reduce the frequency of fibrotic complications in patients with CD. However, recent epidemiological data have revealed that despite the establishment of early immunosuppressive therapy in CD patients at risk for a disabling disease course, the frequency of fibrostenosing complications did not significantly decrease[5].
In recent years, the pathogenetic understanding of intestinal fibrosis has significantly advanced, and common core features of fibrosis in different organs have been identified[6]. Nevertheless, to date, no anti-fibrotic agents for treating intestinal fibrosis in CD have been evaluated in clinical trials[7]. The clinical relevance of intestinal strictures for patients with CD is emphasized by the fact that in population-based studies, approximately 60% of patients require surgery within 20 years after diagnosis[8]. The clinical challenge of managing stricturing CD is further aggravated by the high rate of recurrent stenosis at the surgical site, which larger studies have determined to be approximately 50%[9-11]. Therefore, a significant number of patients must undergo multiple surgeries associated with the risk of developing short bowel syndrome.
Strictures may be subdivided into fibrotic, inflammatory and mixed types[12]. Accordingly, inflammatory strictures can benefit from an anti-inflammatory treatment approach, causing a reduction in the inflammation-mediated edema[13]. In contrast, predominantly fibrotic intestinal strictures do not benefit from anti-inflammatory agents and must be treated using either endoscopic approaches, such as endoscopic balloon dilation, or surgical interventions, e.g., strictureplasty or intestinal resection[14-16].
Altogether, the high frequency of intestinal strictures in patients with CD and the opposed treatment approaches indicate the necessity of accurately diagnosing stricturing CD and differentiating predominantly inflammatory strictures from primarily fibrotic strictures. This review presents various diagnostic approaches for stricturing CD, discusses the current limitations and describes novel attempts to improve the assessment of CD-associated strictures.
In general, CD strictures should be classified into (predominantly) inflammatory or fibromatous strictures. In the case of a fibrostenotic stricture, medical treatment would be ineffective, and therefore, surgical resection or endoscopic dilation therapy is a reasonable treatment option[17]. The term stricture is not defined uniformly with respect to the presence of luminal narrowing and prestenotic dilation[14,18-20]. The European Crohn’s and Colitis Organization (ECCO) guidelines on endoscopy in inflammatory bowel disease have defined a CD stricture as a narrowing of the intestinal lumen[21]. From the clinical perspective, strictures should be subdivided by the number of strictures (single vs multiple) and whether they are endoscopically passable or non-passable strictures (using a standard adult endoscope) but traversable upon dilation. Strictures in the anastomotic region can occur de novo or after surgery and are primarily localized at the ileocecum. Therefore, CD-associated strictures are commonly accessible during colonoscopy[22]. In contrast, single- or double-balloon enteroscopy is required to reach small bowel strictures[23]. In the case of a passable stricture, the length can be easily assessed, and intestinal pathologies that are proximal to the stricture, e.g., additional strictures, should be evaluated. Before endoscopic dilation therapy, the length and angulation of the stricture should be considered, and any fistulas or ulcers located within the stricture should be avoided. The endoscopic criteria predictive of clinical efficacy are as follows: a stricture length of < 4 cm and the absence of ulcers within the stricture[15,21,24]. In addition to the macroscopic evaluation, endoscopy facilitates obtaining biopsy samples to rule out malignancy[21,25-28]. In the case of an impassable stricture, the stricture length can be assessed by applying iodine contrast agents, which are administered into the stricture ostium during endoscopy. Using fluoroscopy, the length and configuration of the stricture can then be determined[29]. Paine and Shen proposed a comprehensive classification system for strictures that includes seven criteria (Table 1)[30].
| Criteria | Classification |
| Etiology | Primary vs secondary (anastomotic); benign vs malignant |
| Number | Single vs multiple |
| Degree | High grade vs low grade |
| Shape | Web like vs spindle shaped; circumferential vs asymmetric |
| Length | Short vs long |
| Location | Esophagus, pylorus, small bowel, ileocecal valve, anastomosis, colon, rectum, anus |
| Associated conditions | Fibrosis, edema, proximal dilation, ulceration, fistula with or without abscess, angulated, prior strictureplasty |
To assess the inflammatory[31] activity in luminal CD, several endoscopic scores are available. The CDEIS (CD endoscopic index of severity) and the SES-CD (simple endoscopic score for CD)[21] are commonly used in clinical trials, are validated and have prognostic value[31,32]. However, they are not routinely used in clinical practice. To evaluate the postoperative recurrence of CD, the Rutgeerts score is the most commonly used score for assessing endoscopic disease activity after ileocecal resection[9,33]. However, the Rutgeerts score has not been validated.
None of these scores has been validated with regard to differentiating the strictures into inflammatory and fibromatous types. In fact, to the best of our knowledge, there is no validated endoscopy CD stricture score for this purpose. Our group defined a combined endoscopic and histological score to differentiate CD strictures into inflammatory, mixed and fibromatous types[12]. Thus far, our score has not been validated by other groups.
Because the available endoscopic approaches are limited to endoluminal findings, cross-sectional imaging can be used as a complementary technique, contributing significant information regarding the bowel wall and the involvement of adjacent tissues, especially when endoscopic modalities are impractical or contraindicated (e.g., capsule endoscopy in the suspected strictures).
Ultrasonography (US) has gained wide acceptance in the diagnostic work-up of patients with CD[34], especially because recent works have shown detection rates for strictures similar to those of magnetic resonance (MR) enterography or fluorine-18-2-fluoro-2-deoxy-D-glucose positron emission tomography and computed tomography (FDG-PET/CT)[12,35]. The major advantage of ultrasound is its ubiquitous availability and non-invasive nature, which simultaneously allow direct interaction with the patient during the examination and the ability to focus on areas of abdominal discomfort. Despite these advantages, ultrasound remains underutilized in many parts of the world[36]. In experienced hands, intestinal ultrasound is a cost-effective, risk-free procedure for monitoring disease activity and assessing complications, especially when evaluating the terminal ileum and the colon, although the proximal ileum, jejunum, transverse colon and the rectum may be difficult to assess due to gas-filled bowel or obesity[37]. Ultrasound examinations primarily focus on detecting bowel wall thickening and increased vascularization on Doppler US, as well as strictures, loss of stratification, haustra coli and conglomeration of intestinal loops and extramural lesions. A systematic review by Panés et al[38] found 85% sensitivity and 91% specificity rates for US in assessing disease activity in patients with CD.
However, despite the introduction of novel ultrasound techniques, such as contrast-enhanced US or real-time elastography, the detection of intramural fibrosis in CD remains challenging[39,40].
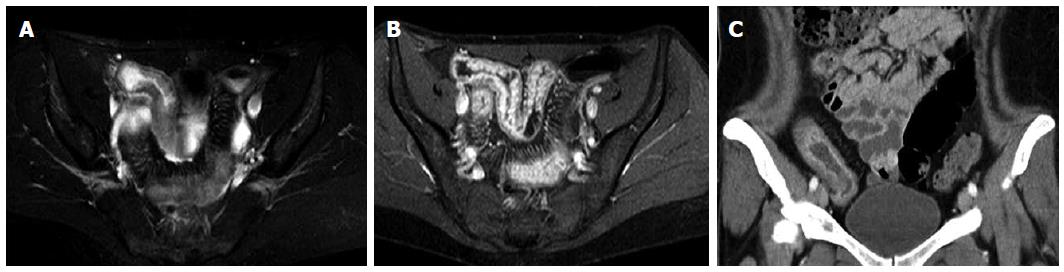
Although computed tomography (enterography) (CT-E) scans are readily available and enable the detection of strictures with or without proximal dilation, as well as signs of penetrating disease, such as fistula and extraluminal abscess formation (Figure 1), this technique inevitably leads to significant patient radiation exposure. Recent data have demonstrated that a significant number of patients with inflammatory bowel disease (IBD) are exposed to a high cumulative effective dose[41]. High radiation exposure and age at the time of exposure both increase the risk of radiation-induced cancer[37,42]. Therefore, it is recommended to monitor the use of CT, particularly in young patients, especially when alternative modalities with acceptable accuracy are available[37,43,44]. Because CT-E has been shown to be equally accurate in depicting active inflammation as MR enterography[45], CT scanning should be restricted to emergency evaluations of patients with new abdominal complaints or a change in their pre-existing condition, whereas in all other settings, radiation-free cross-sectional exams, such as MRI or ultrasound, should be used.
MRI has played a major role in the diagnostic evaluation of CD. It is a radiation-free method with different acquisition protocols, such as unenhanced T2-weighted and gadolinium-enhanced T1-weighted sequences, as well as protocols requiring the intravenous application of contrast medium or the administration of enteric contrast agents (either orally, i.e., MR enterography, or through a nasojejunal tube, i.e., MR enteroclysis), which allow for a quantitative and qualitative diagnostic evaluation of disease activity and severity (Figure 1)[46,47]. In a recently published observational study correlating MRI findings based on enhancement pattern with the pathological analysis of surgically resected intestinal lesions, Rimola and colleagues found that MRI was highly sensitive and specific for detecting severe fibrosis in CD lesions[48]. Various scoring systems have been proposed to improve the interobserver reproducibility of imaging results, and MRI sequences have been used in treatment monitoring by various groups[49-51]. Gadolinum enhancement patterns facilitated detecting the inflammatory activity in patients with CD, but they were unable to exclude the underlying fibrosis[52]. Because inflammation and fibrosis are closely and positively related, the distinction between them and the exclusion of one or the other remains challenging because both conditions may frequently coexist[45,53].
Well-established risk factors for the development of a complicated course of CD include “young age at diagnosis,”“need for steroids at first presentation,”“perianal disease manifestation,”“elevated CRP levels”[54] and “deep colonic ulcers”[54,55]. However, these factors are not exclusively associated with intestinal strictures, although they are generally linked to disabling courses of the disease. The genetic markers, which are stable over time, appear to be promising for predicting fibrotic complications[56]. For example, the nucleotide-binding oligomerization domain-containing 2 (NOD2) gene was evaluated to predict intestinal fibrosis in patients with CD[57]. The IBDchip project, a large multicenter approach, recently assessed more than 70 single nucleotide polymorphisms in 1528 patients with CD and identified NOD2 to be the strongest predictor for a stricturing phenotype[58]. Additionally, JAK2 and ATG16L1 were found to be strongly associated with intestinal stenosis[58].
In addition to genes, antibodies directed against microbial peptides have been proposed to be biomarker candidates for stricturing CD. For example, we found the association between the presence of anti-Saccharomyces cerevisiae antibodies and the stricturing disease course in cohorts of pediatric and adult patients with CD[59-61]. Nevertheless, the routine use of antimicrobial antibody measurements cannot currently be recommended for the daily clinical management of patients with IBD[62]. Furthermore, no extracellular matrix proteins, including collagens and derived peptides, have been found to be specifically predictive of stricturing CD[63,64]. However, it was found that NOD2 exhibited insufficient specificity for the fibrostenotic phenotype in CD and that NOD2 was also linked to other complications[57]. Similarly, the combination of biomarkers was tested to identify fibrotic complications in CD but was also found to possess low specificity[56,65].
Nevertheless, because biomarkers, such as tissue inhibitor of metalloproteinase-1, connective tissue growth factor and others, were found to be predictive of fibrotic alterations in organs other than the gut[66,67], future studies are warranted to identify gut-specific biomarkers of fibrosis.
Non-invasive imaging techniques in patients with CD include MRI-, CT- and US-based techniques. In particular, CTE and MRE have improved the diagnosis of CD. However, these techniques are hampered by the fact that the quantification of inflammation or fibrosis in detected strictures is weak. Consequently, alternative MRI-, CT- and US-based approaches have been investigated to improve the diagnosis of structuring CD.
Contrast-enhanced ultrasound (CEUS) is a non-radiating ultrasound technique for identifying and characterizing strictures. In 2002, a small study described the ability of CEUS to differentiate hyper- from hypovascularized intestinal obstructions, thereby facilitating the identification of cicatricial and inflammatory stenosis[68]. Several recent studies have characterized CEUS[69,70] and demonstrated its potential for distinguishing inflammatory from fibrostenotic lesions in patients with CD[71,72]. A study by Ripollés et al[71] compared the efficacy of preoperative CEUS to characterize intestinal inflammation with postoperative histopathological analysis of the gut segments in patients with CD. CEUS correctly differentiated between the inflammatory and fibrostenotic alterations in 23 of 28 intestinal segments, showing a strong correlation between the sonographic findings and the histological evaluation of inflammation and fibrosis. As described by Kratzer et al[68], there was a significantly negative association between color Doppler vascularization grade and the pathologic fibrostenotic score.
Transabdominal ultrasound elasticity imaging (UEI) investigates the elastic properties of tissue by measuring the strain, which is the extent of deformation of a material upon a given applied force[73]. UEI is an established procedure for investigating liver diseases because the lower strain indicates fibrotic or steatotic remodeling[74]. UEI has also been established to assess other diseases, including prostate cancer[75] and the rejection of renal transplants[76]. Recently, UEI has been investigated in CD to identify intestinal fibrotic tissue. In a CD rat model, ultrasound elastography accurately acutely differentiated inflamed from fibrotic colonic segments[77]. In a second study, the same authors showed that in ex vivo human intestinal specimens, UEI helped discriminate low-grade from high-grade bowel fibrosis in inflammatory bowel disease[78]. Baumgart et al[39] first investigated ultrasound-based real-time elastography (RTE) in patients with stricturing CD. This study demonstrated a significant higher strain measured by RTE in fibrotic than with unaffected intestinal segments. Interestingly, RTE was not associated with the conventional US-based Limberg score, which describes the grade of intestinal wall vascularity in CD[79,80]. This observation might indicate that RTE can evaluate the mechanical properties of the gut in terms of various bowel characteristics, thereby indicating the degree of fibrosis. Nevertheless, there are limitations to this procedure. For example, obesity in patients with IBD might impair the applicability of UEI in these patients by causing inadequate penetration of the high-frequency transducers[79]. However, to date, no clinical trial has been conducted to investigate the diagnostic potential of UEI for stricture differentiation.
Regarding current US techniques, both UEI and CEUS represent promising, non-invasive, non-radiating imaging tools with the potential to improve the diagnostic accuracy of the differentiation of CD strictures. Altogether, the degree of vascularization and the strain appear to be helpful in distinguishing between inflammatory and fibrotic lesions in patients with CD. Although cross-sectional imaging techniques, such as MRI and CT, remain the most accurate noninvasive imaging method for evaluating disease activity in small bowel CD compared to standard US techniques[72], a combination of both US imaging techniques could improve the diagnosis and characterization of stenotic lesions, although such a technique has not yet been investigated.
MRI has become a standard imaging technique in patients with CD, especially when many follow-up examinations are required, and MRI-based treatment monitoring in the evaluation of transmural healing is a promising new alternative approach to endoscopically determined mucosal healing. The hallmark of CD is transmural inflammation, and the persistence of mural inflammation, even in cases of mucosal healing, has been reported[81]. However, the exact distinction between the inflammatory and fibrotic patterns is difficult to determine. Dynamic contrast enhanced (DCE)-MRI quantifies the perfusion by measuring the signal increase in the intestinal wall after the administration of gadolinium[82,83]. Recently, other imaging techniques that provide contrast based on endogenous tissue characteristics have been proposed, especially because achieving imaging contrast by intravenously administered contrast agents, such as gadolinium chelates, increases the cost of the examination and is associated with the risk of nephrogenic systemic fibrosis (NSF) in patients with substantially impaired renal function. Among these techniques are diffusion-weighted, motility and magnetization transfer imaging, which have been employed to enhance the assessment of disease activity and severity in CD[84]. Diffusion weighted imaging (DWI)-MRI provides information regarding tissue perfusion, vascular leakage and water diffusion by quantifying changes in water mobility[82,85]. In a study by Tielbeek et al[82], DWI-MRI and DCE-MRI showed good correlations regarding inflammation and fibrosis to the histopathological scores of surgical CD specimens. Dillmann and colleagues successfully applied magnetization transfer MRI in a rodent model of colonic inflammation and fibrosis to detect bowel wall fibrosis. This method, which generates contrast based on the interaction between the protons of free water and those of fixed macromolecules in the tissue, such as collagen, in addition to T2-weighted sequences, facilitates sufficient detection of bowel wall fibrosis in the setting of superimposed inflammation[86]. In this study, MT-MRI and T2-weighted signal intensity ratios were determined and compared in two groups of rats with either experimentally induced acute colonic inflammation or colonic inflammation and fibrosis. The highly significant results regarding the detection of fibrosis suggest that this approach has great promise. However, the data thus far are limited to experimental studies, and additional studies are required to optimize the detection rates for fibrotic strictures and elucidate the clinical utility. Presumably, no single technique will be sufficient for this distinction; however, a combination of different imaging modalities, together with biochemical markers and experienced clinical judgment, could be useful.
18F-FDG PET/CT represents a modern diagnostic technique in CD and has been investigated in several studies that reported high sensitivity (54%-94%) and specificity rates (55%-81%) for the detection of active CD[87]. According to Louis et al[88], severe endoscopic lesions, such as strictures and deep ulcerations, were detected with a sensitivity of up to 100% using 18F-FDG PET/CT, whereas mild inflammation with superficial and erosive lesions was difficult to determine and had a detection rate of only 7%. These findings were corroborated by a study from our group, which reported high detection rates of advanced inflammatory lesions in patients with colonic CD[89]. Although 18F-FDG PET/CT imaging seems to be able to detect severe colonic inflammation in patients with CD, the discrimination between predominantly inflammatory, fibrotic lesions and muscle hypertrophy in the bowel wall is weak[12,87]. Jacene et al[87] examined the FDG uptake in CD patients with obstructive symptoms and compared the PET/CT findings with the postoperative histopathological analyses of these lesions. They found that FDG accumulates not only in acute inflammatory lesions but also in fibrotic strictures and muscle hypertrophy, indicating the inability of quantitative PET interpretation to further characterize obstructive lesions. Nevertheless, the same study revealed that an additional semiquantitative analysis of the FDG uptake, using the maximum lean standardized uptake value [SUL (max)], allowed the identification patients with predominantly active inflammation. Similarly, a prospective study from our group comparing the diagnostic accuracy of MRI, FDG-PET/CT and US did not identify a single, superior imaging technique for stricture detection and differentiation in 30 CD patients with a total of 37 CD-associated strictures[12]. Notably, in our study, a combined diagnostic approach using FDG PET/CT or MRE and ultrasound resulted in a 100% detection rate of symptomatic strictures requiring interventions[12]. However, additional studies are warranted to investigate 18F-FDG PET/CT and the ability of semiquantitative FDG uptake analyses to distinguish between inflammatory and non-inflammatory obstructive lesions.
New imaging tools focusing on molecular imaging have recently emerged. Atreya et al[90] invented a fluorescent antibody for the colonic targeting of membrane-bound anti-tumor necrosis factor (mTNF) in patients with CD. They found that individuals with a high amount of mTNF+ intestinal cells detected by confocal laser endomicroscopy showed significantly increased response rates to anti-TNF therapy at week 12 compared to patients with low amounts of mTNF+ intestinal cells. Although this study demonstrated that molecular imaging using fluorescent antibodies showed promise as a diagnostic tool, the use of endomicroscopic imaging has not been broadly established. This study assesses the ability to endoscopically detect mucosal targets to identify the markers of fibrotic tissues, such as collagen, connective tissue growth factor, or transforming growth factor beta.
The current guidelines do not provide a standardized approach for evaluating stricturing CD and readily differentiating fibrosis from inflammation in intestinal strictures. In clinical practice, strictures are evaluated using endoscopy combined with imaging techniques. Transabdominal US and MRI represent radiation-free procedures and should, therefore, be preferred for screening examinations. In experienced hands, transabdominal US may be the first imaging procedure used to evaluate patients with CD-associated strictures because of its ubiquitous availability. Additionally, especially in cases of non-conclusive findings or suspected complications (e.g., fistula or abscess), MR-enteroclysis can be performed. Nevertheless, the discrimination of inflammatory and fibromatous strictures remains the most challenging step in the diagnosis of stricturing CD. Because new US-based techniques, such as CEUS and UEI, have shown promise for distinguishing inflammatory from non-inflammatory stenosis because of their advantageous rapid and non-invasive, non-radiating performance, a combination of US-based techniques with established CT-or MR-based techniques could facilitate characterizing stricturing CD and improving the subsequent therapy.
P- Reviewer: Shehata MMM S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, Gendre JP. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis. 2002;8:244-250. [PubMed] |
| 2. | Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1561] [Article Influence: 111.5] [Reference Citation Analysis (1)] |
| 3. | Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El Yafi FA, Belaiche J. Behaviour of Crohn’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777-782. [PubMed] |
| 4. | Solberg IC, Vatn MH, Høie O, Stray N, Sauar J, Jahnsen J, Moum B, Lygren I; IBSEN Study Group. Clinical course in Crohn’s disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol. 2007;5:1430-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 526] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 5. | Cosnes J, Nion-Larmurier I, Beaugerie L, Afchain P, Tiret E, Gendre JP. Impact of the increasing use of immunosuppressants in Crohn’s disease on the need for intestinal surgery. Gut. 2005;54:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 490] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 6. | Friedman SL, Sheppard D, Duffield JS, Violette S. Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med. 2013;5:167sr1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 480] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 7. | Bettenworth D, Rieder F. Medical therapy of stricturing Crohn’s disease: what the gut can learn from other organs - a systematic review. Fibrogenesis Tissue Repair. 2014;7:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Peyrin-Biroulet L, Harmsen WS, Tremaine WJ, Zinsmeister AR, Sandborn WJ, Loftus EV. Surgery in a population-based cohort of Crohn’s disease from Olmsted County, Minnesota (1970-2004). Am J Gastroenterol. 2012;107:1693-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 237] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 9. | Rutgeerts P, Geboes K, Vantrappen G, Kerremans R, Coenegrachts JL, Coremans G. Natural history of recurrent Crohn’s disease at the ileocolonic anastomosis after curative surgery. Gut. 1984;25:665-672. [PubMed] |
| 10. | Shivananda S, Hordijk ML, Pena AS, Mayberry JF. Crohn’s disease: risk of recurrence and reoperation in a defined population. Gut. 1989;30:990-995. [PubMed] |
| 11. | Yamamoto T, Watanabe T. Surgery for luminal Crohn’s disease. World J Gastroenterol. 2014;20:78-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Lenze F, Wessling J, Bremer J, Ullerich H, Spieker T, Weckesser M, Gonschorrek S, Kannengiesser K, Rijcken E, Heidemann J. Detection and differentiation of inflammatory versus fibromatous Crohn’s disease strictures: prospective comparison of 18F-FDG-PET/CT, MR-enteroclysis, and transabdominal ultrasound versus endoscopic/histologic evaluation. Inflamm Bowel Dis. 2012;18:2252-2260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Schoepfer AM, Safroneeva E, Vavricka SR, Peyrin-Biroulet L, Mottet C. Treatment of fibrostenotic and fistulizing Crohn’s disease. Digestion. 2012;86 Suppl 1:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Rieder F, Zimmermann EM, Remzi FH, Sandborn WJ. Crohn‘s disease complicated by strictures: a systematic review. Gut. 2013;62:1072-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 388] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 15. | Hassan C, Zullo A, De Francesco V, Ierardi E, Giustini M, Pitidis A, Taggi F, Winn S, Morini S. Systematic review: Endoscopic dilatation in Crohn’s disease. Aliment Pharmacol Ther. 2007;26:1457-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 197] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 16. | Thienpont C, D’Hoore A, Vermeire S, Demedts I, Bisschops R, Coremans G, Rutgeerts P, Van Assche G. Long-term outcome of endoscopic dilatation in patients with Crohn’s disease is not affected by disease activity or medical therapy. Gut. 2010;59:320-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 17. | Modha K, Navaneethan U. Advanced therapeutic endoscopist and inflammatory bowel disease: dawn of a new role. World J Gastroenterol. 2014;20:3485-3494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Gasche C, Moser G, Turetschek K, Schober E, Moeschl P, Oberhuber G. Transabdominal bowel sonography for the detection of intestinal complications in Crohn’s disease. Gut. 1999;44:112-117. [PubMed] |
| 19. | Fiorino G, Bonifacio C, Peyrin-Biroulet L, Minuti F, Repici A, Spinelli A, Fries W, Balzarini L, Montorsi M, Malesci A. Prospective comparison of computed tomography enterography and magnetic resonance enterography for assessment of disease activity and complications in ileocolonic Crohn’s disease. Inflamm Bowel Dis. 2011;17:1073-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 175] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 20. | Voderholzer WA, Beinhoelzl J, Rogalla P, Murrer S, Schachschal G, Lochs H, Ortner MA. Small bowel involvement in Crohn’s disease: a prospective comparison of wireless capsule endoscopy and computed tomography enteroclysis. Gut. 2005;54:369-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 244] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 21. | Annese V, Daperno M, Rutter MD, Amiot A, Bossuyt P, East J, Ferrante M, Götz M, Katsanos KH, Kießlich R. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013;7:982-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 584] [Article Influence: 48.7] [Reference Citation Analysis (1)] |
| 22. | Gasche C, Scholmerich J, Brynskov J, D’Haens G, Hanauer SB, Irvine EJ, Jewell DP, Rachmilewitz D, Sachar DB, Sandborn WJ. A simple classification of Crohn’s disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm Bowel Dis. 2000;6:8-15. [PubMed] |
| 23. | Fukumoto A, Tanaka S, Yamamoto H, Yao T, Matsui T, Iida M, Goto H, Sakamoto C, Chiba T, Sugano K. Diagnosis and treatment of small-bowel stricture by double balloon endoscopy. Gastrointest Endosc. 2007;66:S108-S112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Hoffmann JC, Heller F, Faiss S, von Lampe B, Kroesen AJ, Wahnschaffe U, Schulzke JD, Zeitz M, Bojarski C. Through the endoscope balloon dilation of ileocolonic strictures: prognostic factors, complications, and effectiveness. Int J Colorectal Dis. 2008;23:689-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Solem CA, Harmsen WS, Zinsmeister AR, Loftus EV. Small intestinal adenocarcinoma in Crohn’s disease: a case-control study. Inflamm Bowel Dis. 2004;10:32-35. [PubMed] |
| 26. | Feldstein RC, Sood S, Katz S. Small bowel adenocarcinoma in Crohn’s disease. Inflamm Bowel Dis. 2008;14:1154-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Kodaira C, Osawa S, Mochizuki C, Sato Y, Nishino M, Yamada T, Takayanagi Y, Takagaki K, Sugimoto K, Kanaoka S. A case of small bowel adenocarcinoma in a patient with Crohn’s disease detected by PET/CT and double-balloon enteroscopy. World J Gastroenterol. 2009;15:1774-1778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Cahill C, Gordon PH, Petrucci A, Boutros M. Small bowel adenocarcinoma and Crohn’s disease: any further ahead than 50 years ago? World J Gastroenterol. 2014;20:11486-11495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Ohmiya N, Arakawa D, Nakamura M, Honda W, Shirai O, Taguchi A, Itoh A, Hirooka Y, Niwa Y, Maeda O. Small-bowel obstruction: diagnostic comparison between double-balloon endoscopy and fluoroscopic enteroclysis, and the outcome of enteroscopic treatment. Gastrointest Endosc. 2009;69:84-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Paine E, Shen B. Endoscopic therapy in inflammatory bowel diseases (with videos). Gastrointest Endosc. 2013;78:819-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Khanna R, Bouguen G, Feagan BG, D’Haens G, Sandborn WJ, Dubcenco E, Baker KA, Levesque BG. A systematic review of measurement of endoscopic disease activity and mucosal healing in Crohn’s disease: recommendations for clinical trial design. Inflamm Bowel Dis. 2014;20:1850-1861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 32. | Ferrante M, Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D’Haens GR, van der Woude CJ. Validation of endoscopic activity scores in patients with Crohn’s disease based on a post hoc analysis of data from SONIC. Gastroenterology. 2013;145:978-986.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (1)] |
| 33. | Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology. 1990;99:956-963. [PubMed] |
| 34. | Strobel D, Goertz RS, Bernatik T. Diagnostics in inflammatory bowel disease: ultrasound. World J Gastroenterol. 2011;17:3192-3197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 35. | Castiglione F, Mainenti PP, De Palma GD, Testa A, Bucci L, Pesce G, Camera L, Diaferia M, Rea M, Caporaso N. Noninvasive diagnosis of small bowel Crohn’s disease: direct comparison of bowel sonography and magnetic resonance enterography. Inflamm Bowel Dis. 2013;19:991-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 36. | Asthana AK, Friedman AB, Maconi G, Maaser C, Kucharzik T, Watanabe M, Gibson PR. Failure of gastroenterologists to apply intestinal ultrasound in inflammatory bowel disease in the Asia-Pacific: a need for action. J Gastroenterol Hepatol. 2015;30:446-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Panes J, Bouhnik Y, Reinisch W, Stoker J, Taylor SA, Baumgart DC, Danese S, Halligan S, Marincek B, Matos C. Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. J Crohns Colitis. 2013;7:556-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 478] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 38. | Panés J, Bouzas R, Chaparro M, García-Sánchez V, Gisbert JP, Martínez de Guereñu B, Mendoza JL, Paredes JM, Quiroga S, Ripollés T. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease. Aliment Pharmacol Ther. 2011;34:125-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 475] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 39. | Baumgart DC, Müller HP, Grittner U, Metzke D, Fischer A, Guckelberger O, Pascher A, Sack I, Vieth M, Rudolph B. US-based Real-time Elastography for the Detection of Fibrotic Gut Tissue in Patients with Stricturing Crohn Disease. Radiology. 2015;275:889-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 40. | Ma X, Li Y, Jia H, Zhang J, Wang G, Liu X, Song Y. Contrast-enhanced ultrasound in the diagnosis of patients suspected of having active Crohn’s disease: meta-analysis. Ultrasound Med Biol. 2015;41:659-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Estay C, Simian D, Lubascher J, Figueroa C, O’Brien A, Quera R. Ionizing radiation exposure in patients with inflammatory bowel disease: are we overexposing our patients? J Dig Dis. 2015;16:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | Hall EJ, Brenner DJ. Cancer risks from diagnostic radiology. Br J Radiol. 2008;81:362-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 577] [Article Influence: 33.9] [Reference Citation Analysis (1)] |
| 43. | Chiorean L, Schreiber-Dietrich D, Braden B, Cui XW, Buchhorn R, Chang JM, Dietrich CF. Ultrasonographic imaging of inflammatory bowel disease in pediatric patients. World J Gastroenterol. 2015;21:5231-5241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 44. | Swanson G, Behara R, Braun R, Keshavarzian A. Diagnostic medical radiation in inflammatory bowel disease: how to limit risk and maximize benefit. Inflamm Bowel Dis. 2013;19:2501-2508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Quencer KB, Nimkin K, Mino-Kenudson M, Gee MS. Detecting active inflammation and fibrosis in pediatric Crohn‘s disease: prospective evaluation of MR-E and CT-E. Abdom Imaging. 2013;38:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 46. | Masselli G, Gualdi G. MR imaging of the small bowel. Radiology. 2012;264:333-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 47. | Mentzel HJ, Reinsch S, Kurzai M, Stenzel M. Magnetic resonance imaging in children and adolescents with chronic inflammatory bowel disease. World J Gastroenterol. 2014;20:1180-1191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 48. | Rimola J, Planell N, Rodríguez S, Delgado S, Ordás I, Ramírez-Morros A, Ayuso C, Aceituno M, Ricart E, Jauregui-Amezaga A. Characterization of inflammation and fibrosis in Crohn’s disease lesions by magnetic resonance imaging. Am J Gastroenterol. 2015;110:432-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 203] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 49. | Van Assche G, Herrmann KA, Louis E, Everett SM, Colombel JF, Rahier JF, Vanbeckevoort D, Meunier P, Tolan D, Ernst O. Effects of infliximab therapy on transmural lesions as assessed by magnetic resonance enteroclysis in patients with ileal Crohn’s disease. J Crohns Colitis. 2013;7:950-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 50. | Rimola J, Ordás I, Rodríguez S, Ricart E, Panés J. Imaging indexes of activity and severity for Crohn’s disease: current status and future trends. Abdom Imaging. 2012;37:958-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Lawrance IC, Welman CJ, Shipman P, Murray K. Correlation of MRI-determined small bowel Crohn’s disease categories with medical response and surgical pathology. World J Gastroenterol. 2009;15:3367-3375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 52. | Makanyanga J, Punwani S, Taylor SA. Assessment of wall inflammation and fibrosis in Crohn’s disease: value of T1-weighted gadolinium-enhanced MR imaging. Abdom Imaging. 2012;37:933-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 53. | D’Incà R, Caccaro R. Measuring disease activity in Crohn’s disease: what is currently available to the clinician. Clin Exp Gastroenterol. 2014;7:151-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 54. | Beaugerie L, Seksik P, Nion-Larmurier I, Gendre JP, Cosnes J. Predictors of Crohn’s disease. Gastroenterology. 2006;130:650-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 640] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 55. | Allez M, Lemann M, Bonnet J, Cattan P, Jian R, Modigliani R. Long term outcome of patients with active Crohn’s disease exhibiting extensive and deep ulcerations at colonoscopy. Am J Gastroenterol. 2002;97:947-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 56. | Rieder F, de Bruyn JR, Pham BT, Katsanos K, Annese V, Higgins PD, Magro F, Dotan I. Results of the 4th scientific workshop of the ECCO (Group II): markers of intestinal fibrosis in inflammatory bowel disease. J Crohns Colitis. 2014;8:1166-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 57. | Adler J, Rangwalla SC, Dwamena BA, Higgins PD. The prognostic power of the NOD2 genotype for complicated Crohn’s disease: a meta-analysis. Am J Gastroenterol. 2011;106:699-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 58. | Cleynen I, González JR, Figueroa C, Franke A, McGovern D, Bortlík M, Crusius BJ, Vecchi M, Artieda M, Szczypiorska M. Genetic factors conferring an increased susceptibility to develop Crohn’s disease also influence disease phenotype: results from the IBDchip European Project. Gut. 2013;62:1556-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 216] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 59. | Targan SR, Landers CJ, Yang H, Lodes MJ, Cong Y, Papadakis KA, Vasiliauskas E, Elson CO, Hershberg RM. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn’s disease. Gastroenterology. 2005;128:2020-2028. [PubMed] |
| 60. | Mow WS, Vasiliauskas EA, Lin YC, Fleshner PR, Papadakis KA, Taylor KD, Landers CJ, Abreu-Martin MT, Rotter JI, Yang H. Association of antibody responses to microbial antigens and complications of small bowel Crohn’s disease. Gastroenterology. 2004;126:414-424. [PubMed] |
| 61. | Amre DK, Lu SE, Costea F, Seidman EG. Utility of serological markers in predicting the early occurrence of complications and surgery in pediatric Crohn’s disease patients. Am J Gastroenterol. 2006;101:645-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 62. | Rieder F, Lawrance IC, Leite A, Sans M. Predictors of fibrostenotic Crohn’s disease. Inflamm Bowel Dis. 2011;17:2000-2007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 63. | Kjeldsen J, Schaffalitzky de Muckadell OB, Junker P. Seromarkers of collagen I and III metabolism in active Crohn’s disease. Relation to disease activity and response to therapy. Gut. 1995;37:805-810. [PubMed] |
| 64. | Loeschke K, Kaltenthaler P. [Procollagen-III-peptide in the serum of patients with Crohn disease]. Z Gastroenterol. 1989;27:137-139. [PubMed] |
| 65. | Dotan I. Disease behavior in adult patients: are there predictors for stricture or fistula formation? Dig Dis. 2009;27:206-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 66. | Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology. 2012;142:1293-1302.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 452] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 67. | Kikuchi K, Kubo M, Sato S, Fujimoto M, Tamaki K. Serum tissue inhibitor of metalloproteinases in patients with systemic sclerosis. J Am Acad Dermatol. 1995;33:973-978. [PubMed] |
| 68. | Kratzer W, von Tirpitz C, Mason R, Reinshagen M, Adler G, Möller P, Rieber A, Kächele V. Contrast-enhanced power Doppler sonography of the intestinal wall in the differentiation of hypervascularized and hypovascularized intestinal obstructions in patients with Crohn’s disease. J Ultrasound Med. 2002;21:149-157; quiz 158-159. [PubMed] |
| 69. | Kumar S, Hakim A, Alexakis C, Chhaya V, Tzias D, Pilcher J, Vlahos J, Pollok R. Small intestinal contrast ultrasonography for the detection of small bowel complications in Crohn’s disease: correlation with intraoperative findings and magnetic resonance enterography. J Gastroenterol Hepatol. 2015;30:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 70. | Paredes JM, Ripollés T, Cortés X, Moreno N, Martínez MJ, Bustamante-Balén M, Delgado F, Moreno-Osset E. Contrast-enhanced ultrasonography: usefulness in the assessment of postoperative recurrence of Crohn’s disease. J Crohns Colitis. 2013;7:192-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 71. | Ripollés T, Rausell N, Paredes JM, Grau E, Martínez MJ, Vizuete J. Effectiveness of contrast-enhanced ultrasound for characterisation of intestinal inflammation in Crohn’s disease: a comparison with surgical histopathology analysis. J Crohns Colitis. 2013;7:120-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 72. | Malagò R, D’Onofrio M, Mantovani W, D’Alpaos G, Foti G, Pezzato A, Caliari G, Cusumano D, Benini L, Pozzi Mucelli R. Contrast-enhanced ultrasonography (CEUS) vs. MRI of the small bowel in the evaluation of Crohn’s disease activity. Radiol Med. 2012;117:268-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 73. | Ophir J, Céspedes I, Ponnekanti H, Yazdi Y, Li X. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991;13:111-134. [PubMed] |
| 74. | Yada N, Kudo M, Kawada N, Sato S, Osaki Y, Ishikawa A, Miyoshi H, Sakamoto M, Kage M, Nakashima O. Noninvasive diagnosis of liver fibrosis: utility of data mining of both ultrasound elastography and serological findings to construct a decision tree. Oncology. 2014;87 Suppl 1:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 75. | Sommerfeld HJ, Garcia-Schürmann JM, Schewe J, Kühne K, Cubick F, Berges RR, Lorenz A, Pesavento A, Scheipers U, Ermert H. [Prostate cancer diagnosis using ultrasound elastography. Introduction of a novel technique and first clinical results]. Urologe A. 2003;42:941-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 76. | Weitzel WF, Kim K, Rubin JM, Wiggins RC, Xie H, Chen X, Emelianov SY, O’Donnell M. Feasibility of applying ultrasound strain imaging to detect renal transplant chronic allograft nephropathy. Kidney Int. 2004;65:733-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 77. | Dillman JR, Stidham RW, Higgins PD, Moons DS, Johnson LA, Rubin JM. US elastography-derived shear wave velocity helps distinguish acutely inflamed from fibrotic bowel in a Crohn disease animal model. Radiology. 2013;267:757-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 78. | Dillman JR, Stidham RW, Higgins PD, Moons DS, Johnson LA, Keshavarzi NR, Rubin JM. Ultrasound shear wave elastography helps discriminate low-grade from high-grade bowel wall fibrosis in ex vivo human intestinal specimens. J Ultrasound Med. 2014;33:2115-2123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 79. | Sasaki T, Kunisaki R, Kinoshita H, Yamamoto H, Kimura H, Hanzawa A, Shibata N, Yonezawa H, Miyajima E, Sakamaki K. Use of color Doppler ultrasonography for evaluating vascularity of small intestinal lesions in Crohn’s disease: correlation with endoscopic and surgical macroscopic findings. Scand J Gastroenterol. 2014;49:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 80. | Limberg B. [Diagnosis of chronic inflammatory bowel disease by ultrasonography]. Z Gastroenterol. 1999;37:495-508. [PubMed] |
| 81. | Zallot C, Peyrin-Biroulet L. Deep remission in inflammatory bowel disease: looking beyond symptoms. Curr Gastroenterol Rep. 2013;15:315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (37)] |
| 82. | Tielbeek JA, Ziech ML, Li Z, Lavini C, Bipat S, Bemelman WA, Roelofs JJ, Ponsioen CY, Vos FM, Stoker J. Evaluation of conventional, dynamic contrast enhanced and diffusion weighted MRI for quantitative Crohn’s disease assessment with histopathology of surgical specimens. Eur Radiol. 2014;24:619-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 159] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 83. | Taylor SA, Punwani S, Rodriguez-Justo M, Bainbridge A, Greenhalgh R, De Vita E, Forbes A, Cohen R, Windsor A, Obichere A. Mural Crohn disease: correlation of dynamic contrast-enhanced MR imaging findings with angiogenesis and inflammation at histologic examination--pilot study. Radiology. 2009;251:369-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 84. | Makanyanga JC, Taylor SA. Current and future role of MR enterography in the management of Crohn disease. AJR Am J Roentgenol. 2013;201:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 85. | Oussalah A, Laurent V, Bruot O, Bressenot A, Bigard MA, Régent D, Peyrin-Biroulet L. Diffusion-weighted magnetic resonance without bowel preparation for detecting colonic inflammation in inflammatory bowel disease. Gut. 2010;59:1056-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 86. | Dillman JR, Swanson SD, Johnson LA, Moons DS, Adler J, Stidham RW, Higgins PD. Comparison of noncontrast MRI magnetization transfer and T2 -Weighted signal intensity ratios for detection of bowel wall fibrosis in a Crohn’s disease animal model. J Magn Reson Imaging. 2015;42:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 87. | Jacene HA, Ginsburg P, Kwon J, Nguyen GC, Montgomery EA, Bayless TM, Wahl RL. Prediction of the need for surgical intervention in obstructive Crohn’s disease by 18F-FDG PET/CT. J Nucl Med. 2009;50:1751-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 88. | Louis E, Ancion G, Colard A, Spote V, Belaiche J, Hustinx R. Noninvasive assessment of Crohn’s disease intestinal lesions with (18)F-FDG PET/CT. J Nucl Med. 2007;48:1053-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 89. | Bettenworth D, Reuter S, Hermann S, Weckesser M, Kerstiens L, Stratis A, Nowacki TM, Ross M, Lenze F, Edemir B. Translational 18F-FDG PET/CT imaging to monitor lesion activity in intestinal inflammation. J Nucl Med. 2013;54:748-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 90. | Atreya R, Neumann H, Neufert C, Waldner MJ, Billmeier U, Zopf Y, Willma M, App C, Münster T, Kessler H. In vivo imaging using fluorescent antibodies to tumor necrosis factor predicts therapeutic response in Crohn’s disease. Nat Med. 2014;20:313-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 295] [Article Influence: 26.8] [Reference Citation Analysis (0)] |